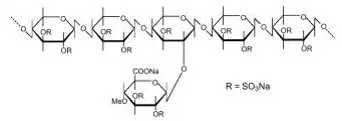
Drug Detail:Elmiron (Pentosan polysulfate sodium [ pen-toe-san-pol-ee-sul-fate-soe-dee-um ])
Drug Class: Miscellaneous genitourinary tract agents
Elmiron - Clinical Pharmacology
Pharmacokinetics
Clinical Studies
ELMIRON ® was evaluated in two clinical trials for the relief of pain in patients with chronic interstitial cystitis (IC). All patients met the NIH definition of IC based upon the results of cystoscopy, cytology, and biopsy. One blinded, randomized, placebo-controlled study evaluated 151 patients (145 women, 5 men, 1 unknown) with a mean age of 44 years (range 18 to 81). Approximately equal numbers of patients received either placebo or ELMIRON ® 100 mg three times a day for 3 months. Clinical improvement in bladder pain was based upon the patient's own assessment. In this study, 28/74 (38%) of patients who received ELMIRON ® and 13/74 (18%) of patients who received placebo showed greater than 50% improvement in bladder pain (p = 0.005).
A second clinical trial, the physician's usage study, was a prospectively designed retrospective analysis of 2499 patients who received ELMIRON ® 300 mg a day without blinding. Of the 2499 patients, 2220 were women, 254 were men, and 25 were of unknown sex. The patients had a mean age of 47 years and 23% were over 60 years of age. By 3 months, 1307 (52%) of the patients had dropped out or were ineligible for analysis, overall, 1192 (48%) received ELMIRON ® for 3 months; 892 (36%) received ELMIRON ® for 6 months; and 598 (24%) received ELMIRON ® for one year.
Patients had unblinded evaluations every 3 months for the patient's rating of overall change in pain in comparison to baseline and for the difference calculated in "pain/discomfort" scores. At baseline, pain/discomfort scores for the original 2499 patients were severe or unbearable in 60%, moderate in 33% and mild or none in 7% of patients. The extent of the patients' pain improvement is shown in Table 1.
At 3 months, 722/2499 (29%) of the patients originally in the study had pain scores that improved by one or two categories. By 6 months, in the 892 patients who continued taking ELMIRON ®, an additional 116/2499 (5%) of patients had improved pain scores. After 6 months, the percent of patients who reported the first onset of pain relief was less than 1.5% of patients who originally entered in the study (see Table 2).
| Efficacy Parameter | 3 months † | 6 months † |
|---|---|---|
|
||
| Patient Rating of Overall Change in Pain (Recollection of difference between current pain and baseline pain) ‡ | N=1161
Median = 3 Mean = 3.44 CI: (3.37, 3.51) | N=724
Median = 4 Mean = 3.91 CI: (3.83, 3.99) |
| Change in Pain/Discomfort Score (Calculated difference in scores at the time point and baseline) § | N=1440
Median = 1 Mean = 0.51 CI: (0.45, 0.57) | N=904
Median = 1 Mean = 0.66 CI: (0.61, 0.71) |
| at 3 months
†
(n=1192) | at 6 months
‡
(n=892) |
|
|---|---|---|
|
||
| Considering only the patients who continued treatment | 722/1192 (61%) | 116/892 (13%) |
| Considering all the patients originally enrolled in the study | 722/2499 (29%) | 116/2499 (5%) |
Warnings
Retinal Pigmentary Changes
Pigmentary changes in the retina, reported in the literature as pigmentary maculopathy, have been identified with long-term use of ELMIRON ® (see ADVERSE REACTIONS). Although most of these cases occurred after 3 years of use or longer, cases have been seen with a shorter duration of use. While the etiology is unclear, cumulative dose appears to be a risk factor. Visual symptoms in the reported cases included difficulty reading, slow adjustment to low or reduced light environments, and blurred vision. The visual consequences of these pigmentary changes are not fully characterized. Caution should be used in patients with retinal pigment changes from other causes in which examination findings may confound the appropriate diagnosis, follow-up, and treatment. Detailed ophthalmologic history should be obtained in all patients prior to starting treatment with ELMIRON ®. If there is a family history of hereditary pattern dystrophy, genetic testing should be considered. For patients with pre-existing ophthalmologic conditions, a comprehensive baseline retinal examination (including color fundoscopic photography, ocular coherence tomography (OCT), and auto-fluorescence imaging) is recommended prior to starting therapy. A baseline retinal examination (including OCT and auto-fluorescence imaging) is suggested for all patients within six months of initiating treatment and periodically while continuing treatment. If pigmentary changes in the retina develop, then risks and benefits of continuing treatment should be re-evaluated, since these changes may be irreversible. Follow-up retinal examinations should be continued given that retinal and vision changes may progress even after cessation of treatment.
Precautions
Hepatic Insufficiency
ELMIRON ® has not been studied in patients with hepatic insufficiency. Because there is evidence of hepatic contribution to the elimination of ELMIRON ®, hepatic impairment may have an impact on the pharmacokinetics of ELMIRON ®. Caution should be exercised when using ELMIRON ® in this patient population.
Mildly (< 2.5 × normal) elevated transaminase, alkaline phosphatase, γ-glutamyl transpeptidase, and lactic dehydrogenase occurred in 1.2% of patients. The increases usually appeared 3 to 12 months after the start of ELMIRON ® therapy, and were not associated with jaundice or other clinical signs or symptoms. These abnormalities are usually transient, may remain essentially unchanged, or may rarely progress with continued use. Increases in PTT and PT (< 1% for both) or thrombocytopenia (0.2%) were noted.
Adverse Reactions/Side Effects
ELMIRON ® was evaluated in clinical trials in a total of 2627 patients (2343 women, 262 men, 22 unknown) with a mean age of 47 [range 18 to 88 with 581 (22%) over 60 years of age]. Of the 2627 patients, 128 patients were in a 3-month trial and the remaining 2499 patients were in a long-term, unblinded trial.
Deaths occurred in 6/2627 (0.2%) patients who received the drug over a period of 3 to 75 months. The deaths appear to be related to other concurrent illnesses or procedures, except in one patient for whom the cause was not known.
Serious adverse events occurred in 33/2627 (1.3%) patients. Two patients had severe abdominal pain or diarrhea and dehydration that required hospitalization. Because there was not a control group of patients with interstitial cystitis who were concurrently evaluated, it is difficult to determine which events are associated with ELMIRON ® and which events are associated with concurrent illness, medicine, or other factors.
| Body System/Adverse Experience | ELMIRON
®
n=128 | Placebo
n=130 |
|---|---|---|
|
||
| CNS Overall Number of Patients * | 3 | 5 |
| Insomnia | 1 | 0 |
| Headache | 1 | 3 |
| Severe Emotional Lability/Depression | 2 | 1 |
| Nystagmus/Dizziness | 1 | 1 |
| Hyperkinesia | 1 | 1 |
| GI Overall Number of Patients * | 7 | 7 |
| Nausea | 3 | 3 |
| Diarrhea | 3 | 6 |
| Dyspepsia | 1 | 0 |
| Jaundice | 0 | 1 |
| Vomiting | 0 | 2 |
| Skin/Allergic Overall Number of Patients * | 2 | 4 |
| Rash | 0 | 2 |
| Pruritus | 0 | 2 |
| Lacrimation | 1 | 1 |
| Rhinitis | 1 | 1 |
| Increased Sweating | 1 | 0 |
| Other Overall Number of Patients * | 1 | 3 |
| Amenorrhea | 0 | 1 |
| Arthralgia | 0 | 1 |
| Vaginitis | 1 | 1 |
| Total Events | 17 | 27 |
| Total Number of Patients Reporting Adverse Events | 13 | 19 |
The adverse events described below were reported in an unblinded clinical trial of 2499 interstitial cystitis patients treated with ELMIRON ®. Of the original 2499 patients, 1192 (48%) received ELMIRON ® for 3 months; 892 (36%) received ELMIRON ® for 6 months; and 598 (24%) received ELMIRON ® for one year, 355 (14%) received ELMIRON ® for 2 years, and 145 (6%) for 4 years.
Frequency (1 to 4%): Alopecia (4%), diarrhea (4%), nausea (4%), headache (3%), rash (3%), dyspepsia (2%), abdominal pain (2%), liver function abnormalities (1%), dizziness (1%).
Frequency (≤ 1%):
Digestive: Vomiting, mouth ulcer, colitis, esophagitis, gastritis, flatulence, constipation, anorexia, gum hemorrhage.
Hematologic: Anemia, ecchymosis, increased prothrombin time, increased partial thromboplastin time, leukopenia, thrombocytopenia.
Hypersensitive Reactions: Allergic reaction, photosensitivity.
Respiratory System: Pharyngitis, rhinitis, epistaxis, dyspnea.
Skin and Appendages: Pruritus, urticaria.
Special Senses: Conjunctivitis, tinnitus, optic neuritis, amblyopia, retinal hemorrhage.
| This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 03/2021 | ||||||
| MEDICATION GUIDE
ELMIRON ® (EL ma ron) (pentosan polysulfate sodium) capsules, for oral use |
||||||
|
What is the most important information I should know about ELMIRON?
|
||||||
|
|
|
||||
Throughout your treatment, regular eye examinations that include retinal examinations are suggested for early detection of retinal/macular changes. Your doctor will discuss with you when to get your first eye examination and follow up exams, and whether the treatment should be continued.
|
||||||
|
|
|
|
|||
| Your risk of bleeding may be increased if you take ELMIRON along with other medicines such as: | ||||||
|
|
|||||
| Tell your healthcare provider if you are taking any of these medicines.
Before you start taking ELMIRON, tell your healthcare provider if you are going to have surgery. Your healthcare provider may stop ELMIRON before you have surgery. Talk to your healthcare provider about when to stop taking ELMIRON and when to start taking it again. |
||||||
What is ELMIRON?
|
||||||
Do not take ELMIRON if you:
|
||||||
Before your take ELMIRON, tell your healthcare provider about all of your medical conditions, including if you:
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
||||||
How should I take ELMIRON?
|
||||||
| What are the possible side effects of ELMIRON?
Serious side effects have been reported with the use of ELMIRON, including: Changes in the retina of the eye Increased bleeding (See " What is the most important information I should know about ELMIRON?") The most common side effects of ELMIRON are: |
||||||
|
|
|
||||
| These are not all of the possible side effects of ELMIRON.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||||
How should I store ELMIRON?
|
||||||
| General information about the safe and effective use of ELMIRON.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ELMIRON for a condition for which it was not prescribed. Do not give ELMIRON to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ELMIRON that is written for health professionals. |
||||||
| What are the ingredients in ELMIRON?
Active ingredient: pentosan polysulfate sodium Inactive ingredients: microcrystalline cellulose, magnesium stearate, gelatin, pharmaceutical glaze (modified) in SD-45, synthetic black iron oxide, FD&C Blue No. 2 aluminum lake, FD&C Red No. 40 aluminum lake, FD&C Blue No. 1 aluminum lake, D&C Yellow No. 10 aluminum lake, n-butyl alcohol, propylene glycol, SDA-3A alcohol, and titanium dioxide. |
||||||
| ELMIRON is a registered trademark of Teva Branded Pharmaceutical Products R&D Inc., used under license.
© 2002, 2021 Janssen Pharmaceutical Companies Product of Germany Manufactured for: Janssen Pharmaceuticals, Inc. Titusville, New Jersey 08560 For more information, go to www.ORTHOELMIRON.com or call 1-800-526-7736. |
||||||
| ELMIRON
pentosan polysulfate sodium capsule, gelatin coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Janssen Pharmaceuticals, Inc. (063137772) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Ortho, LLC | 805887986 | analysis(50458-098) , manufacture(50458-098) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| bene pharmaChem GmbH & Co. KG | 332479380 | api manufacture(50458-098) | |