Drug Detail:Entyvio (Vedolizumab [ ve-doe-liz-ue-mab ])
Drug Class: Selective immunosuppressants
Highlights of Prescribing Information
ENTYVIO (vedolizumab) for injection, for intravenous use
Initial U.S. Approval: 2014
Recent Major Changes
| Dosage and Administration | |
| Important Preparation and Administration Instructions (2.1) | 06/2022 |
| Reconstitution and Dilution Instructions (2.4) | 06/2022 |
Indications and Usage for Entyvio Injection
ENTYVIO is an integrin receptor antagonist indicated in adults for the treatment of:
- moderately to severely active ulcerative colitis. (1)
- moderately to severely active Crohn's disease. (1)
Entyvio Injection Dosage and Administration
- Recommended dosage in UC and CD: 300 mg infused intravenously over approximately 30 minutes at zero, two and six weeks, then every eight weeks thereafter. (2.3)
- Discontinue ENTYVIO in patients who do not show evidence of therapeutic benefit by Week 14. (2.3)
- Reconstitute ENTYVIO lyophilized powder with Sterile Water for Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP. (2.4)
- Dilute in 250 mL of 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP, prior to administration. See Full Prescribing Information for complete reconstitution, dilution and storage instructions. (2.4)
- Bring patients up to date with all immunizations (according to current immunization guidelines) before initiating treatment with ENTYVIO. (2.2)
Dosage Forms and Strengths
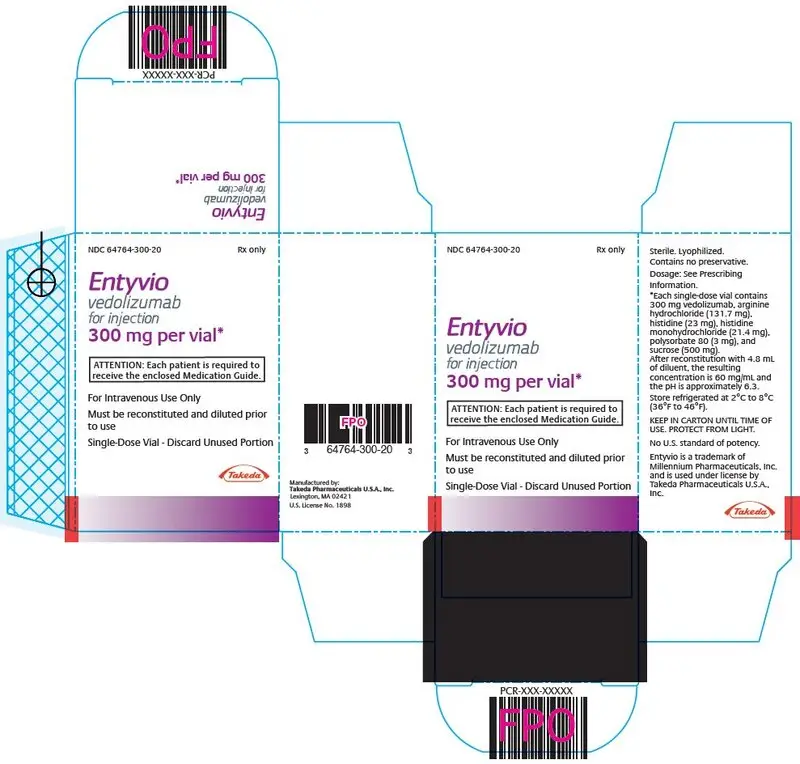
For injection: 300 mg vedolizumab in a single-dose vial. (3)
Contraindications
Patients who have had a known serious or severe hypersensitivity reaction to ENTYVIO or any of its excipients. (4)
Warnings and Precautions
- Infusion-Related Reactions and Hypersensitivity Reactions: Discontinue ENTYVIO and initiate appropriate treatment if serious reactions occur. (5.1)
- Infections: Treatment with ENTYVIO is not recommended in patients with active, severe infections until the infections are controlled. Consider withholding ENTYVIO in patients who develop a severe infection while on treatment with ENTYVIO. (5.2)
- Progressive Multifocal Leukoencephalopathy (PML): Although unlikely, a risk of PML cannot be ruled out. Monitor patients for any new or worsening neurological signs or symptoms. (5.3)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥3% and ≥1% higher than placebo) are: nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, influenza, back pain, rash, pruritus, sinusitis, oropharyngeal pain, and pain in extremities. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2022
Full Prescribing Information
1. Indications and Usage for Entyvio Injection
ENTYVIO is indicated in adults for the treatment of:
- moderately to severely active ulcerative colitis.
- moderately to severely active Crohn's disease.
2. Entyvio Injection Dosage and Administration
2.1 Important Preparation and Administration Instructions
- Administer ENTYVIO as an intravenous infusion over 30 minutes. Do not administer as an intravenous push or bolus.
- Reconstitute ENTYVIO lyophilized powder with Sterile Water for Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP.
- Dilute the reconstituted ENTYVIO solution in 250 mL of 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP, prior to administration [see Dosage and Administration (2.4)].
- After the infusion is complete, flush with 30 mL of 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP.
- ENTYVIO should be administered by a healthcare professional prepared to manage hypersensitivity reactions including anaphylaxis, if they occur [see Warnings and Precautions (5.1)]. Appropriate monitoring and medical support measures should be available for immediate use. Observe patients during infusion and until the infusion is complete.
2.2 Prior to Administration of ENTYVIO
Prior to initiating treatment with ENTYVIO, all patients should be brought up to date with all immunizations according to current immunization guidelines.
2.3 Dosage in Adults with Ulcerative Colitis or Crohn's Disease
The recommended dosage of ENTYVIO in adults with ulcerative colitis or Crohn's disease is 300 mg administered by intravenous infusion at zero, two and six weeks and then every eight weeks thereafter.
Discontinue therapy in patients who show no evidence of therapeutic benefit by Week 14.
2.4 Reconstitution and Dilution Instructions
Storage
Specific storage conditions and timing for the reconstituted solution in vial and diluted solution in the infusion bag are outlined in Table 1.
Do not freeze the reconstituted solution in the vial or the diluted solution in the infusion bag.
| Storage Condition | ||
|---|---|---|
| Refrigeration (2°C to 8°C [36°F to 46°F]) | Room temperature (20°C to 25°C [68°F to 77°F]) |
|
|
||
| Reconstituted Solution (in Sterile Water for Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP, inside vial) | 8 hours | Use immediately after reconstitution |
| Diluted Solution (in 0.9% Sodium Chloride Injection, USP) | 24 hours*,† | 12 hours* |
| Diluted Solution (in Lactated Ringer's Injection, USP) | 6 hours* | Use immediately after dilution |
The combined storage time of reconstituted ENTYVIO solution in the vial and the diluted solution in the infusion bag with 0.9% Sodium Chloride Injection, USP, is a total of 12 hours at room temperature (20°C to 25°C [68°F to 77°F]) or 24 hours refrigerated (2°C to 8°C [36°F to 46°F]). This combined storage time may include up to eight hours of the reconstituted solution in the vial at 2°C to 8°C.
The combined storage time of reconstituted ENTYVIO solution in the vial and the diluted solution in the infusion bag with Lactated Ringer's Injection, USP, is a total of six hours refrigerated (2°C to 8°C [36°F to 46°F]).
3. Dosage Forms and Strengths
For injection: 300 mg of vedolizumab as a white to off-white lyophilized cake in a single-dose vial for reconstitution.
4. Contraindications
ENTYVIO is contraindicated in patients who have had a known serious or severe hypersensitivity reaction to ENTYVIO or any of its excipients (such as dyspnea, bronchospasm, urticaria, flushing, rash and increased heart rate) [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Infusion-Related Reactions and Hypersensitivity Reactions
Infusion-related reactions and hypersensitivity reactions have been reported, including anaphylaxis, dyspnea, bronchospasm, urticaria, flushing, rash, and increased blood pressure and heart rate [see Adverse Reactions (6.1, 6.3)]. These reactions may occur with the first or subsequent infusions of ENTYVIO and may vary in their time of onset from during infusion or up to several hours post-infusion.
If anaphylaxis or other serious infusion-related or hypersensitivity reactions occur, discontinue administration of ENTYVIO immediately and initiate appropriate treatment.
5.2 Infections
Patients treated with ENTYVIO are at increased risk for developing infections [see Adverse Reactions (6.1)]. The most commonly reported infections in clinical trials occurring at a rate greater on ENTYVIO than placebo involved the upper respiratory and nasal mucosa (e.g., nasopharyngitis, upper respiratory tract infection). Serious infections have also been reported in patients treated with ENTYVIO, including anal abscess, sepsis (some fatal), tuberculosis, salmonella sepsis, Listeria meningitis, giardiasis and cytomegaloviral colitis.
ENTYVIO is not recommended in patients with active, severe infections until the infections are controlled. Consider withholding treatment in patients who develop a severe infection while on treatment with ENTYVIO. Exercise caution when considering the use of ENTYVIO in patients with a history of recurring severe infections. Consider screening for tuberculosis (TB) according to the local practice. For progressive multifocal leukoencephalopathy (PML), see Warnings and Precautions (5.3).
5.3 Progressive Multifocal Leukoencephalopathy
PML, a rare and often fatal opportunistic infection of the central nervous system (CNS), has been reported with systemic immunosuppressants, including another integrin receptor antagonist. PML is caused by the John Cunningham (JC) virus and typically only occurs in patients who are immunocompromised. One case of PML in an ENTYVIO-treated patient with multiple contributory factors has been reported in the postmarketing setting (e.g., human immunodeficiency virus [HIV] infection with a CD4 count of 300 cells/mm3 and prior and concomitant immunosuppression). Although unlikely, a risk of PML cannot be ruled out.
Monitor patients on ENTYVIO for any new onset, or worsening, of neurological signs and symptoms. Typical signs and symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. The progression of deficits usually leads to death or severe disability over weeks or months. If PML is suspected, withhold dosing with ENTYVIO and refer to a neurologist; if confirmed, discontinue dosing permanently.
5.4 Liver Injury
There have been reports of elevations of transaminase and/or bilirubin in patients receiving ENTYVIO. In general, the combination of transaminase elevations and elevated bilirubin without evidence of obstruction is generally recognized as an important predictor of severe liver injury that may lead to death or the need for a liver transplant in some patients. ENTYVIO should be discontinued in patients with jaundice or other evidence of significant liver injury [see Adverse Reactions (6.1)].
5.5 Live and Oral Vaccines
Prior to initiating treatment with ENTYVIO, all patients should be brought up to date with all immunizations according to current immunization guidelines [see Dosage and Administration (2.2)]. Patients receiving ENTYVIO may receive non-live vaccines (e.g., influenza vaccine injection) and may receive live vaccines if the benefits outweigh the risks. There are no data on the secondary transmission of infection by live vaccines in patients receiving ENTYVIO [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following topics are also discussed in detail in the Warnings and Precautions section:
- Infusion-Related Reactions and Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Progressive Multifocal Leukoencephalopathy [see Warnings and Precautions (5.3)]
- Liver Injury [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to ENTYVIO in 3,326 patients and healthy volunteers in clinical trials, including 1,396 exposed for greater than one year, and 835 exposed for greater than two years.
The safety data described in Table 2 are derived from four controlled Phase 3 trials (UC Trials I and II, and CD Trials I and III); data from patients receiving open-label ENTYVIO treatment at Weeks 0 and 2 (prior to entry into UC Trial II and CD Trial III) and from Weeks 6 to 52 (non-responders at Week 6 of UC Trial I and CD Trial I) are included [see Clinical Studies (14.1, 14.2)].
In these trials, 1,434 patients received ENTYVIO 300 mg for up to 52 weeks, and 297 patients received placebo for up to 52 weeks. Of these, 769 patients had ulcerative colitis and 962 patients had Crohn's disease. Patients were exposed for a mean duration of 259 days (UC Trials I and II) and 247 days (CD Trials I and III).
Adverse reactions were reported in 52% of patients treated with ENTYVIO and 45% of patients treated with placebo (UC Trials I and II: 49% with ENTYVIO and 37% with placebo; CD Trials I and III: 55% with ENTYVIO and 47% with placebo). Serious adverse reactions were reported in 7% of patients treated with ENTYVIO compared to 4% of patients treated with placebo (UC Trials I and II: 8% with ENTYVIO and 7% with placebo; CD Trials I and III: 12% with ENTYVIO and 9% with placebo).
The most common adverse reactions (reported by ≥3% of patients treated with ENTYVIO in the UC Trials I and II and CD Trials I and III combined group and ≥1% higher than in combined placebo group) were nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, influenza, back pain, rash, pruritus, sinusitis, oropharyngeal pain and pain in extremities (Table 2).
| Adverse Reaction | ENTYVIO†
(N=1434) | Placebo‡
(N=297) |
|---|---|---|
|
||
| Nasopharyngitis | 13% | 7% |
| Headache | 12% | 11% |
| Arthralgia | 12% | 10% |
| Nausea | 9% | 8% |
| Pyrexia | 9% | 7% |
| Upper respiratory tract infection | 7% | 6% |
| Fatigue | 6% | 3% |
| Cough | 5% | 3% |
| Bronchitis | 4% | 3% |
| Influenza | 4% | 2% |
| Back pain | 4% | 3% |
| Rash | 3% | 2% |
| Pruritus | 3% | 1% |
| Sinusitis | 3% | 1% |
| Oropharyngeal pain | 3% | 1% |
| Pain in extremities | 3% | 1% |
Safety data for patients (n=279) in UC Trials I and II and CD Trials I and III who received ENTYVIO at Weeks 0 and 2 and were then randomized to placebo at Week 6 for up to 52 weeks, and for patients (n=416) in CD Trial II, a 10 week Crohn's disease trial, are similar to those listed in Table 2.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to vedolizumab in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
The incidence of anti-vedolizumab antibodies to intravenous ENTYVIO using a drug-tolerant electrochemiluminescence (ECL) method for patients in UC Trials I and II and CD Trials I and III who had continuous treatment for 52 weeks was 6% (86 out of 1,427). Of the 86 patients who tested positive for anti-vedolizumab antibodies, 20 patients were persistently positive (at two or more study visits) and 56 developed neutralizing antibodies to vedolizumab. Among the 20 patients with persistently positive anti-vedolizumab antibody status, 14 had undetectable or reduced vedolizumab serum concentrations [see Clinical Pharmacology (12.3)]. Five of the 20 patients with persistently positive anti-vedolizumab antibody achieved clinical remission at Week 52 in the controlled trials. Overall, there was no apparent correlation of anti-vedolizumab antibody development to adverse reactions following intravenous administration of ENTYVIO.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ENTYVIO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Anaphylaxis [see Warnings and Precautions (5.1)]
Gastrointestinal system disorders: Acute Pancreatitis
7. Drug Interactions
7.1 Natalizumab
Because of the potential for increased risk of PML and other infections, avoid the concomitant use of ENTYVIO with natalizumab.
8. Use In Specific Populations
8.2 Lactation
Data
A milk-only lactation study was conducted in 9 adult lactating women being treated for active ulcerative colitis or Crohn's disease with intravenous ENTYVIO every 8 weeks after reaching steady state and completing the induction phase (ENTYVIO administration at 0, 2, and 6 weeks). Mean concentrations of ENTYVIO in human milk ranged from 0.03 to 0.26 mcg/mL. The mean calculated daily infant oral dosage was 0.02 mg/kg/day calculated as a product of the average concentration over the 8-week dosing interval and the standardized milk consumption of 150 mL/kg/day.
8.4 Pediatric Use
Safety and effectiveness of ENTYVIO in pediatric patients have not been established.
8.5 Geriatric Use
Clinical trials of ENTYVIO did not include sufficient numbers of subjects aged 65 and over (46 Crohn's and ulcerative colitis patients aged 65 and over were treated with ENTYVIO during controlled Phase 3 trials) to determine whether they respond differently from younger subjects. However, no overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients.
11. Entyvio Injection Description
Vedolizumab, an integrin receptor antagonist, is a humanized IgG1 monoclonal antibody produced in Chinese hamster ovary cells that binds to the human α4β7 integrin. ENTYVIO has an approximate molecular weight of 147 kilodaltons.
ENTYVIO (vedolizumab) for injection is supplied as a sterile, white to off-white, preservative-free, lyophilized cake for intravenous infusion. After reconstitution with 4.8 mL Sterile Water for Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP, the resulting concentration is 60 mg/mL with a deliverable volume of 5 mL (300 mg) and the resulting pH is approximately 6.3.
Each single-dose vial contains 300 mg vedolizumab, arginine hydrochloride (131.7 mg), histidine (23 mg), histidine monohydrochloride (21.4 mg), polysorbate 80 (3 mg), and sucrose (500 mg).
12. Entyvio Injection - Clinical Pharmacology
12.1 Mechanism of Action
Vedolizumab is a humanized monoclonal antibody that specifically binds to the α4β7 integrin and blocks the interaction of α4β7 integrin with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and inhibits the migration of memory T-lymphocytes across the endothelium into inflamed gastrointestinal parenchymal tissue. Vedolizumab does not bind to or inhibit function of the α4β1 and αEβ7 integrins and does not antagonize the interaction of α4 integrins with vascular cell adhesion molecule-1 (VCAM-1).
The α4β7 integrin is expressed on the surface of a discrete subset of memory T-lymphocytes that preferentially migrate into the gastrointestinal tract. MAdCAM-1 is mainly expressed on gut endothelial cells and plays a critical role in the homing of T-lymphocytes to gut lymph tissue. The interaction of the α4β7 integrin with MAdCAM-1 has been implicated as an important contributor to the chronic inflammation that is a hallmark of ulcerative colitis and Crohn's disease.
12.2 Pharmacodynamics
In clinical trials with ENTYVIO at doses ranging from 0.2 to 10 mg/kg (which includes doses outside of the recommended dose), saturation of α4β7 receptors on subsets of circulating lymphocytes involved in gut-immune surveillance was observed.
In clinical trials with ENTYVIO at doses ranging from 0.2 to 10 mg/kg and 180 to 750 mg (which include doses outside of the recommended dose) in healthy subjects and in patients with ulcerative colitis or Crohn's disease, vedolizumab did not elevate neutrophils, basophils, eosinophils, B-helper and cytotoxic T-lymphocytes, total memory helper T-lymphocytes, monocytes or natural killer cells.
A reduction in gastrointestinal inflammation was observed in rectal biopsy specimens from Phase 2 ulcerative colitis patients exposed to ENTYVIO for four or six weeks compared to placebo control as assessed by histopathology.
In a study of 14 healthy subjects, ENTYVIO did not affect the CD4+ lymphocyte cell counts, CD8+ lymphocyte cell counts, or the CD4+:CD8+ ratios in the CSF [see Clinical Pharmacology (12.3)].
12.3 Pharmacokinetics
Similar pharmacokinetics were observed in ulcerative colitis and Crohn's disease patients administered 300 mg ENTYVIO as a 30 minute intravenous infusion on Weeks 0 and 2, followed by 300 mg ENTYVIO every eight weeks starting from Week 6 (Table 3).
| Patient Population | Weeks 0 to 6 | Weeks 6 to 52 ENTYVIO Every 8 Weeks |
|---|---|---|
| Trough Serum Concentration at Week 6 (mcg/mL) | Trough Serum Concentration at Week 46† (mcg/mL) | |
|
||
| Ulcerative Colitis | 26.3 ± 12.9 (N=210) | 11.2 ± 7.2 (N=77) |
| Crohn's Disease | 27.4 ± 19.2 (N=198) | 13.0 ± 9.1 (N=72) |
The presence of persistent anti-vedolizumab antibody was observed to substantially reduce serum concentrations of vedolizumab, either to undetectable or negligible levels (n=20) [see Adverse Reactions (6.2)].
Vedolizumab clearance depends on both linear and nonlinear pathways; the nonlinear clearance decreases with increasing concentrations. Population pharmacokinetic analyses indicated that the linear clearance was approximately 0.157 L/day, the serum half-life was approximately 25 days at 300 mg dosage, and the distribution volume was approximately 5 L.
Vedolizumab was not detected in the cerebrospinal fluid (CSF) of 14 healthy subjects at five weeks after a single intravenous administration of 450 mg ENTYVIO (1.5 times the recommended dosage).
14. Clinical Studies
14.1 Clinical Studies in Ulcerative Colitis
The safety and efficacy of ENTYVIO were evaluated in two randomized, double-blind, placebo-controlled trials (UC Trials I and II) in adult patients with moderately to severely active ulcerative colitis (UC) defined as Mayo score of six to 12 with endoscopy subscore of two or three. The Mayo score ranges from zero to 12 and has four subscales that are each scored from zero (normal) to three (most severe): stool frequency, rectal bleeding, findings on endoscopy, and physician global assessment. An endoscopy subscore of two is defined by marked erythema, lack of vascular pattern, friability, and erosions; an endoscopy subscore of three is defined by spontaneous bleeding and ulceration.
Enrolled patients in the U.S. had over the previous five-year period an inadequate response or intolerance to immunomodulator therapy (i.e., azathioprine or 6-mercaptopurine) and/or an inadequate response, loss of response, or intolerance to a TNF blocker. Outside the U.S., prior treatment with corticosteroids was sufficient for entry if over the previous five-year period the patients were corticosteroid dependent (i.e., unable to successfully taper corticosteroids without a return of the symptoms of UC) or had an inadequate response or intolerance to corticosteroids.
Patients that had received natalizumab ever in the past, and patients that had received a TNF blocker in the past 60 days were excluded from enrollment. Concomitant use of natalizumab or a TNF blocker was not allowed.
UC Trial I
In UC Trial I, 374 patients were randomized in a double-blind fashion (3:2) to receive ENTYVIO 300 mg or placebo by intravenous infusion at Week 0 and Week 2. Efficacy assessments were at Week 6. Concomitant stable dosages of aminosalicylates, corticosteroids (prednisone dosage ≤30 mg/day or equivalent), and immunomodulators (azathioprine or 6-mercaptopurine) were permitted through Week 6.
At baseline, patients received corticosteroids (54%), immunomodulators (azathioprine or 6-mercaptopurine) (30%), and/or aminosalicylates (74%). Thirty-nine percent of patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy. Eighteen percent of patients had an inadequate response, inability to taper or intolerance to prior corticosteroid treatment only (i.e., had not received prior immunomodulators or TNF blockers). The median baseline Mayo score was nine in the ENTYVIO group and eight in the placebo group.
In UC Trial I, a greater percentage of patients treated with ENTYVIO compared to patients treated with placebo achieved clinical response at Week 6 (defined in Table 4). A greater percentage of patients treated with ENTYVIO compared to patients treated with placebo also achieved clinical remission at Week 6 (defined in Table 4). In addition, a greater percentage of patients treated with ENTYVIO had improvement of endoscopic appearance of the mucosa at Week 6 (defined in Table 4).
| Endpoint | Placebo N=149 | ENTYVIO N=225 | p-value | Treatment Difference and 95% CI |
|---|---|---|---|---|
|
||||
| Clinical response* at Week 6 | 26% | 47% | <0.001 | 22% (12%, 32%) |
| Clinical remission† at Week 6 | 5% | 17% | 0.001 | 12% (5%, 18%) |
| Improvement of endoscopic appearance of the mucosa‡ at Week 6 | 25% | 41% | 0.001 | 16% (6%, 26%) |
UC Trial II
In order to be randomized to treatment in UC Trial II, patients had to have received ENTYVIO and be in clinical response at Week 6. Patients could have come from either UC Trial I or from a group who received ENTYVIO open-label.
In UC Trial II, 373 patients were randomized in a double-blind fashion (1:1:1) to one of the following regimens beginning at Week 6: ENTYVIO 300 mg every eight weeks, ENTYVIO 300 mg every four weeks or placebo every four weeks. Efficacy assessments were at Week 52. Concomitant aminosalicylates and corticosteroids were permitted through Week 52. Concomitant immunomodulators (azathioprine or 6-mercaptopurine) were permitted outside the U.S. but were not permitted beyond Week 6 in the U.S.
At Week 6, patients were receiving corticosteroids (61%), immunomodulators (azathioprine or 6-mercaptopurine) (32%) and aminosalicylates (75%). Thirty-two percent of patients had an inadequate response, loss of response or intolerance to a TNF blocker therapy. At Week 6, the median Mayo score was eight in the ENTYVIO every eight week group, the ENTYVIO every four week group, and the placebo group. Patients who had achieved clinical response at Week 6 and were receiving corticosteroids were required to begin a corticosteroid-tapering regimen at Week 6.
In UC Trial II, a greater percentage of patients in groups treated with ENTYVIO as compared to placebo achieved clinical remission at Week 52, and maintained clinical response (clinical response at both Weeks 6 and 52) (Table 5). In addition, a greater percentage of patients in groups treated with ENTYVIO as compared to placebo were in clinical remission at both Weeks 6 and 52, and had improvement of endoscopic appearance of the mucosa at Week 52 (Table 5). In the subgroup of patients who achieved clinical response at Week 6 and were receiving corticosteroid medication at baseline, a greater proportion of patients in groups treated with ENTYVIO as compared to placebo discontinued corticosteroids and were in clinical remission at Week 52 (Table 5).
The ENTYVIO every four week dosing regimen did not demonstrate additional clinical benefit over the every eight dosing week regimen. The every four week dosing regimen is not the recommended dosing regimen [see Dosage and Administration (2.3)].
| Endpoint | Placebo†
N=126 | ENTYVIO Every 8 Weeks N=122 | p-value | Treatment Difference and 95% CI |
|---|---|---|---|---|
|
||||
| Clinical remission at Week 52 | 16% | 42% | <0.001 | 26% (15%, 37%) |
| Clinical response at both Weeks 6 and 52 | 24% | 57% | <0.001 | 33% (21%, 45%) |
| Improvement of endoscopic appearance of the mucosa‡ at Week 52 | 20% | 52% | <0.001 | 32% (20%, 44%) |
| Clinical remission at both Weeks 6 and 52 | 9% | 21% | 0.008 | 12% (3%, 21%) |
| Corticosteroid-free clinical remission§ | 14%§ | 31%§ | 0.012 | 18% (4%, 31%) |
14.2 Clinical Studies in Crohn's Disease
The safety and efficacy of ENTYVIO were evaluated in three randomized, double-blind, placebo-controlled clinical trials (CD Trials I, II, and III) in adult patients with moderately to severely active Crohn's disease (CD) (Crohn's Disease Activity Index [CDAI] score of 220 to 450).1
Enrolled patients in the U.S. had over the previous five-year period an inadequate response or intolerance to immunomodulator therapy (i.e., azathioprine, 6-mercaptopurine, or methotrexate) and/or an inadequate response, loss of response, or intolerance to one or more TNF blockers. Outside the U.S., prior treatment with corticosteroids was sufficient for entry if over the previous five-year period the patients were corticosteroid dependent (i.e., unable to successfully taper corticosteroids without a return of the symptoms of CD) or had an inadequate response or intolerance to corticosteroids.
Patients that had received natalizumab ever in the past, and patients that had received a TNF blocker in the past 30 to 60 days were excluded from enrollment. Concomitant use of natalizumab or a TNF blocker was not allowed.
CD Trial II
Compared to CD Trial I, CD Trial II enrolled a higher number of patients who had over the previous five-year period had an inadequate response, loss of response, or intolerance to one or more TNF blockers (76%); this was the primary analysis population. In CD Trial II, 416 patients were randomized in a double-blind fashion (1:1) to receive either ENTYVIO 300 mg or placebo at Weeks 0, 2 and 6. Efficacy assessments were at Weeks 6 and 10. Concomitant aminosalicylates, corticosteroids, and immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) were permitted through Week 10.
At baseline, patients were receiving corticosteroids (54%), immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) (34%), and aminosalicylates (31%). The median baseline CDAI score was 317 in the ENTYVIO group and 301 in the placebo group.
For the primary endpoint (clinical remission at Week 6), treatment with ENTYVIO did not result in statistically significant improvement over placebo (Table 6). Secondary endpoints including assessments at Week 10 were not tested because the primary endpoint was not statistically significant.
| Placebo | ENTYVIO | p-value | Treatment Difference and 95% CI | |
|---|---|---|---|---|
|
||||
| CD Trial I: Clinical Remission* at Week 6 | 7% (10/148) | 15% (32/220) | 0.041† | 8% (1%, 14%) |
| CD Trial II‡: Clinical Remission* at Week 6 | 12% (19/157) | 15% (24/158) | NS§ | 3% (-5%, 11%) |
CD Trial III
In order to be randomized to treatment in CD Trial III, patients had to have received ENTYVIO and be in clinical response (defined as a ≥70-point decrease in CDAI score from baseline) at Week 6. Patients could have come from either CD Trial I or from a group who received ENTYVIO open-label.
In CD Trial III, 461 patients were randomized in a double-blind fashion (1:1:1) to one of the following regimens beginning at Week 6: ENTYVIO 300 mg every eight weeks, ENTYVIO 300 mg every four weeks or placebo every four weeks. Efficacy assessments were at Week 52. Concomitant aminosalicylates and corticosteroids were permitted through Week 52. Concomitant immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) were permitted outside the U.S. but were not permitted beyond Week 6 in the U.S.
At Week 6, patients were receiving corticosteroids (59%), immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) (31%), and aminosalicylates (41%). Fifty-one percent of patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy. At Week 6, the median CDAI score was 322 in the ENTYVIO every eight week group, 316 in the ENTYVIO every four week group, and 315 in the placebo group. Patients who had achieved clinical response (≥70 decrease in CDAI score from baseline) at Week 6 and were receiving corticosteroids were required to begin a corticosteroid-tapering regimen at Week 6.
In CD Trial III, a greater percentage of patients in groups treated with ENTYVIO as compared to placebo were in clinical remission (defined as CDAI score ≤150) at Week 52. A greater percentage of patients in groups treated with ENTYVIO as compared to placebo had a clinical response (defined as ≥100 decrease in CDAI score from baseline) at Week 52 (Table 7). In the subgroup of patients who were receiving corticosteroids at baseline and who were in clinical response at Week 6 (defined as ≥70 decrease in CDAI score from baseline), a greater proportion of patients in groups treated with ENTYVIO as compared to placebo discontinued corticosteroids by Week 52 and were in clinical remission at Week 52 (Table 7).
The ENTYVIO every four week dosing regimen did not demonstrate additional clinical benefit over the every eight dosing week regimen. The every four week dosing regimen is not the recommended dosing regimen [see Dosage and Administration (2.3)].
| Placebo†
N=153 | ENTYVIO Every 8 Weeks N=154 | p-value | Treatment Difference and 95% CI | |
|---|---|---|---|---|
|
||||
| Clinical remission‡ at Week 52 | 22% | 39% | 0.001 | 17% (7%, 28%) |
| Clinical response§ at Week 52 | 30% | 44% | 0.013 | 13% (3%, 24%) |
| Corticosteroid-free clinical remission¶ | 16%¶ | 32%¶ | 0.015 | 16% (3%, 29%) |
15. References
1. Best WR, Becktel JM, Singleton JW, Kern F: Development of a Crohn's Disease Activity Index, National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70(3): 439-444
16. How is Entyvio Injection supplied
ENTYVIO (vedolizumab) for injection is supplied in sterile single-dose glass vials, containing 300 mg of vedolizumab as a white to off-white lyophilized cake.
| NDC 64764-300-20 | 300 mg single-dose vial in individual carton |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | VMB245 R7 | Revised: 06/2022 |
| MEDICATION GUIDE
ENTYVIO (en ti' vee oh) (vedolizumab) for injection, for intravenous use |
||
| What is the most important information I should know about ENTYVIO? ENTYVIO may cause serious side effects, including:
|
||
| What is ENTYVIO?
ENTYVIO is a prescription medicine used in adults for the treatment of:
|
||
| Who should not receive ENTYVIO? Do not receive ENTYVIO if you have had an allergic reaction to ENTYVIO or any of the ingredients in ENTYVIO. See the end of this Medication Guide for a complete list of ingredients in ENTYVIO. |
||
Before receiving ENTYVIO, tell your healthcare provider about all of your medical conditions, including if you:
|
||
How will I receive ENTYVIO?
|
||
| What are the possible side effects of ENTYVIO? ENTYVIO may cause serious side effects, see "What is the most important information I should know about ENTYVIO?". The most common side effects of ENTYVIO include: common cold, headache, joint pain, nausea, fever, infections of the nose and throat, tiredness, cough, bronchitis, flu, back pain, rash, itching, sinus infection, throat pain, and pain in extremities. These are not all of the possible side effects of ENTYVIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
| General information about ENTYVIO
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about ENTYVIO that is written for health professionals. |
||
| What are the ingredients in ENTYVIO?
Active ingredient: vedolizumab Inactive ingredients: arginine hydrochloride, histidine, histidine monohydrochloride, polysorbate 80 and sucrose Manufactured by: Takeda Pharmaceuticals U.S.A., Inc. Lexington, MA 02421 U.S. License No. 1898 ENTYVIO is a trademark of Millennium Pharmaceuticals Inc. and is used under license by Takeda Pharmaceuticals U.S.A., Inc. All other trademark names are the property of their respective owners. ©2022 Takeda Pharmaceuticals U.S.A., Inc. For more information, go to www.ENTYVIO.com or call 1-877-TAKEDA7 (1-877-825-3327). |
||
| ENTYVIO
vedolizumab injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |