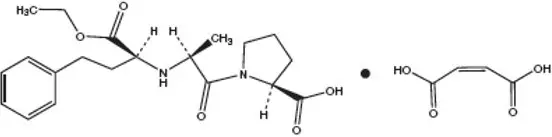
Drug Class: ACE inhibitors with thiazides
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
- When pregnancy is detected, discontinue enalapril maleate and hydrochlorothiazide as soon as possible.
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See WARNINGS: Fetal Toxicity
Enalapril and Hydrochlorothiazide - Clinical Pharmacology
As a result of its diuretic effects, hydrochlorothiazide increases plasma renin activity, increases aldosterone secretion, and decreases serum potassium. Administration of enalapril maleate blocks the renin-angiotensin-aldosterone axis and tends to reverse the potassium loss associated with the diuretic.
In clinical studies, the extent of blood pressure reduction seen with the combination of enalapril maleate and hydrochlorothiazide was approximately additive. The antihypertensive effect of enalapril maleate and hydrochlorothiazide was usually sustained for at least 24 hours.
Concomitant administration of enalapril maleate and hydrochlorothiazide has little, or no effect on the bioavailability of either drug. The combination tablet is bioequivalent to concomitant administration of the separate entities.
Indications and Usage for Enalapril and Hydrochlorothiazide
Enalapril maleate and hydrochlorothiazide is indicated for the treatment of hypertension.
This fixed dose combination is not indicated for initial treatment (see DOSAGE AND ADMINISTRATION).
In using enalapril maleate and hydrochlorothiazide, consideration should be given to the fact that another angiotensin converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease, and that available data are insufficient to show that enalapril does not have a similar risk (see WARNINGS).
In considering use of enalapril maleate and hydrochlorothiazide, it should be noted that black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to non-blacks (see WARNINGS, Head and Neck Angioedema).
Contraindications
Enalapril maleate and hydrochlorothiazide is contraindicated in patients who are hypersensitive to any component of this product and in patients with a history of angioedema related to previous treatment with an angiotensin converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema. Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
Enalapril maleate and hydrochlorothiazide is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer enalapril maleate and hydrochlorothiazide within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor (see WARNINGS).
Do not co-administer aliskiren with enalapril maleate and hydrochlorothiazide in patients with diabetes (see PRECAUTIONS, Drug Interactions).
Warnings
General
Enalapril Maleate
Hypotension
Excessive hypotension was rarely seen in uncomplicated hypertensive patients but is a possible consequence of enalapril use in severely salt/volume depleted persons such as those treated vigorously with diuretics or patients on dialysis.
Syncope has been reported in 1.3 percent of patients receiving enalapril maleate and hydrochlorothiazide. In patients receiving enalapril alone, the incidence of syncope is 0.5 percent. The overall incidence of syncope may be reduced by proper titration of the individual components (see PRECAUTIONS, Drug Interactions, ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION).
In patients with severe congestive heart failure, with or without associated renal insufficiency, excessive hypotension has been observed and may be associated with oliguria and/or progressive azotemia, and rarely with acute renal failure and/or death. Because of the potential fall in blood pressure in these patients, therapy should be started under very close medical supervision. Such patients should be followed closely for the first two weeks of treatment and whenever the dose of enalapril and/or diuretic is increased. Similar considerations may apply to patients with ischemic heart or cerebrovascular disease, in whom an excessive fall in blood pressure could result in a myocardial infarction or cerebrovascular accident.
If hypotension occurs, the patient should be placed in the supine position and, if necessary, receive an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further doses, which usually can be given without difficulty once the blood pressure has increased after volume expansion.
Head and Neck Angioedema
Angioedema of the face, extremities, lips, tongue, glottis and/or larynx has been reported in patients treated with angiotensin converting enzyme inhibitors, including enalapril. This may occur at any time during treatment. In such cases enalapril maleate and hydrochlorothiazide should be promptly discontinued and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms has occurred. In instances where swelling has been confined to the face and lips the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms. Angioedema associated with laryngeal edema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, appropriate therapy, e.g., subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and/or measures necessary to ensure a patent airway, should be promptly provided (see ADVERSE REACTIONS).
Patients receiving coadministration of ACE inhibitor and mTOR (mammalian target of rapamycin) inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema (see PRECAUTIONS).
Anaphylactoid Reactions During Membrane Exposure
Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis, and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Fetal Toxicity
Enalapril Maleate
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue enalapril maleate and hydrochlorothiazide as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue enalapril maleate and hydrochlorothiazide, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to enalapril maleate and hydrochlorothiazide for hypotension, oliguria, and hyperkalemia (see PRECAUTIONS, Pediatric Use).
No teratogenic effects of enalapril were seen in studies of pregnant rats and rabbits. On a body surface area basis, the doses used were 57 times and 12 times, respectively, the MRHDD.
Precautions
General
Enalapril Maleate
Drug Interactions
Enalapril Maleate
Agents Increasing Serum Potassium
Enalapril attenuates diuretic-induced potassium loss. Potassium-sparing diuretics (e.g., spironolactone, triamterene, or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated because of demonstrated hypokalemia they should be used with caution and with frequent monitoring of serum potassium.
Adverse Reactions/Side Effects
Enalapril maleate and hydrochlorothiazide has been evaluated for safety in more than 1500 patients, including over 300 patients treated for one year or more. In clinical trials with enalapril maleate and hydrochlorothiazide no adverse experiences peculiar to this combination drug have been observed. Adverse experiences that have occurred, have been limited to those that have been previously reported with enalapril or hydrochlorothiazide.
The most frequent clinical adverse experiences in controlled trials were: dizziness (8.6 percent), headache (5.5 percent), fatigue (3.9 percent) and cough (3.5 percent). Generally, adverse experiences were mild and transient in nature. Adverse experiences occurring in greater than two percent of patients treated with enalapril maleate and hydrochlorothiazide in controlled clinical trials are shown below.
| Percent of Patients in Controlled Studies | ||
|---|---|---|
| Enalapril maleate and hydrochlorothiazide (n=1580) Incidence (discontinuation) | Placebo (n=230) Incidence |
|
| Dizziness | 8.6 (0.7) | 4.3 |
| Headache | 5.5 (0.4) | 9.1 |
| Fatigue | 3.9 (0.8) | 2.6 |
| Cough | 3.5 (0.4) | 0.9 |
| Muscle Cramps | 2.7 (0.2) | 0.9 |
| Nausea | 2.5 (0.4) | 1.7 |
| Asthenia | 2.4 (0.3) | 0.9 |
| Orthostatic Effects | 2.3 (<0.1) | 0.0 |
| Impotence | 2.2 (0.5) | 0.5 |
| Diarrhea | 2.1 (<0.1) | 1.7 |
Clinical adverse experiences occurring in 0.5 to 2.0 percent of patients in controlled trials included:
Body As A Whole: Syncope, chest pain, abdominal pain
Cardiovascular: Orthostatic hypotension, palpitation, tachycardia
Digestive: Vomiting, dyspepsia, constipation, flatulence, dry mouth
Nervous/Psychiatric: Insomnia, nervousness, paresthesia, somnolence, vertigo
Skin: Pruritus, rash
Other: Dyspnea, gout, back pain, arthralgia, diaphoresis, decreased libido, tinnitus, urinary tract infection
Angioedema: Angioedema has been reported in patients receiving enalapril maleate and hydrochlorothiazide, with an incidence higher in black than in non-black patients. Angioedema associated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue, glottis and/or larynx occurs, treatment with enalapril maleate and hydrochlorothiazide should be discontinued and appropriate therapy instituted immediately (see WARNINGS).
Hypotension: In clinical trials, adverse effects relating to hypotension occurred as follows: hypotension (0.9 percent), orthostatic hypotension (1.5 percent), other orthostatic effects (2.3 percent). In addition syncope occurred in 1.3 percent of patients (see WARNINGS).
Cough: See PRECAUTIONS, Cough.
Enalapril and Hydrochlorothiazide Dosage and Administration
Enalapril and hydrochlorothiazide are effective treatments for hypertension. The usual dosage range of enalapril is 10 to 40 mg per day administered in a single or two divided doses; hydrochlorothiazide is effective in doses of 12.5 to 50 mg daily. The side effects (see WARNINGS) of enalapril are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent phenomena (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. Therapy with any combination of enalapril and hydrochlorothiazide will be associated with both sets of dose-independent side effects but the addition of enalapril in clinical trials blunted the hypokalemia normally seen with diuretics. To minimize dose-independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.
| ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
enalapril maleate and hydrochlorothiazide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
enalapril maleate and hydrochlorothiazide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceutical Industries, Ltd. | 600072078 | MANUFACTURE(51672-4045, 51672-4046) | |