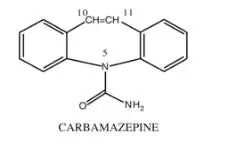
Drug Detail:Equetro (Carbamazepine (oral) [ kar-ba-maz-e-peen ])
Drug Class: Dibenzazepine anticonvulsants
Highlights of Prescribing Information
EQUETRO (carbamazepine) extended-release capsules, for oral use
Initial U.S. Approval: 1968
WARNING: SERIOUS DERMATOLOGIC REACTIONS AND APLASTIC ANEMIA AND AGRANULOCYTOSIS
See full prescribing information for complete boxed warning.
Serious Dermatologic Reactions
• Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN) and Stevens-Johnson Syndrome (SJS), have occurred with EQUETRO (5.1)
• Patients of Asian ancestry have a 10-fold greater risk of TEN/SJS, compared to other populations. In genetically at-risk patients, test for the HLA-B*1502 allele prior to initiating EQUETRO (2.1, 5.1)
• Discontinue EQUETRO if these reactions occur (5.1)
Aplastic Anemia and Agranulocytosis
• Aplastic anemia and agranulocytosis occurred with EQUETRO (5.2)
• Obtain complete pretreatment hematological testing. Consider discontinuing EQUETRO if significant bone marrow depression develops (2.1, 5.2)
Recent Major Changes
| Warnings and Precautions (5.5) 10/2022 |
Indications and Usage for Equetro
EQUETRO is:
- A mood stabilizer indicated for the treatment of acute manic or mixed episodes associated with bipolar I disorder (1.1)
- Indicated for the treatment of the pain associated with trigeminal neuralgia (1.2)
- An anti-epileptic drug (AED) indicated for the treatment of partial seizures with complex symptomatology, generalized tonic-clonic seizures, and mixed seizures (1.3)
Equetro Dosage and Administration
- SEE DOSAGE FOR BIPOLAR DISORDER, TRIGEMINAL NEURALGIA, AND EPILEPSY (2.2, 2.3, 2.4)
- When discontinuing treatment, reduce dose gradually (2.6, 5.6)
- Monitoring serum carbamazepine concentrations may be useful in dose selection and minimizing risk of toxicity (2.7)
- Swallow capsules whole or open capsules and sprinkle beads over food (2.8)
- Do not crush or chew the capsule or beads (2.8)
Dosage Forms and Strengths
Extended-Release Capsules: 100 mg, 200 mg, and 300 mg (3)
Contraindications
- Bone marrow depression (4)
- Known hypersensitivity to carbamazepine (4)
- Known hypersensitivity to tricyclic antidepressants (4)
- Concomitant use with monoamine oxidase inhibitors (MAOIs) or use within 14 days of discontinuing an MAOI (4)
- Concomitant use with delavirdine or other non-nucleoside reverse transcriptase inhibitors that are substrates for CYP3A4. EQUETRO decreases efficacy of these drugs (4, 5.9)
- Concomitant use of nefazodone (4)
Warnings and Precautions
-
Drug Reaction with Eosinophilia and Systemic Symptoms: Monitor for hypersensitivity. Discontinue if another cause can not be established (5.3)
-
Suicidal Behavior and Ideation: Monitor for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior (5.4)
-
Embryofetal Toxicity: Advise women of child-bearing potential of possible risks to the fetus (5.5, 8.1, 8.3)
-
Abrupt Discontinuation and Risk of Seizure: Taper the dose when discontinuing treatment (5.6).
-
Hyponatremia: Consider discontinuing EQUETRO in patients with significant symptomatic hyponatremia (5.7).
-
Cognitive and Motor Impairment: Advise patients not to drive or operate machinery until they have gained sufficient experience on EQUETRO to gauge whether it adversely affects these activities (5.8).
-
Liver Damage: Monitor liver function. Discontinue EQUETRO with aggravated liver dysfunction or active liver disease (5.10).
- Hepatic Porphyria: Avoid EQUETRO use in patients with hepatic porphyria: can cause acute episodes of porphyria (5.12)
Adverse Reactions/Side Effects
Most common (>5% and 2 times placebo) adverse reactions were dizziness, somnolence, nausea, vomiting, ataxia, constipation, pruritus, dry mouth, asthenia, blurred vision, and speech disorder (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Validus Pharmaceuticals LLC at 1-866-982-5438 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Cytochrome (CYP) 3A4 inhibitors, epoxide hydrolase inhibitors, CYP3A4 inducers, drugs metabolized by CYP1A2 or CYP3A4 (oral contraceptives, delavirdine, nefazodone), phenytoin, CNS depressants, lithium, chloroquine, mefloquine (7.1, 7.2, 7.3)
- Equetro may decrease the effectiveness of hormonal contraceptives. Use alternative form of birth control (7.2)
Use In Specific Populations
Pregnancy: Can cause fetal harm. (5.5, 8.1)
Infertility: May impair male fertility (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2022
Full Prescribing Information
WARNING: SERIOUS DERMATOLOGIC REACTIONS AND APLASTIC ANEMIA AND AGRANULOCYTOSIS
Serious Dermatologic Reactions and HLA-B*1502 Allele
Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN) and Stevens-Johnson Syndrome (SJS), have occurred in patients treated with carbamazepine. These syndromes may be accompanied by mucous membrane ulcers, fever, or painful rash. These reactions are estimated to occur in 1 to 6 per 10,000 new users in countries with mainly Caucasian populations, but the risk in patients of Asian descent is estimated to be about 10 times higher. There is a strong association between the risk of developing SJS/TEN and the presence of HLA-B*1502, an inherited allelic variant of the HLA-B gene. Test for HLA-B*1502, prior to initiating EQUETRO in patients with an increased likelihood of carrying this allele. Avoid use of EQUETRO in patients testing positive for the allele unless the benefit clearly outweighs the risk. Discontinue EQUETRO if you suspect that the patient has a serious dermatologic reaction [see Warnings and Precautions (5.1)].
Aplastic Anemia and Agranulocytosis
Aplastic anemia and agranulocytosis can occur during treatment with EQUETRO. The risk of developing these reactions with EQUETRO is 5-8 times greater than in the general population. However, the overall risk in the general population is low (6 cases in a population of one million per year for agranulocytosis and two cases in a population of one million per year for aplastic anemia). Obtain a complete blood count before beginning treatment with EQUETRO, and monitor CBC periodically.
Consider discontinuing EQUETRO if significant bone marrow depression develops [see Warnings and Precautions (5.2)].
1. Indications and Usage for Equetro
1.1 Acute Manic or Mixed Episodes associated with Bipolar I Disorder
EQUETRO is indicated for treatment of patients with acute manic or mixed episodes associated with bipolar I disorder [see Clinical Studies (14.1)].
1.2 Pain of Trigeminal Neuralgia
EQUETRO is indicated in the treatment of the pain associated with trigeminal neuralgia. Beneficial results have also been reported in glossopharyngeal neuralgia. This drug is not a simple analgesic and should not be used for the relief of trivial aches or pains.
1.3 Epilepsy
EQUETRO is indicated for the treatment of partial seizures with complex symptomatology (e.g., psychomotor, temporal lobe), generalized tonic-clonic seizures (grand mal), and mixed seizure patterns, which include the seizure types listed here or other partial or generalized seizures.
Limitations of Usage
EQUETRO is not indicated for the treatment of absence seizures (petit mal).Carbamazepine has been associated with increased frequency of generalized convulsions in these patients.
2. Equetro Dosage and Administration
2.1 Pretreatment Screening
Prior to initiating treatment with EQUETRO, test patients with ancestry in genetically at-risk populations for the presence of the HLA-B*1502 allele. The high resolution genotype test is positive if one or two HLA-B*1502 alleles are present. Avoid use of EQUETRO in patients testing positive for the allele, unless the benefit clearly outweighs the risk [see Boxed Warning, Warnings and Precautions (5.1)].
Complete pretreatment blood counts, including platelets and possibly reticulocytes and serum iron, should be obtained as a baseline. If a patient in the course of treatment exhibits low or decreased white blood cell or platelet counts, the patient should be monitored closely. Discontinuation of EQUETRO should be considered if any evidence of significant bone marrow depression develops [see Warnings and Precautions (5.2)].
Baseline and periodic evaluations of liver function, particularly in patients with a history of liver disease, must be performed during treatment with EQUETRO because liver damage may occur. Discontinue EQUETRO in cases of aggravated liver dysfunction or active liver disease [see Warnings and Precautions (5.10)].
Baseline and periodic eye examinations, including slit-lamp, funduscopy, and tonometry, are recommended since many phenothiazines and related drugs have been shown to cause eye changes [see Warnings and Precautions (5.13)].
Baseline and periodic complete urinalysis and BUN determinations are recommended for patients treated with this agent because of observed renal dysfunction.
2.2 Dosage for Acute Manic or Mixed Episodes Associated with Bipolar I Disorder
The recommended initial dose of EQUETRO is 200 mg administered twice daily. The dose may be increased by 200 mg per day to achieve optimal clinical response. Doses higher than 1600 mg per day have not been studied in mania associated with bipolar disorder.
2.3 Dosage for Pain of Trigeminal Neuralgia
Initial: On the first day, start with one 200 mg capsule once daily. This dose may be increased by up to 200 mg/day using increments of 100 mg every 12 hours only as needed to reach an effective and tolerated dose. Do not exceed a total daily dose of 1200 mg.
Maintenance: Control of pain can be maintained in most patients with 400 mg to 800 mg daily. However, some patients may be maintained on as little as 200 mg daily, while others may require as much as 1200 mg daily. At least once every 3 months throughout the treatment period, attempts should be made to reduce the dose to the minimum effective level or even to discontinue the drug.
2.4 Dosage for Epilepsy
Adults and Children over 12 Years of Age
The recommended initial dose is 200 mg administered twice daily. Increase in weekly increments of 200 mg a day, administered as an equally divided, twice daily dose, until an optimal response is obtained. Dosage generally should not exceed 500 mg twice daily in children 12 to 15 years old; 600 mg twice daily in children 15 to 18 years old; and 800 mg twice daily in adults.
Children Under 12 Years of Age
Ordinarily, optimal clinical response is achieved at daily doses below 35 mg/kg [see Dosage and Administration (2.5)]. No recommendation regarding the safety of EQUETRO for use at doses above 35 mg/kg/24 hours can be made.
Co-Administration with Other AEDs
EQUETRO may be used alone or with other AEDs. When added to existing AEDs, add EQUETRO gradually while the dosage(s) of other AEDs are maintained or gradually decreased. Potential drug interactions should be considered when using carbamazepine with other AEDs [see Drug Interactions (7.1, 7.2)].
2.5 Switching from Immediate-Release Carbamazepine to EQUETRO
EQUETRO is an extended-release formulation for twice a day administration. When converting patients from immediate release carbamazepine to EQUETRO extended-release capsules, the same total daily mg dose of carbamazepine should be administered. Following conversion to EQUETRO, patients should be closely monitored for seizure control. Depending on the therapeutic response after conversion, the total daily dose may need to be adjusted within the recommended dosing instructions.
2.6 Discontinuation of EQUETRO
When discontinuing EQUETRO used for any indication, reduce the dose gradually and avoid abrupt discontinuation in order to decrease the risk of seizure [see Warnings and Precautions (5.6)].
2.7 Monitoring Serum Carbamazepine Concentration
Monitoring serum carbamazepine concentrations may be useful for dose selection, minimizing toxicity, and verifying drug compliance, especially in clinical conditions in which alterations in EQUETRO metabolism can occur (e.g., drug interactions) [see Drug Interactions (7)]. In pediatric patients treated with EQUETRO for epilepsy, if satisfactory clinical response has not been achieved, measure plasma levels to determine whether or not they are in the therapeutic range.
3. Dosage Forms and Strengths
EQUETRO (carbamazepine) extended-release capsules for oral administration is supplied in three dosage strengths:
- 100 mg — Two-piece hard gelatin capsule yellow opaque cap with bluish green opaque body printed with SPD417 on one end and SPD417 and 100 mg on the other in white ink.
- 200 mg — Two-piece hard gelatin capsule yellow opaque cap with blue opaque body printed with SPD417 on one end and SPD417 and 200 mg on the other in white ink.
- 300 mg — Two-piece hard gelatin capsule yellow opaque cap with blue body printed with SPD417 on one end and SPD417 and 300 mg on the other in white ink.
4. Contraindications
- Bone marrow depression [see Warnings and Precautions (5.2)].
- Known hypersensitivity to carbamazepine, such as anaphylaxis or serious hypersensitivity reaction [see Warnings and Precautions (5.3)].
- Known hypersensitivity to any of the tricyclic compounds (e.g., amitriptyline, desipramine, imipramine, protriptyline, and nortriptyline.) Hypersensitivity reactions include anaphylaxis and serious rash.
- Concomitant use of delavirdine or other non-nucleoside reverse transcriptase inhibitors that are substrates for CYP3A4. EQUETRO can substantially reduce the concentrations of these drugs through induction of CYP3A4. This can lead to loss of virologic response and possible resistance to these medications [see Warnings and Precautions (5.9) and Drug Interactions (7.2)].
- Concomitant use of monoamine oxidase inhibitors (MAOIs). Before beginning treatment with EQUETRO, MAOIs should be discontinued for a minimum of 14 days. Concomitant use can cause serotonin syndrome.
- Concomitant use of nefazodone. This may result in insufficient plasma concentrations of nefazodone and its active metabolite to achieve a therapeutic effect.
5. Warnings and Precautions
5.1 Serious Dermatologic Reactions
Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), have been reported with carbamazepine treatment. These syndromes may be accompanied by mucous membrane ulcers, fever, or painful rash. Over 90% of carbamazepine-treated patients who experienced SJS/TEN developed these reactions within the first few months of treatment. The risk of these reactions is estimated to be about 1 to 6 per 10,000 new users in countries with mainly Caucasian populations. However, the risk in some Asian countries is estimated to be about 10 times higher. Discontinue EQUETRO if you suspect that the patient has a serious dermatologic reaction. If signs or symptoms suggest SJS/TEN, do not resume treatment with EQUETRO.
SJS, TEN, and HLA-B*1502 Allele
Retrospective case-control studies have found that in patients of Chinese ancestry there is a strong association between the risk of developing SJS/TEN with EQUETRO treatment and the presence of the HLA-B*1502 allele (an inherited variant of the HLA-B gene). Prior to initiating EQUETRO therapy in patients at higher likelihood for this allele, perform testing for HLA-B*1502. The high resolution genotype test is positive if one or two HLA-B*1502 alleles are present. Avoid use of EQUETRO in patients positive for the HLA-B*1502 allele unless the benefits clearly outweighs the risks of serious dermatologic reactions. Tested patients who are found to be negative for the allele are thought to have a low risk of SJS/TEN associated with carbamazepine treatment.
The prevalence of the HLA-B*1502 allele may be higher in Asian populations: Hong Kong, Thailand, Malaysia, and parts of the Philippines (greater than 15%); Taiwan (10%), North China (4%); south Asians, including Indians (2 to 4%); and Japan and Korea (less than 1%). HLA-B*1502 is largely absent in individuals not of Asian origin (e.g., Caucasians, African-Americans, Hispanics, and Native Americans). The accuracy of estimated rates of the HLA-B*1502 allele in these populations may be limited by wide variability in rates within ethnic groups, the difficulty in ascertaining ethnic ancestry, and the likelihood of mixed ancestry.
The HLA-B*1502 allele has not been found to predict risk of less severe adverse cutaneous reactions from carbamazepine, such as maculopapular eruption (MPE) or to predict Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.3)].
Limited evidence suggests that HLA-B*1502 may be a risk factor for the development of SJS/TEN in patients of Chinese ancestry taking other anti-epileptic drugs associated with SJS/TEN, including phenytoin. Consideration should be given to avoiding use of other drugs associated with SJS/TEN in HLA-B*1502 positive patients, when alternative therapies are otherwise equally acceptable [see Dosage and Administration (2.1)].
Hypersensitivity Reactions and HLA-A*3101 Allele
Retrospective case-control studies in patients of European, Korean, and Japanese ancestry have found a moderate association between the risk of developing hypersensitivity reactions and the presence of HLA-A*3101, an inherited allelic variant of the HLA-A gene, in patients using carbamazepine. These hypersensitivity reactions include SJS/TEN, maculopapular eruptions, and Drug Reaction with Eosinophilia and Systemic Symptoms [see Warnings and Precautions (5.3)].
HLA-A*3101 is expected to be present in the following frequencies: greater than 15% in patients of Japanese, Native American, Southern Indian (for example, Tamil Nadu) and some Arabic ancestry; up to about 10% in patients of Han Chinese, Korean, European, Latin American, and other Indian ancestry; and up to about 5% in African-Americans and patients of Thai, Taiwanese, and Chinese (Hong Kong) ancestry.
The risks and benefits of EQUETRO therapy should be weighed before considering EQUETRO in patients known to be positive for HLA-A*3101.
Hypersensitivity and Limitations of HLA Genotyping
Application of HLA genotyping as a screening tool has important limitations and must never substitute for appropriate clinical vigilance and patient management. Many HLA-B*1502-positive and HLA-A*3101- positive patients treated with EQUETRO will not develop SJS/TEN or other hypersensitivity reactions, and these reactions can still occur infrequently in HLA-B*1502-negative and HLA-A*3101-negative patients of any ethnicity. The role of other possible factors in the development of, and morbidity from, SJS/TEN and other hypersensitivity reactions, such as AED dose, compliance, concomitant medications, co-morbidities, and the level of dermatologic monitoring have not been studied.
5.2 Aplastic Anemia and Agranulocytosis
Aplastic anemia and agranulocytosis have occurred in patients treated with carbamazepine. Data from a population-based case-control study suggest that the risk of developing these reactions is 5-8 times greater than in the general population. However, the overall risk of these reactions in the untreated general population is low, approximately six patients per one million population per year for agranulocytosis and two patients per one million population per year for aplastic anemia.
Although reports of transient or persistent decreased platelet or white blood cell counts are not uncommon in association with the use of carbamazepine, data are not available to estimate accurately their incidence or outcome. However, the vast majority of the cases of leukopenia have not progressed to the more serious conditions of aplastic anemia or agranulocytosis.
Because of the very low incidence of agranulocytosis and aplastic anemia, the vast majority of minor hematologic changes observed in monitoring of patients on EQUETRO are unlikely to signal the occurrence of either abnormality. Nonetheless, complete pretreatment hematological testing should be obtained as a baseline. If a patient in the course of treatment exhibits low or decreased white blood cell or platelet counts, the patient should be monitored closely. Consider discontinuing EQUETRO if any evidence of significant bone marrow depression develops. Clinical features can include fever, dyspnea on exertion, fatigue, easy bruising, petechiae, epistaxis, gingival bleeding, and heavy menses.
5.3 Drug Reaction with Eosinophilia and Systemic Symptoms/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan Hypersensitivity, have occurred with carbamazepine. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity (e.g., fever, lymphadenopathy) may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. EQUETRO should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
Hypersensitivity
Hypersensitivity reactions to carbamazepine have been reported in patients who previously experienced this reaction to anticonvulsants including phenytoin, primidone, and phenobarbital. If such history is present, benefits and risks should be carefully considered, and, if EQUETRO is initiated, the signs and symptoms of hypersensitivity should be carefully monitored.
In patients who have exhibited hypersensitivity reactions to carbamazepine, approximately 25 to 30% may experience hypersensitivity reactions with oxcarbazepine.
5.4 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including EQUETRO, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to100 years) in the clinical trials analyzed. Table 1 presents the absolute and relative risk by indication for all evaluated AEDs.
| Placebo | Antiepileptic Drugs | |||
| Indication | Reactions Per 1000 Patients | Reactions Per 1000 Patients | Relative Risk: Incidence of Reactions in AED Group/ Incidence of Reactions in Placebo Group | Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
| Epilepsy Psychiatric Other Total | 1.0 5.7 1.0 2.4 | 3.4 8.5 1.8 4.3 | 3.5 1.5 1.9 1.8 | 2.4 2.9 0.9 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing EQUETRO or any other AED must balance the risk of suicidal thought or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behaviors and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
5.5 Embryofetal Toxicity
EQUETRO can cause fetal harm when administered to a pregnantfemale. [see Use in Specific Populations (8.1)]. Pregnancy registries and epidemiological data suggest a potential association between the use of carbamazepine during pregnancy and major congenital malformations, including neural tube defects and malformations involving other body systems (e.g., craniofacial defects and cardiovascular malformations). These available data suggest that, compared with monotherapy, there may be a higher prevalence of teratogenic effects associated with the use of anticonvulsants in combination therapy.
In animal studies, administration of carbamazepine at clinically relevant doses during pregnancy resulted in developmental toxicity, including increased incidences of fetal malformations.
Inform females who may become pregnant about the potential increased risk of major congenital malformations with EQUETRO use during pregnancy. Assess the risks and benefits of EQUETRO and discuss with the patient to determine if an alternative treatment should be considered. EQUETRO can reduce the effectiveness of hormonal contraceptives. Females of reproductive potential should be counseled on the consistent use of effective nonhormonal contraception or barrier methods during treatment with EQUETRO [see Drug Interactions (7.2) and Use in Specific Populations (8.1, 8.3)].
5.6 Abrupt Discontinuation and Risk of Seizure
Do not discontinue EQUETRO abruptly, because of the risk of seizure and other withdrawal signs/symptoms. Patients with seizure disorder are at increased risk of developing seizure and status epilepticus with attendant hypoxia and threat to life. However, in the event of an allergic or hypersensitivity reaction, more rapid substitution of alternative therapy may be necessary.
5.7 Hyponatremia
Hyponatremia can occur as a result of treatment with EQUETRO. In many cases, the hyponatremia appears to be caused by the syndrome of inappropriate antidiuretic hormone secretion (SIADH). The risk of developing SIADH with EQUETRO treatment appears to be dose-related. Elderly patients and patients treated with diuretics are at greater risk of developing hyponatremia. Signs and symptoms of hyponatremia include headache, new or increased seizure frequency, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Consider discontinuing EQUETRO in patients with symptomatic hyponatremia.
5.8 Potential for Cognitive and Motor Impairment
EQUETRO has the potential to cause impairment in judgment, cognition, and motor function. Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain the EQUETRO does not affect them adversely. Adverse reactions in the clinical trials in bipolar disorder included (EQUETRO, N= 251 and Placebo, N= 248): somnolence (32% vs. 13%), ataxia (15% vs. 0.4%), dizziness (44% vs. 12%), vertigo (2% vs. 1%), thinking abnormal (2% vs. 0.4%), tremor (3% vs. 1%), and blurred vision (6% vs. 2%) [see Adverse Reactions (6.1)].
5.9 Potential for Loss of Virologic Response to Non-nucleoside Reverse Transcriptase Inhibitors that are substrates for CYP3A4 with Concomitant Use of EQUETRO
Coadministration of EQUETRO with non-nucleoside reverse transcriptase inhibitors, including delavirdine, is contraindicated because it may lead to loss of virologic response and possible resistance. Through induction of CYP3A4, EQUETRO can markedly decrease the concentrations of these drugs. Coadministration of delavirdine, an NNRTI and a substrate of CYP3A4, and EQUETRO can decrease delavirdine concentrations by 90% [see Contraindications (4), Drug Interactions (7.2)].
5.10 Liver Damage
Hepatic effects, ranging from slight elevations in liver enzymes to rare cases of hepatic failure have been reported. In some cases, hepatic effects may progress despite discontinuation of the drug. In addition rare instances of vanishing bile duct syndrome have been reported. This syndrome consists of a cholestatic process with a variable clinical course ranging from fulminant to indolent, involving the destruction and disappearance of the intrahepatic bile ducts. Some, but not all, cases are associated with features that overlap with other immunoallergenic syndromes such as serious dermatologic reactions and Drug Reaction with Eosinophilia and Systemic Symptoms/Multiorgan Hypersensitivity [see Warnings and Precautions (5.1, 5.2)].
Baseline and periodic evaluations of liver function, particularly in patients with a history of liver disease, must be performed during treatment with this drug since liver damage may occur. The drug should be discontinued immediately in cases of aggravated liver dysfunction or active liver disease.
5.11 AV Heart Block
AV heart block, including second and third degree block, have been reported following carbamazepine treatment. This occurred generally, but not solely, in patients with underlying EKG abnormalities or risk factors for conduction disturbances.
5.12 Hepatic Porphyria
The use of EQUETRO should be avoided in patients with a history of hepatic porphyria (e.g., acute intermittent porphyria, variegate porphyria, porphyria cutanea tarda). Acute attacks have been reported in such patients receiving carbamazepine therapy. EQUETRO administration has also been demonstrated to increase porphyrin precursors in rodents, a presumed mechanism for the induction of acute attacks of porphyria.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Serious Dermatologic Reactions: Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome [see Warnings and Precautions (5.1)
- Aplastic anemia/agranulocytosis [see Warnings and Precautions (5.2)]
- Drug Reaction with Eosinophilia and Systemic Symptoms/Multiorgan Hypersensitivity [see Warnings and Precautions (5.3)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.4)]
- Embryofetal Toxicity [see Warnings and Precautions (5.5)]
- Abrupt Discontinuation and Seizure Risk [see Warnings and Precautions (5.6)]
- Hyponatremia [see Warnings and Precautions (5.7)]
- Cognitive and Motor Impairment [see Warnings and Precautions (5.8)]
- Drug Interaction with Non-Nucleoside Reverse Transcriptase Inhibitors [see Warnings and Precautions (5.9)]
- Liver Damage [see Warnings and Precautions (5.10)]
- AV Heart Block [see Warnings and Precautions (5.11)]
- Hepatic Porphyria [see Warnings and Precautions (5.12)]
- Increased Intraocular Pressure [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most commonly reported adverse reactions (>5% in the EQUETRO group and at least twice placebo) in the pooled 3-week placebo-controlled trials in patients with acute mania associated with Bipolar I Disorder (Studies 1 and 2) were dizziness, somnolence, nausea, vomiting, ataxia, constipation, pruritus, dry mouth, asthenia, blurred vision, and speech disorder [see Clinical Studies (14.1)]. The EQUETRO doses used were 400 to 1600 mg per day.
| Adverse Reactions | EQUETRO®
(N = 251) | Placebo
(N = 248) |
| Dizziness | 44% | 12% |
| Somnolence | 32% | 13% |
| Nausea | 29% | 10% |
| Vomiting | 18% | 3% |
| Ataxia | 15% | 0.4% |
| Constipation | 10% | 5% |
| Pruritus | 8% | 2% |
| Dry Mouth | 8% | 3% |
| Asthenia | 8% | 4% |
| Rash | 7% | 4% |
| Blurred vision | 6% | 2% |
| Speech Disorder | 6% | 0.4% |
| Hypertension | 3% | 0.4% |
| Paresthesia | 2% | 1% |
| Thinking abnormal | 2% | 0.4% |
| Tremor | 3% | 1% |
| Twitching | 2% | 1% |
| Vertigo | 2% | 1% |
7. Drug Interactions
7.1 Pharmacokinetic Effects of other Drugs on EQUETRO
Drugs that Inhibit Cytochrome P450 3A4 (CYP3A4)
EQUETRO is metabolized primarily by CYP3A4 to the active carbamazepine-10,11-epoxide, which is further metabolized to the trans-diol by epoxide hydrolase. Inhibitors of CYP 3A4 and/or epoxide hydrolase can increase plasma levels of EQUETRO and its active metabolites, increasing plasma concentrations of EQUETRO and the risk of adverse reactions. It may be necessary to reduce the EQUETRO dose if used concomitantly with inhibitors of CYP3A4 and/or epoxide hydrolase. The following drugs are CYP3A4 inhibitors:
Acetazolamide, aprepitant, azole antifungals (e.g., ketoconazole, itraconazole, fluconazole, voriconazole), cimetidine, ciprofloxacin, clarithromycin, dalfopristin, danazol, dantrolene, delavirdine, diltiazem, erythromycin, fluoxetine, fluvoxamine, grapefruit juice, ibuprofen, isoniazid, loratadine, nefazodone, niacinamide, nicotinamide, olanzapine, omeprazole, oxybutynin, quinine, quinupristin, ticlopidine, troleandomycin, valproate, verapamil, zileuton.
Drugs that Inhibit Epoxide Hydrolase and CYP3A4
Clarithromycin, erythromycin, loxapine, quetiapine, and valproate also inhibit epoxide hydrolase, resulting in increased levels of the active metabolite carbamazepine-10,11-epoxide [see Clinical Pharmacology (12.3)].
Drugs that Induce CYP3A4
CYP3A4 inducers can decrease serum concentrations of EQUETRO and decrease its effectiveness. It may be necessary to increase the dose of EQUETRO if used concomitantly with a CYP3A4 inducer. Such drugs include the following:
Aminophylline, cisplatin, doxorubicin, felbamate, phosphenytoin, methsuximide, phenobarbital, phenytoin, primidone, rifampin and theophylline.
7.2 Pharmacokinetic Effects of EQUETRO on other Drugs
EQUETRO is a potent inducer of hepatic 3A4 and is also known to be an inducer of CYP1A2, 2B6, 2C9/19 and may therefore reduce plasma concentrations of co-medications mainly metabolized by CYP 1A2, 2B6, 2C9/19 and 3A4, through induction of their metabolism. When used concomitantly with EQUETRO, monitoring of concentrations or dosage adjustment of these agents may be necessary.
EQUETRO decreases the concentrations of the following drugs through induction of their metabolism:
Hormonal Contraceptives (CYP3A4 Substrates)
EQUETRO is a strong inducer of CYP3A4. EQUETRO can increase the metabolism of certain hormonal contraceptives (through CYP3A4 induction) such as oral and subdermal implant contraceptives, leading to significantly lower plasma concentrations of hormones. This can cause contraceptive failure or breakthrough bleeding. Consider alternatives to oral and subdermal implant contraceptives that are significantly affected by induction of CYP3A4; or consider alternatives to EQUETRO [see Warnings and Precautions (5.5) and Use in Specific Populations (8.3)].
Delavirdine and other Non-Nucleoside Reverse Transcriptase Inhibitors (CYP3A4 Substrates)
Through induction of CYP3A4, EQUETRO increases the metabolism of delavirdine and certain non-nucleoside reverse transcriptase inhibitors and significantly reduces the plasma concentrations of these drugs. This can cause inadequate antiviral activity, loss of virologic response, and possible resistance to delavirdine or other non-nucleoside reverse transcriptase inhibitors. Therefore, the use of EQUETRO with these non-nucleoside reverse transcriptase inhibitors is contraindicated [see Contraindications (4) and Warnings and Precautions (5.9)].
Nefazodone (CYP3A4 Substrate)
The use of EQUETRO is contraindicated with the use of nefazodone because the concomitant use may result in insufficient plasma concentrations of nefazodone and its active metabolite to achieve a therapeutic effect of nefazodone.
Warfarin (CYP1A2 and CYP3A4 Substrate)
Through induction of CYP1A2 and CYP3A4, EQUETRO decreases the concentration of warfarin and decreases its anticoagulant effectiveness.
Aripiprazole
When carbamazepine is added to aripiprazole, the aripiprazole dose should be doubled. Additional dose increases should be based on clinical evaluation. If carbamazepine is later withdrawn, the aripiprazole dose should be reduced.
Tacrolimus
When carbamazepine is used with tacrolimus, monitoring of tacrolimus blood concentrations and appropriate dosage adjustments are recommended.
Temsirolimus
The use of concomitant strong CYP3A4 inducers such as carbamazepine should be avoided with temsirolimus. If patients must be coadministered carbamazepine with temsirolimus, an adjustment of temsirolimus dosage should be considered.
Lapatinib
The use of carbamazepine with lapatinib should generally be avoided. If carbamazepine is started in a patient already taking lapatinib, the dose of lapatinib should be gradually titrated up. If carbamazepine is discontinued, the lapatinib dose should be reduced.
HIV Protease Inhibitors
Due to strong induction of CYP3A4 caused by carbamazepine, use of EQUETRO with HIV protease inhibitors is not recommended.
Other CYP1A2 and CYP3A4 Substrates
EQUETRO induces CYP1A2 and CYP3A4, leading to decreased concentrations of drugs metabolized by CYP3A4 or CYP1A2. It may be necessary to increase the doses of such drugs when used concomitantly with EQUETRO. Drugs metabolized by CYP3A4 or CYP1A2 include the following:
Acetaminophen, albendazole, alprazolam, aprepitant, buprenorphone, bupropion, buspirone, citalopram, clobazam, clonazepam, clozapine, cyclosporin, delavirdine, desipramine, diazepam, dicumarol, dihydropyridine calcium channel blockers (e.g., felodipine), doxycycline, ethosuximide, everolimus, felbamate, glucocorticoids, haloperidol, imatinib, itraconazole, lamotrigine, levothyroxine, lorazepam, methadone, methsuxamide, mianserin, midazolam, mirtazapine, nefazodone, olanzapine, oral and other hormonal contraceptives, oxcarbazepine, paliperidone, phenytoin, praziquantel, quetiapine, risperidone, sertraline, sirolimus, tadalafil, theophylline, topiramate, tiagabine, tramadol, triazolam, tricyclic antidepressants (e.g., imipramine, amitriptyline, nortriptyline), trazodone, valproate, warfarin , ziprasidone, and zonisamide.
EQUETRO increases the plasma levels of the following drugs by inhibition of their metabolism:
Clomipramine, Phenytoin, and Primidone
EQUETRO can increase the concentrations of clomipramine, phenytoin, and primidone. If a patient has been titrated to a stable dosage on one of these agents in this category, and then begins treatment with EQUETRO, it may be necessary to decrease the dose of these drugs.
Phenytoin
Phenytoin levels have been reported to increase or decrease in the presence of carbamazepine. There are multiple pharmacokinetic mechanisms for changes in phenytoin levels when used concomitantly with EQUETRO. Monitor phenytoin serum levels carefully when used concomitantly with EQUETRO.
Cyclophosphamide
Cyclophosphamide is an inactive prodrug and is converted to its active metabolite in part by CYP3A. The rate of metabolism and the leukopenic activity of cyclophosphamide are reportedly increased by chronic co-administration of CYP3A4 inducers. There is a potential for increased cyclophosphamide toxicity when coadministered with carbamazepine.
7.3 Pharmacodynamic Drug Interactions
Monoamine Oxidase Inhibitors
Concomitant treatment with EQUETRO is contraindicated during use of an MAOI or within 14 days after discontinuing an MAOI. Concomitant use can cause serotonin syndrome.
Lithium
Concomitant administration of EQUETRO and lithium can increase the risk of neurotoxic adverse reactions. Consider reducing the dose of lithium or EQUETRO when using these drugs concomitantly.
Isoniazid
Concomitant use of carbamazepine and isoniazid has been reported to increase isoniazid-induced hepatotoxicity.
CNS Depressants
The concomitant use of EQUETRO and other CNS depressants can increase the risk of respiratory depression, profound sedation, hypotension, and syncope. CNS depressants include: alcohol, opioid analgesics, benzodiazepines, tricyclic antidepressants, sedative/hypnotics, anticonvulsants, antipsychotics, antihistamines, anticholinergics, alpha and beta blockers, general anesthetics, muscle relaxants, and illicit CNS depressants. Consider reducing the dose of CNS depressants or EQUETRO when using these drugs concomitantly.
Chloroquine and Mefloquine
The anti-malarial drugs chloroquine and mefloquine can antagonize the activity of EQUETRO.
Neuromuscular Blocking Agents
Resistance to the neuromuscular blocking action of the nondepolarizing neuromuscular blocking agents pancuronium, vecuronium, rocuronium and cisatracurium has occurred in patients chronically administered carbamazepine. Whether or not carbamazepine has the same effect on other nondepolarizing agents is unknown. Patients should be monitored closely for more rapid recovery from neuromuscular blockade than expected, and infusion rate requirements may be higher.
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as EQUETRO, during pregnancy. Healthcare providers are encouraged to recommend that pregnant patients taking EQUETRO enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling 1-888-233-2334 or online at http://www.aedpregnancyregistry.org/.
Risk Summary
EQUETRO can cause fetal harm when administered to a pregnantfemale [see Warnings and Precautions (5.5)]. Pregnancy registry and epidemiological data suggest a potential association between the use of carbamazepine during pregnancy and major congenital malformations, including neural tube defects and malformations involving other body systems (e.g., craniofacial defects and cardiovascular malformations). The available data are insufficient to identify an association with carbamazepine use and miscarriage (see Data). There are risks to the mother and fetus associated with untreated bipolar I disorder or epilepsy and with exposure to carbamazepine during pregnancy (see Clinical Considerations). In animal studies, administration of carbamazepine at clinically relevant doses during pregnancy resulted in developmental toxicity, including increased incidences of fetal malformation. Advise pregnant females of the potential risk of major congenital malformations with use of EQUETRO during pregnancy. Assess the risk and benefits of EQUETRO and discuss with the patient to determine if an alternative treatment should be considered during pregnancy.
Dietary folic acid supplementation both prior to conception and during pregnancy should be recommended for patients using carbamazepine. However, it is not known whether the risk of neural tube defects in the offspring of women receiving carbamazepine is reduced by folic supplementation. Evidence suggests that folic acid supplementation prior to conception and during the first trimester of pregnancy decreases the risk for congenital neural tube defects in the general population.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Disease-associated Maternal and/or Embryofetal Risk
There are risks to the mother from untreated bipolar I disorder, including increased risk of relapse, hospitalization, and suicide. Bipolar I disorder is associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Epilepsy, with or without exposure to antiepileptic drugs, has been associated with several adverse outcomes during pregnancy, including preeclampsia, preterm labor, antepartum and postpartum hemorrhage, placental abruption, poor fetal growth, prematurity, fetal death, and maternal mortality. The risk of maternal or fetal injury may be greatest for patients with untreated or poorly controlled convulsive seizures. Females with epilepsy should not discontinue carbamazepine abruptly due to the risk of status epilepticus and less severe seizures which may be life-threatening [see Warnings and Precautions (5.6)].
Fetal/Neonatal Adverse Reactions
There have been a few cases of neonatal seizures and/or respiratory depression associated with maternal carbamazepine and other concomitant anticonvulsant drug use. A few cases of neonatal vomiting, diarrhea, and/or decreased feeding have also been reported in association with maternal carbamazepine use. These symptoms may represent a neonatal withdrawal syndrome. Published studies have demonstrated that carbamazepine crosses the placenta.
Data
Human Data
Pregnancy registry and epidemiological data suggest a potential association between the use of carbamazepine during pregnancy and major congenital malformations, including neural tube defects and malformations involving other body systems (e.g., craniofacial defects and cardiovascular malformations). These available data suggest that, compared with monotherapy, there may be a higher prevalence of teratogenic effects associated with the use of anticonvulsants in combination therapy.
The North American Antiepileptic Drug (NAAED) Pregnancy Registry has reported a rate of major congenital malformations of 3.0% (95% CI: 2.1, 4.2) among pregnant females exposed to carbamazepine monotherapy (n=1,033) during the first trimester with a relative risk of 2.7 (95% CI: 1.0, 7.0) compared to pregnant females not taking an epileptic drug. The European Registry of Antiepileptic Drugs and Pregnancy (EURAP), a large international pregnancy registry focused outside of North America, reported major birth defects in 5.5% (95% CI: 4.5, 6.6) of 1,957 exposures to carbamazepine monotherapy in the first trimester. The UK Epilepsy and Pregnancy Register reported major birth defects in 2.6% (95% CI: 1.9-3.5) of 1,657 exposures to carbamazepine during the first three months of pregnancy.
Animal Data
In studies in which pregnant rats were administered carbamazepine orally during organogenesis, dose-related increases in the rates of fetal structural abnormalities (craniofacial, skeletal, cardiac, and urogenital defects), intrauterine growth retardation, and embryofetal death occurred at clinically relevant doses.
8.3 Females and Males of Reproductive Potential
In females currently on EQUETRO who may become pregnant, assess the risks and benefits of continuing on EQUETRO and discuss with the patient if an alternative treatment should be considered. Dietary folic acid supplementation both prior to conception and during pregnancy should be recommended for patients using carbamazepine [see Warnings and Precautions (5.5), Use in Specific Populations (8.1)].
Contraception
EQUETRO can increase metabolism of certain hormonal contraceptives (through CYP3A4 induction) such as oral and subdermal implant contraceptives, leading to significant lower plasma concentrations of hormones. This can cause contraceptive failure or breakthrough bleeding. Consider alternatives to oral and subdermal implant contraceptives that are significantly affected by induction of CYP3A4; or consider alternatives to EQUETRO [see Drug Interactions (7.2)].
Infertility
Based on human and animal findings, EQUETRO may impair male fertility. Published clinical studies have reported a reduction in semen quality in males of reproductive potential with epilepsy that were treated with carbamazepine [see Nonclinical Toxicology (13.1)].
12. Equetro - Clinical Pharmacology
12.3 Pharmacokinetics
Carbamazepine (CBZ)
Absorption: Following a single 200 mg oral extended-release dose of carbamazepine, peak plasma concentration was 1.9 ± 0.3 mcg/mL and the time to reach the peak was 19 ± 7 hours. Following repeat dose administration (800 mg every 12 hours), the peak levels were 11.0 ± 2.5 mcg/mL and the time to reach the peak was 5.9 ± 1.8 hours. The pharmacokinetics of extended-release carbamazepine is linear over the single dose range of 200–800 mg.
Carbamazepine is 76% bound to plasma proteins. Carbamazepine is primarily metabolized in the liver. Cytochrome P450 3A4 was identified as the major isoform responsible for the formation of carbamazepine-10,11-epoxide. Since carbamazepine induces its own metabolism, the half-life is also variable. The average half-life ranged from 35 to 40 hours following a single extended-release dose of carbamazepine and from 12 to 17 hours following repeated dosing. The apparent oral clearance was 25 ± 5 mL/min following a single dose and 80 ± 30 mL/min following multiple dosing.
Carbamazepine-10,11-epoxide (CBZ-E): Carbamazepine-10,11-epoxide is considered to be an active metabolite of carbamazepine. Following a single 200 mg oral extended-release dose of carbamazepine, the peak plasma concentration of carbamazepine-10,11-epoxide was 0.11 ± 0.012 mcg/mL and the time to reach the peak was 36 ± 6 hours. Following chronic administration of an extended-release dose of carbamazepine (800 mg every 12 hours), the peak levels of carbamazepine-10,11-epoxide were 2.2 ± 0.9 mcg/mL and the time to reach the peak was 14 ± 8 hours. The plasma half-life of carbamazepine-10,11-epoxide following administration of carbamazepine is 34 ± 9 hours. Following a single oral dose of extended-release carbamazepine (200–800 mg) the AUC and Cmax of carbamazepine-10,11-epoxide were less than 10% of carbamazepine. Following multiple dosing of extended-release carbamazepine (800–1600 mg daily for 14 days), the AUC and Cmax of carbamazepine-10,11-epoxide were dose-related, ranging from 15.7 mcg.hr/mL and 1.5 mcg/mL at 800 mg/day to 32.6 mcg.hr/mL and 3.2 mcg/mL at 1600 mg/day, respectively, and were less than 30% those of carbamazepine. Carbamazepine-10,11-epoxide is 50% bound to plasma proteins.
Food Effect: A high-fat meal diet increased the rate of absorption of a single 400 mg dose (mean Tmax was reduced from 24 hours, in the fasting state, to 14 hours, and Cmax increased from 3.2 to 4.3 mcg/mL) but not the extent (AUC) of absorption. The elimination half-life remained unchanged between fed and fasting state. The multiple-dose study conducted in the fed state showed that the steady-state Cmax values were within the therapeutic concentration range. The pharmacokinetic profile of extended-release carbamazepine was similar when given by sprinkling the beads over applesauce compared to the intact capsule administered in the fasted state.
Elimination: After oral administration of 14C-carbamazepine, 72% of the administered radioactivity was found in the urine and 28% was found in the feces. This urinary radioactivity was composed largely of hydroxylated and conjugated metabolites, with only 3% of unchanged carbamazepine.
Metabolism: In vitro data indicate carbamazepine is metabolized mainly by cytochrome P450 (CYP) 3A4 to the active carbamazepine-10,11-epoxide, which is further metabolized to the transdiol by epoxide hydrolase. Human microsomal epoxide hydrolase has been identified as the enzyme responsible for the formation of the 10,11-transdiol derivative from carbamazepine-10,11-epoxide.
Renal Impairment: The effect of renal impairment on the pharmacokinetics of carbamazepine is not known.
Hepatic Impairment: The effect of hepatic impairment on the pharmacokinetics of carbamazepine is not known. Consider reducing the dosage in patients with hepatic impairment.
Effect of Age: Carbamazepine is more rapidly metabolized to carbamazepine-10,11-epoxide in young children than in adults. In children below the age of 15, there is an inverse relationship between CBZ-E/CBZ ratio and increasing age. The safety and effectiveness of EQUETRO have not been established in pediatric patients for indications other than Epilepsy [see Indications and Usage (1.3)].
Effect of Gender: No difference in the mean AUC and Cmax of carbamazepine and carbamazepine-10,11-epoxide was found between males and females.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenicity
Oral administration of carbamazepine to rats for 2 years at doses of 25, 75, and 250 mg/kg/day resulted in a dose-related increase in the incidence of hepatocellular tumors (females) and of benign interstitial cell adenomas in the testes.
Mutagenicity
Carbamazepine was negative in in vitro bacterial and mammalian genotoxicity.
Impairment of Fertility
The effects of carbamazepine on male and female fertility have not been adequately studied in animals. However, testicular atrophy and/or aspermatogenesis were observed in rats with repeat oral administration of carbamazepine at clinically relevant doses.
14. Clinical Studies
14.1 Bipolar I Disorder (Acute Manic or Mixed Episodes)
The efficacy of EQUETRO in the acute treatment of manic or mixed symptoms associated with bipolar I disorder was established in two 3-week, multicenter, randomized, double-blind, placebo-controlled, flexible-dose studies (Studies 1 and 2) in adult patients who met the DSM-IV criteria for bipolar I disorder, manic or mixed episode. In both studies, patients must have had a history of at least one previous manic or mixed episode. They must have had a Young Mania Rating Scale (YMRS) baseline score of at least 20. The YMRS is an 11-item instrument, ranging from 0 to 60 (greater score indicates a more severe manic disorder) that measures symptoms associated with a manic state: elevated mood, increased motor activity/energy, sexual interest, sleep, irritability, speech, language-thought disorder, content, disruptive/aggressive behavior, appearance, and insight.
In Studies 1 and 2, patients were hospitalized for at least one week. They received placebo during a 5-day lead-in period and subsequently were randomized to receive placebo or EQUETRO, initially at a dose of 200 mg twice daily (400 mg per day). If tolerated, the total daily dose could be increased by 200 mg once daily to a maximum dose of 800 mg twice daily (1600 mg/day). The mean EQUETRO dose during the last week was 952 mg/day in Study 1 and 726 mg/day in Study 2.
Patients were permitted to receive lorazepam for agitation or insomnia up to 6 mg/day during the placebo-lead in period, up to 4 mg/day during the first week of controlled treatment, and up to 2 mg/day during the second week of treatment; no lorazepam was permitted during the third week of treatment. They were permitted to continue their routine psychotherapy. Patients were not allowed to use antipsychotics, lithium, antidepressants, or sedatives/hypnotics (other than lorazepam) during the studies. There were no significant differences in lorazepam use between the EQUETRO and placebo groups in both studies.
In Studies 1 and 2, the primary endpoint was the mean change from baseline in the YMRS total score at Day 21. In both studies, treatment with EQUETRO was statistically significantly superior to placebo, as measured by the mean decrease in YMRS score at Day 21 (Table 3).
The key secondary efficacy endpoint in both trials was the change in Clinical Global Impression-Severity (CGI-S) Scale score. The CGI-S an investigator-rated global assessment of symptom severity that is scored on a 7-point scale (1 = normal, not ill); (7 = severely ill). In both studies, there was a statistically significant decrease from baseline in the mean CGI-S score at Day 21, compared to placebo (Table 3).
| Study 1 | Study 2 | |||
| EQUETRO
(n=94) | Placebo
(n=98) | EQUETRO
(n=120) | Placebo
(n=115) |
|
| Young Mania Rating Scale (YMRS) | ||||
| Baseline YMRS | 26.6 | 27.3 | 28.5 | 27.9 |
| Week 3 YMRS | 17.9 | 22.1 | 13.4 | 20.8 |
| LS mean change | -7.8 | -4.8 | -14.8 | -7.0 |
| LS mean difference from placebo* | -3.5 | ─ | -8.0 | ─ |
| p-value | P= 0.033 | < 0.0001 | ||
| Clinical Global Impression-Severity Scale (CGI-S) | ||||
| Baseline CGI-S | 4.4 | 4.4 | 4.5 | 4.5 |
| Week 3 CGI-S | 3.7 | 4.1 | 3 | 3.9 |
| Change from Baseline at Week 3 | -0.7 | -0.3 | -1.5 | -0.6 |
| Difference (p-value) | -0.4 (0.025) | ─ | -0.9 (< 0.0001) | ─ |
* Least squares mean for the difference defined as the change from baseline at Week 3 in the EQUETRO group minus that in the placebo group.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
-
Serious Dermatologic Reactions
Inform patients and caregivers about the risk of potentially fatal, serious skin reactions and the signs and symptoms that may signal a serious skin reaction. Instruct patients to consult with their healthcare provider immediately if a skin reaction occurs during treatment with EQUETRO [see Warnings and Precautions (5.1)].
-
Agranulocytosis and Aplastic Anemia
Inform patients and caregivers about the risk of potentially fatal agranulocytosis and aplastic anemia and the signs and symptoms that may signal these reactions. Instruct them to contact their healthcare provider immediately if symptoms occur [see Warnings and Precautions (5.2)].
-
Drug Reaction with Eosinophilia and Systemic Symptoms
Inform patients of the early toxic signs and symptoms of a potential hematologic, dermatologic, hypersensitivity, or hepatic reactions. Advise patients that these signs and symptoms may signal a serious reaction and to report any occurrence immediately to their healthcare provider [see Warnings and Precautions (5.3)].
-
Suicidal Ideation and Behavior
Counsel patients, their caregivers, and families that AEDs, including EQUETRO, may increase the risk of suicidal thinking and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients, caregivers and families to report behaviors of concern immediately to healthcare providers [see Warnings and Precautions (5.4)].
-
Abrupt Discontinuation and Risk of Seizure
Inform patients that abrupt discontinuation of EQUETRO can cause seizures or an increase in seizure frequency. Advise patients that the drug should be tapered when discontinued [see Warnings and Precautions (5.6)].
-
Hyponatremia
Advise patients that EQUETRO may reduce serum sodium concentrations especially if they are taking other medications that can lower sodium. Advise patients to report symptoms of low sodium like nausea, tiredness, lack of energy, confusion, seizures, or more frequent or more severe seizures [see Warnings and Precautions (5.7)].
-
Potential for Cognitive and Motor Impairment
Advise patients not to drive or operate machinery until they have gained sufficient experience on EQUETRO to gauge whether it adversely affects their ability to drive or operate machinery.
Advise patients to exercise caution if alcohol is taken in combination with EQUETRO therapy, due to a possible additive sedative effect [see Warnings and Precautions (5.8)].
-
Concomitant Use with other Carbamazepine Products
Inform patients that EQUETRO contains carbamazepine and should not be used in combination with any other medications containing carbamazepine.
-
Embryofetal Toxicity
EQUETRO may cause fetal harm. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy. [see Warning and Precautions (5.5) and Use in Specific Populations (8.1)].
-
Pregnancy
Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to EQUETRO during pregnancy [see Use in Specific Populations (8.1)].
-
Decreased Effectiveness of Hormonal Contraceptives
Inform patients that EQUETRO can significantly decrease the effectiveness of hormonal contraceptives, such as oral contraceptives and subdermal implants. This can cause contraceptive failure or breakthrough bleeding [see Drug Interactions (7.2) and Use in Specific Populations (8.3)].
-
Infertility
Advise males of reproductive potential that EQUETRO may impair fertility [see Use in Specific Populations (8.3)].
Manufactured for and Distributed by:
Validus Pharmaceuticals LLC
Parsippany, NJ 07054
[email protected]
http://www.equetro.com
1-866-982-5438
Product of India
© 2022 Validus Pharmaceuticals LLC
60079-04 October 2022
| EQUETRO
carbamazepine capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| EQUETRO
carbamazepine capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| EQUETRO
carbamazepine capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Validus Pharmaceuticals LLC (801194619) |