Drug Detail:Eloxatin (Oxaliplatin [ ox-al-i-pla-tin ])
Drug Class: Alkylating agents
Highlights of Prescribing Information
ELOXATIN (oxaliplatin) injection for intravenous use
Initial U.S. Approval: 2002
WARNING: ANAPHYLACTIC REACTIONS
See full prescribing information for complete boxed warning.
Anaphylactic reactions to ELOXATIN have been reported, and may occur within minutes of ELOXATIN administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms. (5.1)
Recent Major Changes
| Dosage and Administration (2.2) | 10/2015 |
| Warnings and Precautions (5.3, 5.6, 5.7) | 10/2015 |
Indications and Usage for Eloxatin
ELOXATIN is a platinum-based drug used in combination with infusional 5-fluorouracil /leucovorin, which is indicated for:
- adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor. (1)
- treatment of advanced colorectal cancer. (1)
Eloxatin Dosage and Administration
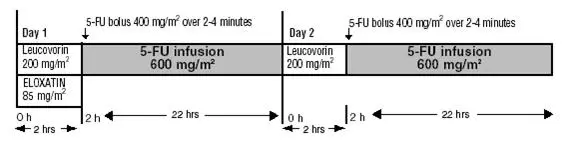
- Administer ELOXATIN in combination with 5-fluorouracil/leucovorin every 2 weeks. (2.1):
- –
- Day 1: ELOXATIN 85 mg/m2 intravenous infusion in 250–500 mL 5% Dextrose Injection, USP and leucovorin 200 mg/m2 intravenous infusion in 5% Dextrose Injection, USP both given over 120 minutes at the same time in separate bags using a Y-line, followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2–4 minutes, followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion.
- –
- Day 2: leucovorin 200 mg/m2 intravenous infusion over 120 minutes, followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2–4 minutes, followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion.
- Reduce the dose of ELOXATIN to 75 mg/m2 (adjuvant setting) or 65 mg/m2 (advanced colorectal cancer) (2.2):
- –
- if there are persistent grade 2 neurosensory events that do not resolve.
- –
- after recovery from grade 3/4 gastrointestinal toxicities (despite prophylactic treatment) or grade 4 neutropenia or febrile neutropenia or grade 3/4 thrombocytopenia. Delay next dose until neutrophils ≥1.5 × 109/L and platelets ≥75 × 109/L.
- For patients with severe renal impairment (creatinine clearance <30 mL/min), the initial recommended dose is 65 mg/m2. (2.2)
- Discontinue ELOXATIN if there are persistent Grade 3 neurosensory events. (2.2)
- Never prepare a final dilution with a sodium chloride solution or other chloride-containing solutions. (2.3)
Dosage Forms and Strengths
Single-use vials of 50 mg or 100 mg oxaliplatin as a sterile, preservative-free, aqueous solution at a concentration of 5 mg/mL. (3)
Contraindications
- Known allergy to ELOXATIN or other platinum compounds. (4, 5.1)
Warnings and Precautions
- Allergic Reactions: Monitor for development of rash, urticaria, erythema, pruritis, bronchospasm, and hypotension. (5.1)
- Neuropathy: Reduce the dose or discontinue ELOXATIN if necessary. (5.2)
- Severe Neutropenia: Delay ELOXATIN until neutrophils are ≥1.5 × 109/L. Withhold ELOXATIN for sepsis. (5.3)
- Pulmonary Toxicity: May need to discontinue ELOXATIN until interstitial lung disease or pulmonary fibrosis are excluded. (5.4)
- Hepatotoxicity: Monitor liver function tests. (5.5)
- Cardiovascular Toxicity: Correct hypokalemia or hypomagnesemia prior to initiating ELOXATIN. (5.6)
- Rhabdomyolysis: Discontinue ELOXATIN if rhabdomyolysis occurs. (5.7)
- Pregnancy. Fetal harm can occur when administered to a pregnant woman. Women should be apprised of the potential harm to the fetus. (5.8, 8.1)
Adverse Reactions/Side Effects
- Most common adverse reactions (incidence ≥ 40%) were peripheral sensory neuropathy, neutropenia, thrombocytopenia, anemia, nausea, increase in transaminases and alkaline phosphatase, diarrhea, emesis, fatigue and stomatitis. Other adverse reactions, including serious adverse reactions, have been reported. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2015
Full Prescribing Information
WARNING: ANAPHYLACTIC REACTIONS
Anaphylactic reactions to ELOXATIN have been reported, and may occur within minutes of ELOXATIN administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms of anaphylaxis [see Warnings and Precautions (5.1)].
1. Indications and Usage for Eloxatin
ELOXATIN, used in combination with infusional 5-fluorouracil/leucovorin, is indicated for:
- adjuvant treatment of stage III colon cancer in patients who have undergone complete resection of the primary tumor.
- treatment of advanced colorectal cancer.
2. Eloxatin Dosage and Administration
ELOXATIN (oxaliplatin injection) should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate diagnostic and treatment facilities are readily available.
2.2 Dose Modification Recommendations
Prior to subsequent therapy cycles, patients should be evaluated for clinical toxicities and recommended laboratory tests [see Warnings and Precautions (5.9)]. Prolongation of infusion time for ELOXATIN from 2 hours to 6 hours may mitigate acute toxicities. The infusion times for 5-fluorouracil and leucovorin do not need to be changed.
2.3 Preparation of Infusion Solution
Do not freeze and protect from light the concentrated solution.
A final dilution must never be performed with a sodium chloride solution or other chloride-containing solutions.
The solution must be further diluted in an infusion solution of 250-500 mL of 5% Dextrose Injection, USP.
After dilution with 250-500 mL of 5% Dextrose Injection, USP, the shelf life is 6 hours at room temperature [20-25°C (68-77°F)] or up to 24 hours under refrigeration [2-8°C (36-46°F)].
After final dilution, protection from light is not required.
ELOXATIN is incompatible in solution with alkaline medications or media (such as basic solutions of 5-fluorouracil) and must not be mixed with these or administered simultaneously through the same infusion line. The infusion line should be flushed with 5% Dextrose Injection, USP prior to administration of any concomitant medication.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and discarded if present.
Needles or intravenous administration sets containing aluminum parts that may come in contact with ELOXATIN should not be used for the preparation or mixing of the drug. Aluminum has been reported to cause degradation of platinum compounds.
3. Dosage Forms and Strengths
ELOXATIN is supplied in single-use vials containing 50 mg or 100 mg of oxaliplatin as a sterile, preservative-free, aqueous solution at a concentration of 5 mg/mL.
4. Contraindications
ELOXATIN should not be administered to patients with a history of known allergy to ELOXATIN or other platinum compounds [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Allergic Reactions
See boxed warning
Grade 3/4 hypersensitivity, including anaphylactic/anaphylactoid reactions, to ELOXATIN has been observed in 2–3% of colon cancer patients. These allergic reactions which can be fatal, can occur within minutes of administration and at any cycle, and were similar in nature and severity to those reported with other platinum-containing compounds, such as rash, urticaria, erythema, pruritus, and, rarely, bronchospasm and hypotension. The symptoms associated with hypersensitivity reactions reported in the previously untreated patients were urticaria, pruritus, flushing of the face, diarrhea associated with oxaliplatin infusion, shortness of breath, bronchospasm, diaphoresis, chest pains, hypotension, disorientation and syncope. These reactions are usually managed with standard epinephrine, corticosteroid, antihistamine therapy, and require discontinuation of therapy. Rechallenge is contraindicated in these patients [see Contraindications (4)]. Drug-related deaths associated with platinum compounds from anaphylaxis have been reported.
5.3 Severe Neutropenia
Grade 3 or 4 neutropenia occurred in 41–44% of patients with colorectal cancer treated with ELOXATIN in combination with 5-flurouracil (5-FU) and leucovorin compared to 5% with 5-FU plus leucovorin alone. Sepsis, neutropenic sepsis and septic shock have been reported in patients treated with ELOXATIN, including fatal outcomes [see Adverse Reactions (6.1)].
Delay ELOXATIN until neutrophils are ≥ 1.5 × 109/L. Withhold ELOXATIN for sepsis or septic shock. Dose reduce ELOXATIN after recovery from Grade 4 neutropenia or febrile neutropenia [see Dosage and Administration (2.2)].
5.4 Pulmonary Toxicity
ELOXATIN has been associated with pulmonary fibrosis (<1% of study patients), which may be fatal. The combined incidence of cough and dyspnea was 7.4% (any grade) and <1% (grade 3) with no grade 4 events in the ELOXATIN plus infusional 5-fluorouracil/leucovorin arm compared to 4.5% (any grade) and no grade 3 and 0.1% grade 4 events in the infusional 5-fluorouracil/leucovorin alone arm in adjuvant colon cancer patients. In this study, one patient died from eosinophilic pneumonia in the ELOXATIN combination arm. The combined incidence of cough, dyspnea and hypoxia was 43% (any grade) and 7% (grade 3 and 4) in the ELOXATIN plus 5-fluorouracil/leucovorin arm compared to 32% (any grade) and 5% (grade 3 and 4) in the irinotecan plus 5-fluorouracil/leucovorin arm of unknown duration for patients with previously untreated colorectal cancer. In case of unexplained respiratory symptoms such as non-productive cough, dyspnea, crackles, or radiological pulmonary infiltrates, ELOXATIN should be discontinued until further pulmonary investigation excludes interstitial lung disease or pulmonary fibrosis.
5.5 Hepatotoxicity
Hepatotoxicity as evidenced in the adjuvant study, by increase in transaminases (57% vs. 34%) and alkaline phosphatase (42% vs. 20%) was observed more commonly in the ELOXATIN combination arm than in the control arm. The incidence of increased bilirubin was similar on both arms. Changes noted on liver biopsies include: peliosis, nodular regenerative hyperplasia or sinusoidal alterations, perisinusoidal fibrosis, and veno-occlusive lesions. Hepatic vascular disorders should be considered, and if appropriate, should be investigated in case of abnormal liver function test results or portal hypertension, which cannot be explained by liver metastases [see Clinical Trials Experience (6.1)].
5.6 Cardiovascular Toxicity
QT prolongation and ventricular arrhythmias including fatal Torsade de Pointes have been reported in postmarketing experiences following ELOXATIN administration. ECG monitoring is recommended if therapy is initiated in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating ELOXATIN and monitor these electrolytes periodically during therapy. Avoid ELOXATIN in patients with congenital long QT syndrome [see Adverse Reactions (6.2)].
5.7 Rhabdomyolysis
Rhabdomyolysis, including fatal cases, has been reported in patients treated with ELOXATIN. Discontinue ELOXATIN if any signs or symptoms of rhabdomyolysis occur. [see Adverse Reactions (6.2)].
5.8 Use in Pregnancy
Pregnancy Category D
ELOXATIN may cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of ELOXATIN in pregnant women. Women of childbearing potential should be advised to avoid becoming pregnant while receiving treatment with ELOXATIN. [see Use in Specific Populations (8.1)].
5.9 Recommended Laboratory Tests
Standard monitoring of the white blood cell count with differential, hemoglobin, platelet count, and blood chemistries (including ALT, AST, bilirubin and creatinine) is recommended before each ELOXATIN cycle [see Dosage and Administration (2)].
There have been reports while on study and from post-marketing surveillance of prolonged prothrombin time and INR occasionally associated with hemorrhage in patients who received ELOXATIN plus 5-fluorouracil/leucovorin while on anticoagulants. Patients receiving ELOXATIN plus 5-fluorouracil/leucovorin and requiring oral anticoagulants may require closer monitoring.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Anaphylaxis and Allergic reactions [see Boxed Warning, Warnings and Precautions (5.1).]
- Neuropathy [see Warnings and Precautions (5.2).]
- Severe Neutropenia [see Warnings and Precautions (5.3).]
- Pulmonary Toxicities [see Warnings and Precautions (5.4).]
- Hepatotoxicity [see Warnings and Precautions (5.5).]
- Cardiovascular Toxicities [see Warnings and Precautions (5.6).]
- Rhabdomyolysis [see Warnings and Precautions (5.7).]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
More than 1100 patients with stage II or III colon cancer and more than 4,000 patients with advanced colorectal cancer have been treated in clinical studies with ELOXATIN. The most common adverse reactions in patients with stage II or III colon cancer receiving adjuvant therapy were peripheral sensory neuropathy, neutropenia, thrombocytopenia, anemia, nausea, increase in transaminases and alkaline phosphatase, diarrhea, emesis, fatigue and stomatitis. The most common adverse reactions in previously untreated and treated patients were peripheral sensory neuropathies, fatigue, neutropenia, nausea, emesis, and diarrhea [see Warnings and Precautions (5)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ELOXATIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a whole:
angioedema, anaphylactic shock
Cardiovascular disorders:
QT prolongation leading to ventricular arrhythmias including fatal Torsade de Pointes
Central and peripheral nervous system disorders:
loss of deep tendon reflexes, dysarthria, Lhermitte's sign, cranial nerve palsies, fasciculations, convulsion, Reversible Posterior Leukoencephalopathy Syndrome (RPLS, also known as PRES).
Hearing and vestibular system disorders:
deafness
Infections:
septic shock, including fatal outcomes
Infusion reactions/hypersensitivity:
laryngospasm
Liver and Gastrointestinal system disorders:
severe diarrhea/vomiting resulting in hypokalemia, colitis (including Clostridium difficile diarrhea), metabolic acidosis; ileus; intestinal obstruction, pancreatitis; veno-occlusive disease of liver also known as sinusoidal obstruction syndrome, and perisinusoidal fibrosis which rarely may progress.
Musculoskeletal and connective tissue disorders
rhabdomyolysis, including fatal outcomes.
Platelet, bleeding, and clotting disorders:
immuno-allergic thrombocytopenia
prolongation of prothrombin time and of INR in patients receiving anticoagulants
Red Blood Cell disorders:
hemolytic uremic syndrome, immuno-allergic hemolytic anemia
Renal disorders:
Acute tubular necrosis, acute interstitial nephritis and acute renal failure.
Respiratory system disorders:
pulmonary fibrosis, and other interstitial lung diseases (sometimes fatal)
Vision disorders:
decrease of visual acuity, visual field disturbance, optic neuritis and transient vision loss (reversible following therapy discontinuation)
7. Drug Interactions
No specific cytochrome P-450-based drug interaction studies have been conducted. No pharmacokinetic interaction between 85 mg/m2 ELOXATIN and 5-fluorouracil/leucovorin has been observed in patients treated every 2 weeks. Increases of 5-fluorouracil plasma concentrations by approximately 20% have been observed with doses of 130 mg/m2 ELOXATIN dosed every 3 weeks. Because platinum-containing species are eliminated primarily through the kidney, clearance of these products may be decreased by coadministration of potentially nephrotoxic compounds; although, this has not been specifically studied [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.3 Nursing Mothers
It is not known whether ELOXATIN or its derivatives are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ELOXATIN, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The effectiveness of oxaliplatin in children has not been established. Oxaliplatin has been tested in 2 Phase 1 and 2 Phase 2 trials in 235 patients ages 7 months to 22 years with solid tumors (see below) and no significant activity observed.
In a Phase 1/2 study, oxaliplatin was administered as a 2-hour intravenous infusion on Days 1, 8 and 15 every 4 weeks (1 cycle), for a maximum of 6 cycles, to 43 patients with refractory or relapsed malignant solid tumors, mainly neuroblastoma and osteosarcoma. Twenty eight pediatric patients in the Phase 1 study received oxaliplatin at 6 dose levels starting at 40 mg/m2 with escalation to 110 mg/m2. The dose limiting toxicity (DLT) was sensory neuropathy at the 110 mg/m2 dose. Fifteen patients received oxaliplatin at a dose of 90 mg/m2 intravenous in the Phase 2 portion of the study. At this dose, paresthesia (60%, G3/4: 7%), fever (40%, G3/4: 7%) and thrombocytopenia (40%, G3/4: 27%) were the main adverse reactions. No responses were observed.
In a second Phase 1 study, oxaliplatin was administered to 26 pediatric patients as a 2-hour intravenous infusion on day 1 every 3 weeks (1 cycle) at 5 dose levels starting at 100 mg/m2 with escalation to 160 mg/m2, for a maximum of 6 cycles. In a separate cohort, oxaliplatin 85 mg/m2 was administered on day 1 every 2 weeks, for a maximum of 9 doses. Patients had metastatic or unresectable solid tumors mainly neuroblastoma and ganglioneuroblastoma. No responses were observed. The DLT was sensory neuropathy at the 160 mg/m2 dose. Based on these studies, oxaliplatin 130 mg/m2 as a 2-hour intravenous infusion on day 1 every 3 weeks (1 cycle) was used in subsequent Phase II studies. A dose of 85 mg/m2 on day 1 every 2 weeks was also found to be tolerable.
In one Phase 2 study, 43 pediatric patients with recurrent or refractory embryonal CNS tumors received oxaliplatin 130 mg/m2 every 3 weeks for a maximum of 12 months in absence of progressive disease or unacceptable toxicity. In patients < 10 kg the oxaliplatin dose used was 4.3 mg/kg. The most common adverse reactions reported were leukopenia (67%, G3/4: 12%), anemia (65%, G3/4: 5%), thrombocytopenia (65%, G3/4: 26%), vomiting (65%, G3/4: 7%), neutropenia (58%, G3/4: 16%) and sensory neuropathy (40%, G3/4: 5%). One partial response was observed.
In a second Phase 2 study, 123 pediatric patients with recurrent solid tumors, including neuroblastoma, osteosarcoma, Ewing sarcoma or peripheral PNET, ependymoma, rhabdomyosarcoma, hepatoblastoma, high grade astrocytoma, Brain stem glioma, low grade astrocytoma, malignant germ cell tumor and other tumors of interest received oxaliplatin 130 mg/m2 every 3 weeks for a maximum of 12 months or 17 cycles. In patients ≤ 12 months old the oxaliplatin dose used was 4.3 mg/kg. The most common adverse reactions reported were sensory neuropathy (52%, G3/4: 12%), thrombocytopenia (37%, G3/4: 17%), anemia (37%, G3/4: 9%), vomiting (26%, G3/4:4%), ALT increased (24%, G3/4: 6%), AST increased (24%, G3/4: 2%), and nausea (23%, G3/:4 3%). Two partial responses were observed.
The pharmacokinetic parameters of ultrafiltrable platinum have been evaluated in 105 pediatric patients during the first cycle. The mean clearance in pediatric patients estimated by the population pharmacokinetic analysis was 4.7 L/h. The inter-patient variability of platinum clearance in pediatric cancer patients was 41%. Mean platinum pharmacokinetic parameters in ultrafiltrate were Cmax of 0.75 ± 0.24 mcg/mL, AUC0–48 of 7.52 ± 5.07 mcg∙h/mL and AUCinf of 8.83 ± 1.57 mcg∙h/mL at 85 mg/m2 of oxaliplatin and Cmax of 1.10 ± 0.43 mcg/mL, AUC0–48 of 9.74 ± 2.52 mcg∙h/mL and AUCinf of 17.3 ± 5.34 mcg∙h/mL at 130 mg/m2 of oxaliplatin.
8.5 Geriatric Use
No significant effect of age on the clearance of ultrafilterable platinum has been observed.
In the adjuvant therapy colon cancer randomized clinical trial, [see Clinical Studies (14)] 723 patients treated with ELOXATIN and infusional 5-fluorouracil/leucovorin were <65 years and 400 patients were ≥65 years.
A descriptive subgroup analysis demonstrated that the improvement in DFS for the ELOXATIN combination arm compared to the infusional 5-fluorouracil/leucovorin alone arm appeared to be maintained across genders. The effect of ELOXATIN in patients ≥65 years of age was not conclusive. Insufficient subgroup sizes prevented analysis by race.
Patients ≥ 65 years of age receiving the ELOXATIN combination therapy experienced more grade 3–4 granulocytopenia than patients < 65 years of age (45% versus 39%).
In the previously untreated for advanced colorectal cancer randomized clinical trial [see Clinical Studies (14)] of ELOXATIN, 160 patients treated with ELOXATIN and 5-fluorouracil/leucovorin were < 65 years and 99 patients were ≥65 years. The same efficacy improvements in response rate, time to tumor progression, and overall survival were observed in the ≥65 year old patients as in the overall study population. In the previously treated for advanced colorectal cancer randomized clinical trial [see Clinical Studies (14)] of ELOXATIN, 95 patients treated with ELOXATIN and 5-fluorouracil/leucovorin were <65 years and 55 patients were ≥65 years. The rates of overall adverse reactions, including grade 3 and 4 events, were similar across and within arms in the different age groups in all studies. The incidence of diarrhea, dehydration, hypokalemia, leukopenia, fatigue and syncope were higher in patients ≥65 years old. No adjustment to starting dose was required in patients ≥65 years old.
8.6 Patients with Renal Impairment
The exposure (AUC) of unbound platinum in plasma ultrafiltrate tends to increase in renally impaired patients [see Pharmacokinetics (12.3)]. Caution and close monitoring should be exercised when ELOXATIN is administered to patients with renal impairment. The starting ELOXATIN dose does not need to be reduced in patients with mild (creatinine clearance=50-80 mL/min) or moderate (creatinine clearance=30-49 mL/min) renal impairment. However, the starting dose of ELOXATIN should be reduced in patients with severe renal impairment (creatinine clearance < 30 mL/min) [see Dosage and Administration (2.2)].
11. Eloxatin Description
ELOXATIN® (oxaliplatin injection) is an antineoplastic agent with the molecular formula C8H14N2O4Pt and the chemical name of cis-[(1 R,2 R)-1,2-cyclohexanediamine-N,N'] [oxalato(2-)- O,O'] platinum. Oxaliplatin is an organoplatinum complex in which the platinum atom is complexed with 1,2-diaminocyclohexane(DACH) and with an oxalate ligand as a leaving group.
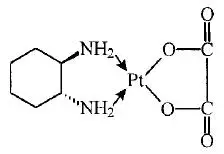
The molecular weight is 397.3. Oxaliplatin is slightly soluble in water at 6 mg/mL, very slightly soluble in methanol, and practically insoluble in ethanol and acetone.
ELOXATIN is supplied in vials containing 50 mg or 100 mg of oxaliplatin as a sterile, preservative-free, aqueous solution at a concentration of 5 mg/mL. Water for Injection, USP is present as an inactive ingredient.
12. Eloxatin - Clinical Pharmacology
12.1 Mechanism of Action
Oxaliplatin undergoes nonenzymatic conversion in physiologic solutions to active derivatives via displacement of the labile oxalate ligand. Several transient reactive species are formed, including monoaquo and diaquo DACH platinum, which covalently bind with macromolecules. Both inter- and intrastrand Pt-DNA crosslinks are formed. Crosslinks are formed between the N7 positions of two adjacent guanines (GG), adjacent adenine-guanines (AG), and guanines separated by an intervening nucleotide (GNG). These crosslinks inhibit DNA replication and transcription. Cytotoxicity is cell-cycle nonspecific.
In vivo studies have shown antitumor activity of oxaliplatin against colon carcinoma. In combination with 5-fluorouracil, oxaliplatin exhibits in vitro and in vivo antiproliferative activity greater than either compound alone in several tumor models [HT29 (colon), GR (mammary), and L1210 (leukemia)].
12.3 Pharmacokinetics
The reactive oxaliplatin derivatives are present as a fraction of the unbound platinum in plasma ultrafiltrate. The decline of ultrafilterable platinum levels following oxaliplatin administration is triphasic, characterized by two relatively short distribution phases (t1/2α; 0.43 hours and t1/2β; 16.8 hours) and a long terminal elimination phase (t1/2γ; 391 hours). Pharmacokinetic parameters obtained after a single 2-hour intravenous infusion of ELOXATIN at a dose of 85 mg/m2 expressed as ultrafilterable platinum were Cmax of 0.814 mcg/mL and volume of distribution of 440 L.
Interpatient and intrapatient variability in ultrafilterable platinum exposure (AUC0–48hr) assessed over 3 cycles was moderate to low (23% and 6%, respectively). A pharmacodynamic relationship between platinum ultrafiltrate levels and clinical safety and effectiveness has not been established.
14. Clinical Studies
14.1 Combination Adjuvant Therapy with ELOXATIN and Infusional 5-fluorouracil/leucovorin in Patients with Colon Cancer
An international, multicenter, randomized study compared the efficacy and evaluated the safety of ELOXATIN in combination with an infusional schedule of 5-fluorouracil/leucovorin to infusional 5-fluorouracil/leucovorin alone, in patients with stage II (Dukes' B2) or III (Dukes' C) colon cancer who had undergone complete resection of the primary tumor. The primary objective of the study was to compare the 3-year disease-free survival (DFS) in patients receiving ELOXATIN and infusional 5-fluorouracil/leucovorin to those receiving 5-fluorouracil/leucovorin alone. Patients were to be treated for a total of 6 months (i.e., 12 cycles). A total of 2246 patients were randomized; 1123 patients per study arm. Patients in the study had to be between 18 and 75 years of age, have histologically proven stage II (T3–T4 N0 M0; Dukes' B2) or III (any T N1–2 M0; Dukes' C) colon carcinoma (with the inferior pole of the tumor above the peritoneal reflection, i.e., ≥15 cm from the anal margin) and undergone (within 7 weeks prior to randomization) complete resection of the primary tumor without gross or microscopic evidence of residual disease. Patients had to have had no prior chemotherapy, immunotherapy or radiotherapy, and have an ECOG performance status of 0,1, or 2 (KPS ≥ 60%), absolute neutrophil count (ANC) > 1.5x109/L, platelets ≥100×109/L, serum creatinine ≤ 1.25 × ULN total bilirubin < 2 × ULN, AST/ALT < 2 × ULN and carcino-embyrogenic antigen (CEA) < 10 ng/mL. Patients with preexisting peripheral neuropathy (NCI grade ≥ 1) were ineligible for this trial.
The following table shows the dosing regimens for the two arms of the study.
| Treatment | ||
|---|---|---|
| Arm | Dose | Regimen |
|
ELOXATIN + 5-FU/LV (FOLFOX4) (N =1123) | Day 1: ELOXATIN: 85 mg/m2 (2-hour infusion) + LV: 200 mg/m2 (2-hour infusion), followed by 5-FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) Day 2: LV: 200 mg/m2 (2-hour infusion), followed by 5-FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks 12 cycles |
|
5-FU/LV (N=1123) | Day 1: LV: 200 mg/m2 (2-hour infusion), followed by 5-FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) Day 2: LV: 200 mg/m2 (2-hour infusion), followed by 5-FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks 12 cycles |
The following tables show the baseline characteristics and dosing of the patient population entered into this study. The baseline characteristics were well balanced between arms.
| ELOXATIN + infusional 5-FU/LV N=1123 | Infusional 5-FU/LV N=1123 |
|
|---|---|---|
| Sex: Male (%) | 56.1 | 52.4 |
| Female (%) | 43.9 | 47.6 |
| Median age (years) | 61.0 | 60.0 |
| <65 years of age (%) | 64.4 | 66.2 |
| ≥65 years of age (%) | 35.6 | 33.8 |
| Karnofsky Performance Status (KPS) (%) | ||
| 100 | 29.7 | 30.5 |
| 90 | 52.2 | 53.9 |
| 80 | 4.4 | 3.3 |
| 70 | 13.2 | 11.9 |
| ≤60 | 0.6 | 0.4 |
| Primary site (%) | ||
| Colon including cecum | 54.6 | 54.4 |
| Sigmoid | 31.9 | 33.8 |
| Recto sigmoid | 12.9 | 10.9 |
| Other including rectum | 0.6 | 0.9 |
| Bowel obstruction (%) | ||
| Yes | 17.9 | 19.3 |
| Perforation (%) | ||
| Yes | 6.9 | 6.9 |
| Stage at Randomization (%) | ||
| II (T=3,4 N=0, M=0) | 40.1 | 39.9 |
| III (T=any, N=1,2, M=0) | 59.6 | 59.3 |
| IV (T=any, N=any, M=1) | 0.4 | 0.8 |
| Staging – T (%) | ||
| T1 | 0.5 | 0.7 |
| T2 | 4.5 | 4.8 |
| T3 | 76.0 | 75.9 |
| T4 | 19.0 | 18.5 |
| Staging – N (%) | ||
| N0 | 40.2 | 39.9 |
| N1 | 39.4 | 39.4 |
| N2 | 20.4 | 20.7 |
| Staging – M (%) | ||
| M1 | 0.4 | 0.8 |
| ELOXATIN + infusional 5-FU/LV N=1108 | Infusional 5-FU/LV N=1111 |
|
|---|---|---|
| Median Relative Dose Intensity (%) | ||
| 5-FU | 84.4 | 97.7 |
| ELOXATIN | 80.5 | N/A |
| Median Number of Cycles | 12 | 12 |
| Median Number of cycles with ELOXATIN | 11 | N/A |
The following table and figures summarize the disease-free survival (DFS) results in the overall randomized population and in patients with stage II and III disease based on an ITT analysis. The median duration of follow-up was approximately 77 months.
| ELOXATIN + Infusional 5-FU/LV | Infusional 5-FU/LV |
|
|---|---|---|
|
||
| Parameter | ||
| Overall | ||
| N | 1123 | 1123 |
| Number of events – relapse or death (%) | 304 (27.1) | 360 (32.1) |
| Disease-free survival % [95% CI]† | 73.3 [70.7, 76.0] | 67.4 [64.6, 70.2] |
| Hazard ratio [95% CI]‡ | 0.80 [0.68, 0.93] | |
| Stratified Logrank test | p=0.003 | |
| Stage III (Dukes' C) | ||
| N | 672 | 675 |
| Number of events –relapse or death (%) | 226 (33.6) | 271 (40.1) |
| Disease-free survival % [95% CI]† | 66.4 [62.7, 70.0] | 58.9 [55.2, 62.7] |
| Hazard ratio [95% CI]‡ | 0.78 [0.65, 0.93] | |
| Logrank test | p=0.005 | |
| Stage II (Dukes' B2) | ||
| N | 451 | 448 |
| Number of events – relapse or death (%) | 78 (17.3) | 89 (19.9) |
| Disease-free survival % [95% CI] † | 83.7 [80.2, 87.1] | 79.9 [76.2, 83.7] |
| Hazard ratio [95% CI]‡ | 0.84 [0.62, 1.14] | |
| Logrank test | p=0.258 | |
In the overall and stage III colon cancer populations DFS was statistically significantly improved in the ELOXATIN combination arm compared to infusional 5-fluorouracil/leucovorin alone. However, a statistically significant improvement in DFS was not noted in Stage II patients.
Figure 2 shows the DFS Kaplan-Meier curves for the comparison of ELOXATIN and infusional 5-fluorouracil/leucovorin combination and infusional 5-fluorouracil/leucovorin alone for the overall population (ITT analysis).
Figure 3 shows the DFS Kaplan-Meier curves for the comparison of ELOXATIN and infusional 5-fluorouracil/leucovorin combination and infusional 5-fluorouracil/leucovorin alone in Stage III patients.
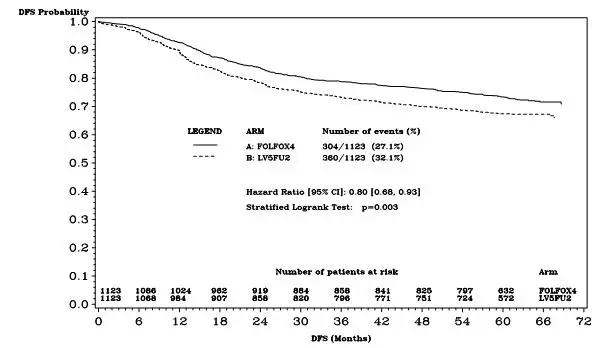
Figure 2 - DFS Kaplan-Meier curves by treatment arm (cutoff: 1 June 2006) – ITT population
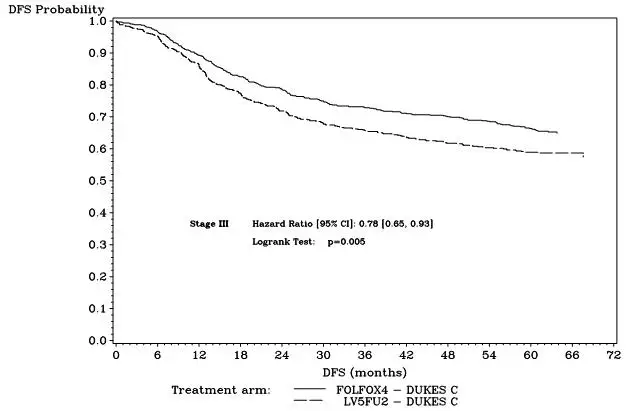
Figure 3 - DFS Kaplan-Meier curves by treatment arm in Stage III patients (cutoff: 1 June 2006) – ITT population
The following table summarizes the overall survival (OS) results in the overall randomized population and in patients with stage II and III disease, based on the ITT analysis.
|
||
| Parameter | Eloxatin + Infusional 5-FU/LV | Infusional 5-FU/LV |
| Overall | ||
| N | 1123 | 1123 |
| Number of death events (%) | 245 (21.8) | 283 (25.2) |
| Hazard ratio†[95% CI] | 0.84 [0.71 , 1.00] | |
| Stage III (Dukes' C) | ||
| N | 672 | 675 |
| Number of death events (%) | 182 (27.1) | 220 (32.6) |
| Hazard ratio† [95% CI] | 0.80 [0.65 , 0.97] | |
| Stage II (Dukes' B2) | ||
| N | 451 | 448 |
| Number of death events (%) | 63 (14.0) | 63 (14.1) |
| Hazard ratio† [95% CI] | 1.00 [0.70 , 1.41] | |
14.2 Combination Therapy with ELOXATIN and 5-fluorouracil/leucovorin in Patients Previously Untreated for Advanced Colorectal Cancer
A North American, multicenter, open-label, randomized controlled study was sponsored by the National Cancer Institute (NCI) as an intergroup study led by the North Central Cancer Treatment Group (NCCTG). The study had 7 arms at different times during its conduct, four of which were closed due to either changes in the standard of care, toxicity, or simplification. During the study, the control arm was changed to irinotecan plus 5-fluorouracil/leucovorin. The results reported below compared the efficacy and safety of two experimental regimens, ELOXATIN in combination with infusional 5-fluorouracil/leucovorin and a combination of ELOXATIN plus irinotecan, to an approved control regimen of irinotecan plus 5-fluorouracil/leucovorin in 795 concurrently randomized patients previously untreated for locally advanced or metastatic colorectal cancer. After completion of enrollment, the dose of irinotecan plus 5-fluorouracil/leucovorin was decreased due to toxicity. Patients had to be at least 18 years of age, have known locally advanced, locally recurrent, or metastatic colorectal adenocarcinoma not curable by surgery or amenable to radiation therapy with curative intent, histologically proven colorectal adenocarcinoma, measurable or evaluable disease, with an ECOG performance status 0,1, or 2. Patients had to have granulocyte count ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, hemoglobin ≥9.0 gm/dL, creatinine ≤ 1.5 × ULN, total bilirubin ≤ 1.5 mg/dL, AST ≤ 5 × ULN, and alkaline phosphatase ≤ 5 × ULN. Patients may have received adjuvant therapy for resected Stage II or III disease without recurrence within 12 months. The patients were stratified for ECOG performance status (0, 1 vs. 2), prior adjuvant chemotherapy (yes vs. no), prior immunotherapy (yes vs. no), and age (<65 vs. ≥65 years). Although no post study treatment was specified in the protocol, 65 to 72% of patients received additional post study chemotherapy after study treatment discontinuation on all arms. Fifty-eight percent of patients on the ELOXATIN plus 5-fluorouracil/leucovorin arm received an irinotecan-containing regimen and 23% of patients on the irinotecan plus 5-fluorouracil/leucovorin arm received oxaliplatin-containing regimens. Oxaliplatin was not commercially available during the trial.
The following table presents the dosing regimens of the three arms of the study.
| Treatment Arm | Dose | Regimen |
|---|---|---|
| ELOXATIN + 5-FU/LV (FOLFOX4) (N=267) | Day 1: ELOXATIN: 85 mg/m2 (2-hour infusion) + LV 200 mg/m2 (2-hour infusion), followed by 5-FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) Day 2: LV 200 mg/m2 (2-hour infusion), followed by 5-FU: 400 mg/m2 (bolus), 600 mg/m2 (22-hour infusion) | every 2 weeks |
| Irinotecan + 5-FU/LV (IFL) (N=264) | Day 1: irinotecan 125 mg/m2 as a 90–min infusion + LV 20 mg/m2 as a 15-min infusion or intravenous push, followed by 5-FU 500 mg/m2 intravenous bolus weekly × 4 | every 6 weeks |
| ELOXATIN + Irinotecan (IROX) (N=264) | Day 1: ELOXATIN: 85 mg/m2 intravenous (2-hour infusion) + irinotecan 200 mg/m2 intravenous over 30 minutes | every 3 weeks |
The following table presents the demographics of the patient population entered into this study.
| ELOXATIN + 5-FU/LV N=267 | Irinotecan + 5-FU/LV N=264 | ELOXATIN + irinotecan N=264 |
|
|---|---|---|---|
| Sex: Male (%) | 58.8 | 65.2 | 61.0 |
| Female (%) | 41.2 | 34.8 | 39.0 |
| Median age (years) | 61.0 | 61.0 | 61.0 |
| <65 years of age (%) | 61 | 62 | 63 |
| ≥65 years of age (%) | 39 | 38 | 37 |
| ECOG (%) | |||
| 0–1 | 94.4 | 95.5 | 94.7 |
| 2 | 5.6 | 4.5 | 5.3 |
| Involved organs (%) | |||
| Colon only | 0.7 | 0.8 | 0.4 |
| Liver only | 39.3 | 44.3 | 39.0 |
| Liver + other | 41.2 | 38.6 | 40.9 |
| Lung only | 6.4 | 3.8 | 5.3 |
| Other (including lymph nodes) | 11.6 | 11.0 | 12.9 |
| Not reported | 0.7 | 1.5 | 1.5 |
| Prior radiation (%) | 3.0 | 1.5 | 3.0 |
| Prior surgery (%) | 74.5 | 79.2 | 81.8 |
| Prior adjuvant (%) | 15.7 | 14.8 | 15.2 |
The length of a treatment cycle was 2 weeks for the ELOXATIN and 5-fluorouracil/leucovorin regimen; 6 weeks for the irinotecan plus 5-fluorouracil/leucovorin regimen; and 3 weeks for the ELOXATIN plus irinotecan regimen. The median number of cycles administered per patient was 10 (23.9 weeks) for the ELOXATIN and 5-fluorouracil/leucovorin regimen, 4 (23.6 weeks) for the irinotecan plus 5-fluorouracil/leucovorin regimen, and 7 (21.0 weeks) for the ELOXATIN plus irinotecan regimen. Patients treated with the ELOXATIN and 5-fluorouracil/leucovorin combination had a significantly longer time to tumor progression based on investigator assessment, longer overall survival, and a significantly higher confirmed response rate based on investigator assessment compared to patients given irinotecan plus 5-fluorouracil/leucovorin. The following table summarizes the efficacy results.
| ELOXATIN + 5-FU/LV N=267 | irinotecan + 5-FU/LV N=264 | ELOXATIN + irinotecan N=264 |
|
|---|---|---|---|
|
|||
| Survival (ITT) | |||
| Number of deaths N (%) | 155 (58.1) | 192 (72.7) | 175 (66.3) |
| Median survival (months) | 19.4 | 14.6 | 17.6 |
| Hazard Ratio and (95% confidence interval) | 0.65 (0.53–0.80)† | ||
| P-value | <0.0001† | - | - |
| TTP (ITT, investigator assessment) | |||
| Percentage of progressors | 82.8 | 81.8 | 89.4 |
| Median TTP (months) | 8.7 | 6.9 | 6.5 |
| Hazard Ratio and (95% confidence interval)‡ | 0.74 (0.61–0.89)† | ||
| P-value | 0.0014† | - | - |
| Response Rate (investigator assessment)§ | |||
| Patients with measurable disease | 210 | 212 | 215 |
| Complete response N (%) | 13 (6.2) | 5 (2.4) | 7 (3.3) |
| Partial response N (%) | 82 (39.0) | 64 (30.2) | 67 (31.2) |
| Complete and partial response N (%) | 95 (45.2) | 69 (32.5) | 74 (34.4) |
| 95% confidence interval | (38.5 – 52.0) | (26.2 – 38.9) | (28.1 – 40.8) |
| P-value | 0.0080† | - | - |
Figure 4 illustrates the Kaplan-Meier survival curves for the comparison of ELOXATIN and 5-fluorouracil/leucovorin combination and ELOXATIN plus irinotecan to irinotecan plus 5-fluorouracil/leucovorin.
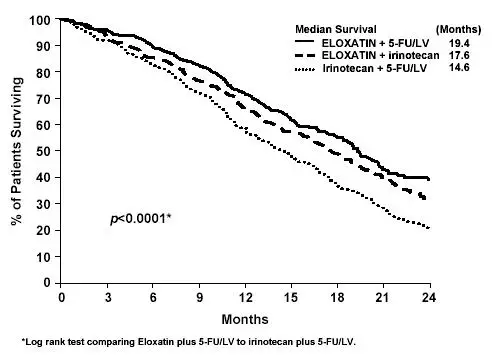
Figure 4– Kaplan-Meier Overall Survival by treatment arm
A descriptive subgroup analysis demonstrated that the improvement in survival for ELOXATIN plus 5-fluorouracil/leucovorin compared to irinotecan plus 5-fluorouracil/leucovorin appeared to be maintained across age groups, prior adjuvant therapy, and number of organs involved. An estimated survival advantage in ELOXATIN plus 5-fluorouracil/leucovorin versus irinotecan plus 5-fluorouracil/leucovorin was seen in both genders; however it was greater among women than men. Insufficient subgroup sizes prevented analysis by race.
15. References
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004–165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. (2006) ASHP Guidelines on Handling Hazardous Drugs.
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society.
FDA-Approved Patient Labeling
| This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 10/2015 Issue date: 10/07/2015 |
||
| Patient Information ELOXATIN® (eh-LOX-ah-tin) (oxaliplatin) injection for intravenous use |
||
| Read this Patient Information leaflet carefully before you start receiving ELOXATIN. There may be new information. It will help you learn more about ELOXATIN. This leaflet does not take the place of talking to your doctor about your medical condition or your treatment. Ask your doctor about any questions you have. | ||
|
What is the most important information I should know about ELOXATIN? ELOXATIN can cause serious allergic reactions, including allergic reactions that can lead to death. ELOXATIN is a platinum base medicine. Serious allergic reactions including death can happen in people who take ELOXATIN and who have had previous allergic reactions to platinum medicines. Serious allergic reactions can happen within a few minutes of your ELOXATIN infusion or any time during your treatment with ELOXATIN. Get emergency help right away if you:
Call your doctor right away if you have any of the following signs or symptoms of an allergic reaction: |
||
|
|
|
| See "What are the possible side effects of ELOXATIN?" for information about other serious side effects. | ||
|
What is ELOXATIN? ELOXATIN is an anti-cancer (chemotherapy) medicine that is used with other anti-cancer medicines called 5-fluorouracil and leucovorin to treat people with:
It is not known if ELOXATIN is effective in children. |
||
|
Who should not receive ELOXATIN? Do not receive ELOXATIN if you are allergic to any of the ingredients in ELOXATIN or other medicines that contain platinum. See the end of this leaflet for a complete list of the ingredients ELOXATIN. Ask your doctor if you are not sure if you take a medicine that contains platinum. |
||
|
What should I tell my doctor before receiving ELOXATIN? Before receiving ELOXATIN, tell your doctor about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. |
||
|
How will I receive ELOXATIN?
Treatment Day 1:
Treatment Day 2: You will not get ELOXATIN on Day 2. Leucovorin and 5-fluorouracil will be given the same way as on Day 1. The 5-fluorouracil will be given through your IV with a pump. If you have any problems with the pump or the tube, call your doctor, your nurse, or the person who is responsible for your pump. Do not let anyone other than a healthcare provider touch your infusion pump or tubing. |
||
|
What should I avoid while receiving ELOXATIN?
See "How can I reduce the side effects caused by cold temperatures?" for more information. Talk with your doctor and nurse about your level of activity during treatment with ELOXATIN. Follow their instructions. |
||
|
What are the possible side effects of ELOXATIN? ELOXATIN can cause serious side effects, including:
For information on ways to lessen or help with the nerve problems, see the end of this leaflet, "How can I reduce the side effects caused by cold temperatures?"
|
||
|
|
|
The most common side effects of ELOXATIN include:
Tell your doctor if you have any side effect that bothers your or that does not go away. These are not all the possible side effects of ELOXATIN. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How can I reduce the side effects caused by cold temperatures?
Your doctor may have other useful tips for helping you with side effects. |
||
|
General information about the safe and effective use of ELOXATIN Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. This Patient Information leaflet summarizes the most important information about ELOXATIN. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about ELOXATIN that is written for health professionals. |
||
| What are the ingredients in ELOXATIN?
Active ingredient: oxaliplatin Inactive ingredient: water for injection Manufactured by: sanofi-aventis U.S. LLC Bridgewater, NJ 08807, A SANOFI COMPANY © 2015 sanofi-aventis U.S. LLC |
||
| ELOXATIN
oxaliplatin injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ELOXATIN
oxaliplatin injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| sanofi-aventis Deutschland GmbH | 313218430 | MANUFACTURE(0024-0590, 0024-0591) , ANALYSIS(0024-0590, 0024-0591) , PACK(0024-0590, 0024-0591) , LABEL(0024-0590, 0024-0591) | |