Drug Detail:Evrysdi (Risdiplam)
Drug Class: Miscellaneous uncategorized agents
Highlights of Prescribing Information
EVRYSDI® (risdiplam) for oral solution
Initial U.S. Approval: 2020
Indications and Usage for Evrysdi
EVRYSDI is a survival of motor neuron 2 (SMN2) splicing modifier indicated for the treatment of spinal muscular atrophy (SMA) in patients 2 months of age and older. (1)
Evrysdi Dosage and Administration
EVRYSDI must be constituted by a pharmacist prior to dispensing.
Administer orally once daily after a meal using the provided oral syringe. (2.1, 2.4)
| Age and Body Weight | Recommended Daily Dosage |
|---|---|
| 2 months to less than 2 years of age | 0.2 mg/kg |
| 2 years of age and older weighing less than 20 kg | 0.25 mg/kg |
| 2 years of age and older weighing 20 kg or more | 5 mg |
See Full Prescribing Information for important preparation and administration instructions. (2.1, 2.4)
Dosage Forms and Strengths
For Oral Solution: 60 mg of risdiplam as a powder for constitution to provide 0.75 mg/mL solution. (3)
Contraindications
None. (4)
Adverse Reactions/Side Effects
The most common adverse reactions in later-onset SMA (incidence at least 10% of patients treated with EVRYSDI and more frequent than control) were fever, diarrhea, and rash. (6.1)
The most common adverse reactions in infantile-onset SMA were similar to those observed in later-onset SMA patients. Additionally, adverse reactions with an incidence of at least 10% were upper respiratory tract infection, pneumonia, constipation, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Avoid coadministration with drugs that are substrates of multidrug and toxin extrusion (MATE) transporters. (7.1)
Use In Specific Populations
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2021
Related/similar drugs
nusinersen, Spinraza, risdiplam, ZolgensmaFull Prescribing Information
1. Indications and Usage for Evrysdi
EVRYSDI is indicated for the treatment of spinal muscular atrophy (SMA) in patients 2 months of age and older.
2. Evrysdi Dosage and Administration
2.2 Dosing Information
EVRYSDI is administered orally once daily. The recommended dosage is determined by age and body weight (see Table 1).
| Age and Body Weight | Recommended Daily Dosage |
|---|---|
| 2 months to less than 2 years of age | 0.2 mg/kg |
| 2 years of age and older weighing less than 20 kg | 0.25 mg/kg |
| 2 years of age and older weighing 20 kg or more | 5 mg |
2.3 Missed Dose
If a dose of EVRYSDI is missed, EVRYSDI should be administered as soon as possible if still within 6 hours of the missed dose, and the usual dosing schedule can be resumed on the next day. Otherwise, the missed dose should be skipped, and the next dose should be taken at the regularly scheduled time on the next day.
If a dose is not fully swallowed or vomiting occurs after taking a dose of EVRYSDI, another dose should not be administered to make up for the lost dose. The patient should wait until the next day to take the next dose at the regularly scheduled time.
3. Dosage Forms and Strengths
EVRYSDI for oral solution: 60 mg as a light yellow, yellow, greyish yellow, greenish yellow, or light green powder for constitution. Following constitution, the volume of the greenish yellow to yellow solution is 80 mL, providing 60 mg/80 mL (0.75 mg/mL) risdiplam.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials including patients with infantile-onset SMA and later-onset SMA, a total of 337 patients (52% female, 72% Caucasian) were exposed to EVRYSDI for up to a maximum of 32 months, with 209 patients receiving treatment for more than 12 months. Forty-seven (14%) patients were 18 years and older, 74 (22%) were 12 years to less than 18 years, 154 (46%) were 2 years to less than 12 years, and 62 (18%) 2 months to less than 2 years.
Clinical Trial in Later-Onset SMA
The safety of EVRYSDI for later-onset SMA is based on data from a randomized, double-blinded, placebo-controlled study (Study 2 Part 2) in patients with SMA Type 2 or 3 (n = 180) [see Clinical Studies (14.2)]. The patient population in Study 2 Part 2 ranged in age from 2 to 25 years at the time of treatment start.
The most common adverse reactions (reported in at least 10% of patients treated with EVRYSDI and at an incidence greater than on placebo) in Study 2 Part 2 were fever, diarrhea, and rash. Table 2 lists the adverse reactions that occurred in at least 5% of patients treated with EVRYSDI and at an incidence ≥ 5% greater than on placebo in Study 2 Part 2.
| Adverse Reaction | EVRYSDI (N = 120) % | Placebo (N = 60) % |
|---|---|---|
|
||
| Fever* | 22 | 17 |
| Diarrhea | 17 | 8 |
| Rash† | 17 | 2 |
| Mouth and aphthous ulcers | 7 | 0 |
| Arthralgia | 5 | 0 |
| Urinary tract infection‡ | 5 | 0 |
7. Drug Interactions
7.1 Effect of EVRYSDI on Substrates of Multidrug and Toxin Extrusion (MATE) Protein Transporters
Based on in vitro data, EVRYSDI may increase plasma concentrations of drugs eliminated via MATE1 or MATE2-K [see Clinical Pharmacology (12.3)], such as metformin. Avoid coadministration of EVRYSDI with MATE substrates. If coadministration cannot be avoided, monitor for drug-related toxicities and consider dosage reduction of the coadministered drug (based on the labeling of that drug) if needed.
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
Studies of risdiplam in juvenile and adult rats and in monkeys demonstrated adverse effects on the reproductive organs, including germ cells, in males at clinically-relevant plasma exposures [see Use in Specific Populations (8.4) and Nonclinical Toxicology (13.1)].
11. Evrysdi Description
EVRYSDI for oral solution contains risdiplam, which is a survival of motor neuron 2 (SMN2)-directed RNA splicing modifier.
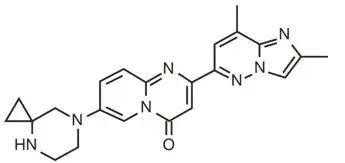
The chemical name of risdiplam is 7-(4,7-diazaspiro[2.5]octan-7-yl)-2-(2,8 dimethylimidazo[1,2-b]pyridazin-6-yl)pyrido-4H-[1,2-a]pyrimidin-4-one. Risdiplam has a molecular weight of 401.46 g/mol.
The molecular formula of risdiplam is C22H23N7O and the chemical structure is shown below.
EVRYSDI is supplied as a powder in an amber glass bottle. Each bottle contains 60 mg of risdiplam. The inactive ingredients of EVRYSDI are: ascorbic acid, disodium edetate dihydrate, isomalt, mannitol, polyethylene glycol 6000, sodium benzoate, strawberry flavor, sucralose, and tartaric acid.
The powder is constituted with purified water to yield 60 mg/80 mL (0.75 mg/mL) of risdiplam after constitution [see Dosage and Administration (2.4)].
12. Evrysdi - Clinical Pharmacology
12.1 Mechanism of Action
Risdiplam is a survival of motor neuron 2 (SMN2) splicing modifier designed to treat patients with spinal muscular atrophy (SMA) caused by mutations in chromosome 5q that lead to SMN protein deficiency. Using in vitro assays and studies in transgenic animal models of SMA, risdiplam was shown to increase exon 7 inclusion in SMN2 messenger ribonucleic acid (mRNA) transcripts and production of full-length SMN protein in the brain.
In vitro and in vivo data indicate that risdiplam may cause alternative splicing of additional genes, including FOXM1 and MADD. FOXM1 and MADD are thought to be involved in cell cycle regulation and apoptosis, respectively, and have been identified as possible contributors to adverse effects seen in animals.
12.2 Pharmacodynamics
In clinical trials, EVRYSDI led to an increase in SMN protein with a greater than 2-fold median change from baseline within 4 weeks of treatment initiation. The increase was sustained throughout the treatment period (of at least 12 months) across all SMA types.
12.3 Pharmacokinetics
Pharmacokinetics of EVRYSDI have been characterized in healthy adult subjects and in patients with SMA.
After administration of EVRYSDI as an oral solution, pharmacokinetics of risdiplam were approximately linear between 0.6 and 18 mg in a single-ascending-dose study in healthy adult subjects, and between 0.02 and 0.25 mg/kg once daily in a multiple-ascending-dose study in patients with SMA. Following once-daily oral administration of risdiplam in healthy subjects, approximately 3-fold accumulation of peak plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC0-24h) was observed. Risdiplam exposures reach steady state 7 to 14 days after once-daily administration.
Elimination
The apparent clearance (CL/F) of risdiplam is 2.1 L/h for a 14.9 kg patient.
The terminal elimination half-life of risdiplam was approximately 50 hours in healthy adults.
13. Nonclinical Toxicology
14. Clinical Studies
The efficacy of EVRYSDI for the treatment of patients with infantile-onset and later-onset SMA was evaluated in two clinical studies, Study 1 (NCT02913482) and Study 2 (NCT02908685).
The overall findings of these studies support the effectiveness of EVRYSDI in SMA patients 2 months of age and older and appear to support the early initiation of treatment with EVRYSDI.
14.1 Infantile-Onset SMA
Study 1 was an open-label, 2-part study to investigate the efficacy, safety, pharmacokinetics, and pharmacodynamics of EVRYSDI in patients with Type 1 SMA (symptom onset between 28 days and 3 months of age). Part 1 of Study 1 (n = 21) provides efficacy and safety data in patients with Type 1 SMA. Additional safety information is provided by Part 2 of Study 1 (n = 41) in patients with Type 1 SMA [see Adverse Reactions (6.1)].
In Part 1 of Study 1, patients (n = 21) were enrolled in one of two dosage cohorts. Patients in the higher-dosage cohort (n = 17) had their dosage adjusted to the recommended dosage of 0.2 mg/kg/day before 12 months of treatment, while patients in the low-dosage cohort (n = 4) did not.
Effectiveness was established based on the ability to sit without support for at least 5 seconds (as measured by Item 22 of the Bayley Scales of Infant and Toddler Development – Third Edition (BSID-III) gross motor scale) and on the basis of survival without permanent ventilation. Permanent ventilation was defined as requiring a tracheostomy or more than 21 consecutive days of either non-invasive ventilation (≥ 16 hours per day) or intubation, in the absence of an acute reversible event.
The median age of onset of clinical signs and symptoms of Type 1 SMA in patients enrolled in Part 1 of Study 1 was 2.0 months (range: 0.9 to 3.0); 71% of patients were female, 81% were Caucasian, and 19% were Asian. The median age at enrollment was 6.7 months (range: 3.3 to 6.9), and the median time between onset of symptoms and first dose was 4.0 months (range: 2.0 to 5.8). All patients had genetic confirmation of homozygous deletion or compound heterozygosity predictive of loss of function of the SMN1 gene, and two SMN2 gene copies.
In Study 1 Part 1, the median duration of EVRYSDI treatment was 14.8 months (range: 0.6 to 26.0), and 19 patients were treated for a minimum duration of 12 months.
Of the patients who were treated with the recommended dosage of EVRYSDI 0.2 mg/kg/day, 41% (7/17) were able to sit independently for ≥ 5 seconds (BSID-III, Item 22) after 12 months of treatment. These results indicate a clinically meaningful deviation from the natural history of untreated infantile-onset SMA. As described in the natural history of untreated infantile-onset SMA, patients would not be expected to attain the ability to sit independently, and no more than 25% of these patients would be expected to survive without permanent ventilation beyond 14 months of age. After 12 months of treatment with EVRYSDI, 90% (19/21) of patients were alive without permanent ventilation (and reached 15 months of age or older). After a minimum of 23 months of treatment with EVRYSDI, 81% (17/21) of all patients were alive without permanent ventilation (and reached an age of 28 months or older; median 32 months; range 28 to 45 months).
14.2 Later-Onset SMA
Study 2 was a 2-part, multicenter trial to investigate the efficacy, safety, pharmacokinetics, and pharmacodynamics of EVRYSDI in patients diagnosed with SMA Type 2 or Type 3. Part 1 of Study 2 was dose-finding and exploratory in 51 patients (14% ambulatory). Part 2 was randomized, double-blind, placebo-controlled, and is described below.
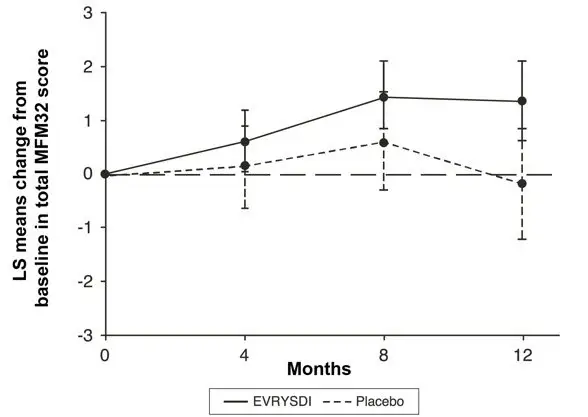
The primary endpoint in Study 2 Part 2 was the change from baseline to Month 12 in the Motor Function Measure 32 (MFM32) score. A key secondary endpoint was the proportion of patients with a 3-point or greater change from baseline to Month 12 in the MFM32 total score. The MFM32 measures motor function abilities that relate to daily functions. The total MFM32 score is expressed as a percentage (range: 0 to 100) of the maximum possible score, with higher scores indicating greater motor function. Another key secondary endpoint was the Revised Upper Limb Module (RULM). The RULM is a tool used to assess motor performance of the upper limb in SMA patients. It tests proximal and distal motor functions of the arm. The total score ranges from 0 (all the items cannot be performed) to 37 (all the activities are achieved fully without any compensatory maneuvers).
Study 2 Part 2 enrolled 180 non-ambulatory patients with Type 2 (71%) or Type 3 (29%) SMA. Patients were randomized 2:1 to receive EVRYSDI at the recommended dosage [see Dosage and Administration (2.2)] or placebo. Randomization was stratified by age group (2 to 5, 6 to 11, 12 to 17, or 18 to 25 years of age).
The median age of patients at the start of treatment was 9.0 years (range 2 to 25), and the median time between onset of initial SMA symptoms and first treatment was 102.6 months (range 1 to 275). Of the 180 patients included in the trial, 51% were female, 67% were Caucasian, and 19% were Asian. At baseline, 67% of patients had scoliosis (32% of them with severe scoliosis). Patients had a mean baseline MFM32 score of 46.1, and RULM score of 20.1. Overall baseline demographic characteristics were reasonably balanced between the treatment groups (EVRYSDI and placebo), with the exception of scoliosis (63% in the EVRYSDI arm vs. 73% in the placebo group).
The primary analysis on the change from baseline in MFM32 total score at Month 12 showed a clinically meaningful and statistically significant difference between patients treated with EVRYSDI and placebo. The results of the primary analysis and key secondary endpoints are shown in Table 3 and Figure 1.
| Endpoint | EVRYSDI (N = 120) | Placebo (N = 60) |
|---|---|---|
|
||
| Primary Endpoint: | ||
| Change from baseline in total MFM32 score at Month 12, LS means (95% CI) *,†,‡ | 1.36 (0.61, 2.11) | -0.19 (-1.22, 0.84) |
| Difference from Placebo, Estimate (95% CI)*
p-value | 1.55 (0.30, 2.81) 0.0156 |
|
| Secondary Endpoints: | ||
| Proportion of patients with a change from baseline MFM32 total score of 3 or more at Month 12 (95% CI)†,‡ | 38.3% (28.9, 47.6) | 23.7% (12.0, 35.4) |
| Odds ratio for overall response (95% CI) adjusted§ (unadjusted) p-value¶ | 2.35 (1.01, 5.44) 0.0469 (0.0469) |
|
| Change from baseline in total score of RULM at Month 12, LS means (95% CI)*, # | 1.61 (1.00, 2.22) | 0.02 (-0.83, 0.87) |
| Difference from Placebo, Estimate (95% CI) adjusted§ (unadjusted) p-value* | 1.59 (0.55, 2.62) 0.0469 (0.0028) |
|
|
|
Figure 1 Mean Change from Baseline in Total MFM32 Score Over 12 Months (Study 2 Part 2)*,† |
|
|
16. How is Evrysdi supplied
16.1 How Supplied
Each amber glass bottle of EVRYSDI is packaged with a bottle adapter, two 6 mL reusable oral syringes, and two 12 mL reusable oral syringes. EVRYSDI for oral solution is a light yellow, yellow, greyish yellow, greenish yellow, or light green powder. Each bottle contains 60 mg of risdiplam (NDC 50242-175-07).
16.2 Storage and Handling
Store the dry powder at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP controlled room temperature]. Keep in the original carton.
Keep the constituted oral solution of EVRYSDI in the original amber bottle to protect from light. Store in a refrigerator at 2°C to 8°C (36°F to 46°F) [see Dosage and Administration (2.4)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Approved: 4/2021 | ||
| Patient Information EVRYSDI® [ev-RIZ-dee] (risdiplam) for oral solution |
|||
What is EVRYSDI?
|
|||
Before taking EVRYSDI, tell your healthcare provider about all of your medical conditions, including if you:
|
|||
| How should I take EVRYSDI? See the detailed Instructions for Use that comes with EVRYSDI for information on how to take or give EVRYSDI oral solution.
|
|||
| What are the possible side effects of EVRYSDI? The most common side effects of EVRYSDI include:
|
|||
|
|
|
|
|
|||
|
|
|
|
| These are not all of the possible side effects of EVRYSDI. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
How should I store EVRYSDI?
|
|||
| General information about the safe and effective use of EVRYSDI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EVRYSDI for a condition for which it was not prescribed. Do not give EVRYSDI to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about EVRYSDI that is written for health professionals. |
|||
| What are the ingredients in EVRYSDI? Active ingredient: risdiplam Inactive ingredients: ascorbic acid, disodium edetate dihydrate, isomalt, mannitol, polyethylene glycol 6000, sodium benzoate, strawberry flavor, sucralose, and tartaric acid. Distributed by: Genentech, Inc., A Member of the Roche Group, 1 DNA Way, South San Francisco, CA 94080-4990 EVRYSDI is a registered trademark of Genentech, Inc. ©2021 Genentech, Inc. All rights reserved. For more information, go to www.EVRYSDI.com or call 1-833-387-9734. |
|||
INSTRUCTIONS FOR USE
EVRYSDI® [ev-RIZ-dee]
(risdiplam)
for oral solution
Please read and understand this Instructions for Use and the Patient Information leaflet before you start taking EVRYSDI for information about EVRYSDI and how to prepare and give EVRYSDI through an oral syringe, gastrostomy tube (G-tube), or nasogastric tube (NG-tube).
If you have any questions about how to take EVRYSDI, contact your healthcare provider.
EVRYSDI should come as a liquid in a bottle when you receive it from the pharmacy. Do not take EVRYSDI and contact your pharmacist if the medicine in the bottle is a powder.
Each EVRYSDI carton contains (see Figure A):
 | 1 Cap |  Figure A Figure A |
 | 1 Bottle adapter | |
 | 1 EVRYSDI bottle | |
 | 2 Reusable oral syringes | |
 | 1 Instructions for Use (not shown) | |
 | 1 Prescribing Information and Patient Information (not shown) | |
| Reusable Oral Syringe Overview (see Figure B) |  Figure B Figure B |
|
Important information about EVRYSDI
- Ask your healthcare provider to show you the correct oral syringe you should use and how to measure your prescribed daily dose.
- Always use the reusable oral syringes that come with EVRYSDI to measure your prescribed daily dose. If your carton does not contain two identical syringes, contact your pharmacist.
- Always take EVRYSDI exactly as your healthcare provider tells you to take it.
- Take EVRYSDI 1 time daily after a meal at approximately the same time each day.
- Do not take EVRYSDI if the bottle adapter is not in the bottle. If the bottle adapter is not in the bottle, contact your pharmacist.
- Do not mix EVRYSDI into food or liquids. Do not mix EVRYSDI with formula or milk.
- Do not take EVRYSDI if the bottle or oral syringes are damaged.
- Avoid getting EVRYSDI on your skin or in your eyes. If EVRYSDI gets on your skin, wash the area with soap and water. If EVRYSDI gets in your eyes, rinse your eyes with water.
- If you spill EVRYSDI, dry the area with a dry paper towel and then clean with water. Throw away the paper towel in the trash and wash your hands well with soap and water.
- If there is not enough EVRYSDI left in the bottle for your prescribed dose, throw away (discard) the bottle with remaining EVRYSDI and used oral syringes according to your local requirements.
- Use a new bottle of EVRYSDI to get your prescribed dose.
Do not mix EVRYSDI from the new bottle with the bottle you are currently using.
How to store EVRYSDI
| 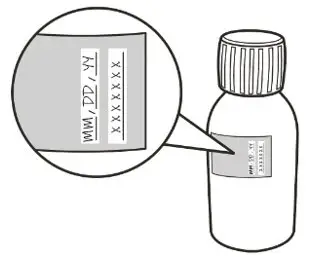 Figure C Figure C |
A) Preparing and withdrawing your dose
How to prepare your dose of EVRYSDI
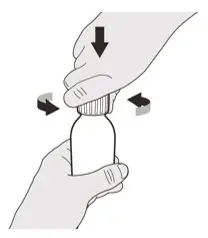 Figure D Figure D | Step A1
Remove the cap by pushing it down and then twisting the cap to the left (counterclockwise) (See Figure D). Do not throw away the cap. |
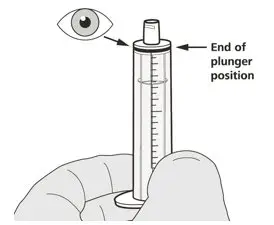 Figure E Figure E | Step A2
Push the plunger of the oral syringe all the way down to remove any air in the oral syringe (See Figure E). |
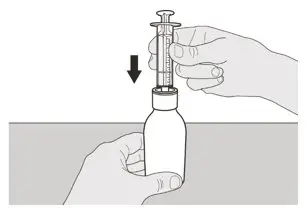 Figure F Figure F | Step A3
Place the EVRYSDI bottle on a flat surface. While keeping the bottle in an upright position, insert the syringe tip into the bottle adapter (See Figure F). |
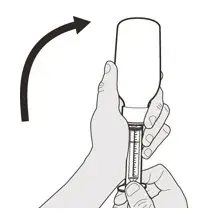 Figure G Figure G | Step A4
Carefully turn the bottle upside down with the syringe tip firmly inserted into the bottle adapter (See Figure G). |
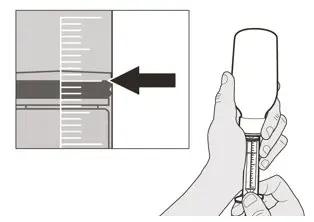 Figure H Figure H | Step A5
Slowly pull back on the plunger to withdraw your prescribed dose of EVRYSDI. The top of the black plunger stopper must line up with the mL marking on the oral syringe for your prescribed daily dose (See Figure H). After the correct dose is withdrawn, hold the plunger in place to keep the plunger from moving. |
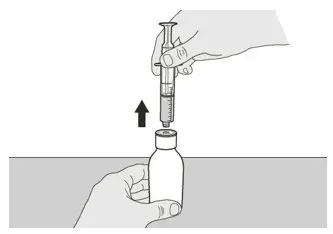 Figure I Figure I | Step A6 Continue to hold the plunger in place to keep the plunger from moving. Leave the oral syringe in the bottle adapter and turn the bottle to an upright position. Place the bottle onto a flat surface. Remove the oral syringe from the bottle adapter by gently pulling straight up on the oral syringe while holding the plunger in place (See Figure I). |
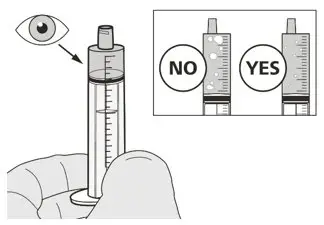 Figure J Figure J | Step A7
Hold the oral syringe with the syringe tip pointing up. Check the EVRYSDI in the oral syringe. If there are large air bubbles in the oral syringe (See Figure J) or if you have drawn up the wrong dose of EVRYSDI, insert the syringe tip firmly into the bottle adapter while the bottle is in an upright position. Push the plunger all the way down so that EVRYSDI flows back into the bottle and repeat Steps A4 through A7. Take or give EVRYSDI right away after it is drawn up into the oral syringe. If it is not taken within 5 minutes, throw away EVRYSDI liquid from your oral syringe into the household trash. Do this by pushing the plunger all the way down to remove EVRYSDI from the oral syringe. Prepare a new dose starting with Step A2. |
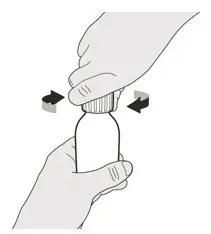 Figure K Figure K | Step A8
Put the cap back on the bottle. Turn the cap to the right (clockwise) to tightly close the bottle (See Figure K). Do not remove the bottle adapter from the bottle. |
If you are taking your dose of EVRYSDI by mouth, follow the instructions in "B) How to take a dose of EVRYSDI by mouth".
If you are taking your dose of EVRYSDI through a gastrostomy tube, follow the instructions in "C) How to give a dose of EVRYSDI through a gastrostomy tube".
If you are taking your dose of EVRYSDI through a nasogastric tube, follow the instructions in "D) How to give a dose of EVRYSDI through a nasogastric tube".
B) How to take a dose of EVRYSDI by mouth
Sit upright when taking a dose of EVRYSDI by mouth.
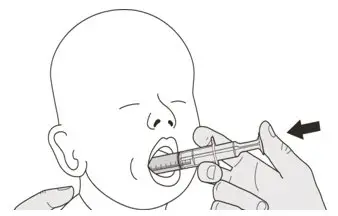 Figure L Figure L | Step B1
Place the oral syringe into the mouth with the tip along either cheek. Slowly push the plunger all the way down to give the full dose of EVRYSDI (See Figure L). Giving EVRYSDI into the throat or too fast may cause choking. |
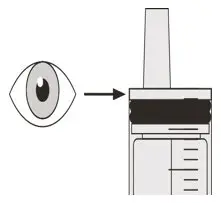 Figure M Figure M | Step B2
Check that there is no EVRYSDI left in the oral syringe (See Figure M). |
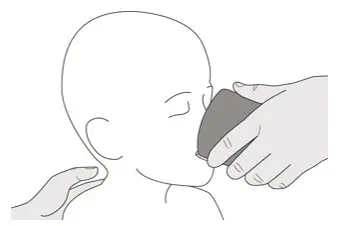 Figure N Figure N | Step B3
Drink about a tablespoon (15 mL) of water right after taking the prescribed dose of EVRYSDI to ensure the drug has been completely swallowed (See Figure N). Go to Step E for cleaning of the syringe. |
C) How to give a dose of EVRYSDI through a gastrostomy tube
If you are giving EVRYSDI through a gastrostomy tube, ask your healthcare provider to show you how to inspect the gastrostomy tube before giving EVRYSDI.
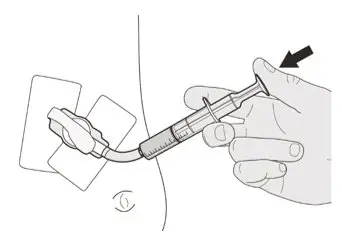 Figure O Figure O | Step C1
Place the oral syringe tip into the gastrostomy tube. Slowly push the plunger all the way down to give the full dose of EVRYSDI (See Figure O). |
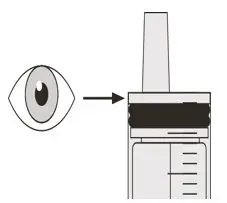 Figure P Figure P | Step C2
Check that there is no EVRYSDI left in the oral syringe (See Figure P). |
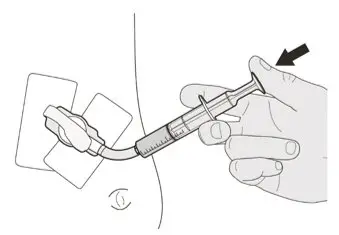 Figure Q Figure Q | Step C3
Flush the gastrostomy tube with 10 mL to 20 mL of water right after giving the prescribed dose of EVRYSDI (See Figure Q). Go to Step E for cleaning of the syringe. |
D) How to give a dose of EVRYSDI through a nasogastric tube
If you are giving EVRYSDI through a nasogastric tube, ask your healthcare provider to show you how to inspect the nasogastric tube before giving EVRYSDI.
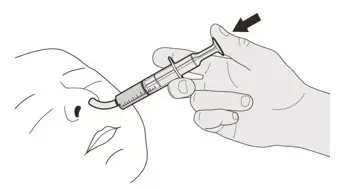 Figure R Figure R | Step D1
Place the oral syringe tip into the nasogastric tube. Slowly press the plunger all the way down to give the full dose of EVRYSDI (See Figure R). |
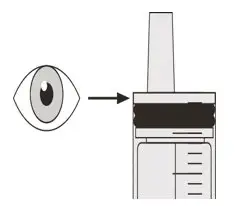 Figure S Figure S | Step D2
Check that there is no EVRYSDI left in the oral syringe (See Figure S). |
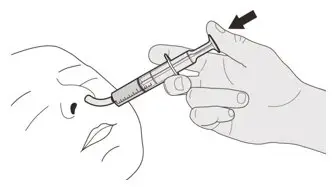 Figure T Figure T | Step D3
Flush the nasogastric tube with 10 mL to 20 mL of water right after giving the prescribed dose of EVRYSDI (See Figure T). Go to Step E for cleaning of the syringe. |
E) How to clean the oral syringe after use
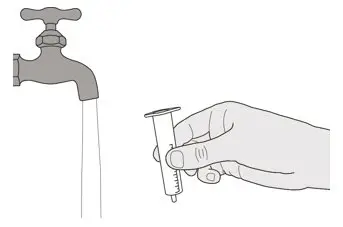 Figure U Figure U | Step E1
Remove the plunger from the oral syringe by pulling the plunger away from the syringe until the plunger comes out of the syringe. Rinse the oral syringe barrel well under clean water (See Figure U). |
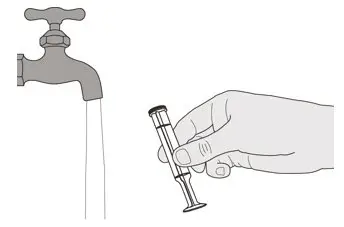 Figure V Figure V | Step E2
Rinse the plunger well under clean water (See Figure V). |
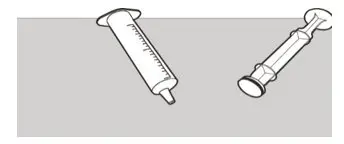 Figure W Figure W | Step E3
Check that the oral syringe barrel and plunger are clean. Place the oral syringe barrel and plunger on a clean surface in a safe place to dry (See Figure W). Wash your hands with soap and water. After the oral syringe barrel and plunger are dry, put the plunger back into the oral syringe barrel and store the syringe with your medicine. |
EVRYSDI is a registered trademark of Genentech, Inc.
Distributed by:
Genentech, Inc.
1 DNA Way
South San Francisco, CA 94080-4990
Approved: 4/2021
This Instructions for Use has been Approved by the U.S. Food and Drug Administration.
©2021 Genentech, Inc. All Rights Reserved
EVRYSDI®
(risdiplam) for oral solution
Instructions for Constitution
(FOR PHARMACISTS ONLY)
Each EVRYSDI carton contains (see Figure A):
 | 1 Cap | 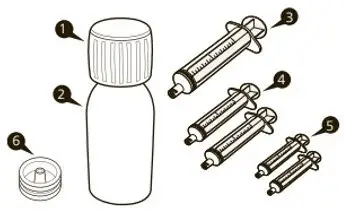 Figure A Figure A |
 | 1 EVRYSDI bottle | |
 | 2 Reusable oral syringes 12 mL | |
 | 2 Reusable oral syringes 6 mL | |
 | 1 Press-in bottle adapter | |
 | 1 Prescribing Information (not shown) | |
 | 1 Instructions for Constitution (not shown) | |
 | 1 Instructions for Use (not shown) |
Important information about EVRYSDI
- Do not use if the powder expiration date has passed. The powder expiration date is printed on the bottle label.
- Do not use the medicine if any of the supplies are damaged or missing.
- Use Purified Water to constitute the medicine.
- Select the appropriate oral syringes (6 mL or 12 mL) based on the patient's dose and provide instruction to the patient/caregiver on how to administer their dose.
- Do not add oral syringes other than the ones provided in the carton. The oral syringes supplied are intended to be reusable.
How to store EVRYSDI
- Store the dry powder at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP controlled room temperature]. Keep in the original carton.
- Store the constituted oral solution of EVRYSDI upright in the original amber bottle in a refrigerator at 2°C to 8°C (36°F to 46°F). Do not freeze.
Important precautions for preparation of EVRYSDI
- Avoid inhalation and direct contact with skin or mucous membranes with the dry powder and the constituted solution. If such contact occurs, wash thoroughly with soap and water; rinse eyes with water.
- Wear disposable gloves during the preparation and clean up procedure.
Constitution
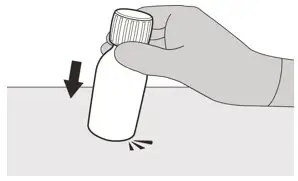 Figure B Figure B | Step 1
Gently tap the bottom of the bottle to loosen the powder (See Figure B). |
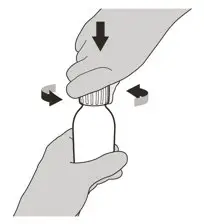 Figure C Figure C | Step 2
Remove the cap by pushing it down and then twisting to the left (counter-clockwise) (See Figure C). Do not throw away the cap. |
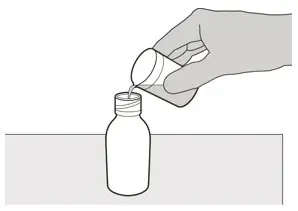 Figure D Figure D | Step 3
Carefully pour 79 mL of Purified Water into the medicine bottle (See Figure D). |
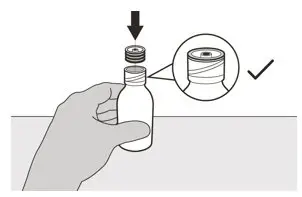 Figure E Figure E | Step 4
Hold the medicine bottle on a table with one hand. Insert the press-in bottle adapter into the opening by pushing it down with the other hand. Ensure it is completely pressed against the bottle lip (See Figure E). |
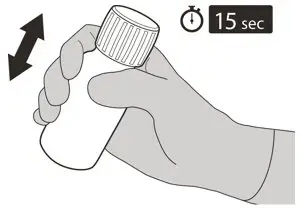 Figure F Figure F | Step 5
Put the cap back on the bottle. Turn the cap to the right (clockwise) to close the bottle. Ensure it is completely closed and then shake well for 15 seconds (See Figure F). Wait for 10 minutes. You should have obtained a clear solution. If not, shake well again for another 15 seconds. |
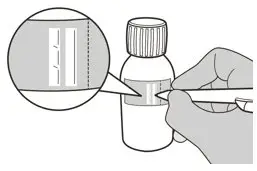 Figure G Figure G | Step 6
Calculate the Discard After date of the oral solution as 64 days after constitution (Note: The day of constitution is counted as day 0. For example, if constitution is on the 1st of April, the Discard After date will be the 4th of June). Write the Discard After date of the solution and the Lot number on the bottle label (See Figure G). Do not dispense the constituted solution if the solution's Discard After date exceeds the original powder expiration date. |
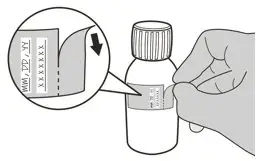 Figure H Figure H | Step 7
Remove and discard the peel-off part of the bottle label with the expiration date of the powder (See Figure H). |
Selecting the oral syringe for the prescribed daily dose of EVRYSDI
For the calculation of dosing volume, the syringe increments need to be considered. Round the dose volume to the closest increment marked on the selected oral syringe.
Select the correct oral syringe(s) (6 mL or 12 mL) for the calculated dosing volume according to the table below and remove the other oral syringes.
| Dose Strength | Syringe Size | Dosing Volume | Syringe Increments |
|---|---|---|---|
| 0.75 mg/mL | 6 mL | 1 mL to 6 mL | 0.1 mL |
| 12 mL | 6.2 mL to 6.6 mL | 0.2 mL |
Put the bottle back in its original carton with the correct oral syringes, Prescribing Information, and Instructions for Use.
Store the constituted oral solution of EVRYSDI upright in the original amber bottle in a refrigerator at 2°C to 8°C (36°F to 46°F). Do not freeze. Discard any unused portion 64 days after constitution.
EVRYSDI is a registered trademark of Genentech, Inc.
Distributed by:
Genentech, Inc.
1 DNA Way
South San Francisco, CA 94080-4990
Revision Date: 4/2021
©2021 Genentech, Inc. All Rights Reserved.
| EVRYSDI
risdiplam powder, for solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Genentech Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F. Hoffmann-La Roche AG | 482242971 | ANALYSIS(50242-175) , API MANUFACTURE(50242-175) , LABEL(50242-175) , MANUFACTURE(50242-175) , PACK(50242-175) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F. Hoffmann-La Roche Ltd. | 485244961 | ANALYSIS(50242-175) , LABEL(50242-175) , PACK(50242-175) | |