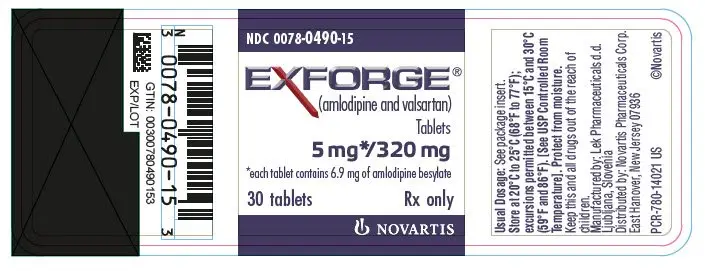
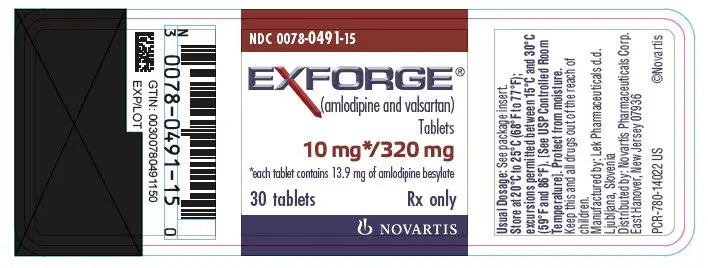
Drug Detail:Exforge (Amlodipine and valsartan [ am-loe-de-peen-val-sar-tan ])
Drug Class: Angiotensin II inhibitors with calcium channel blockers
Highlights of Prescribing Information
EXFORGE® (amlodipine and valsartan) tablets, for oral use
Initial U.S. Approval: 2007
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
- When pregnancy is detected, discontinue Exforge as soon as possible. (5.1)
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1)
Indications and Usage for Exforge
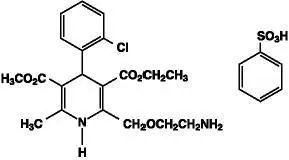
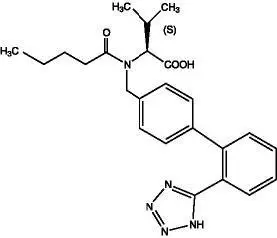
Exforge is the combination tablet of amlodipine, a dihydropyridine calcium channel blocker (DHP CCB), and valsartan, an angiotensin II receptor blocker (ARB). Exforge is indicated for the treatment of hypertension, to lower blood pressure:
- In patients not adequately controlled on monotherapy (1)
- As initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals (1)
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.
Exforge Dosage and Administration
General Considerations:
- Majority of effect attained within 2 weeks (2.1)
- May be administered with other antihypertensive agents (2.1)
Hypertension:
- May be used as add-on therapy for patients not controlled on monotherapy (2.2)
- Patients who experience dose-limiting adverse reactions on monotherapy may be switched to Exforge containing a lower dose of that component (2.2)
- May be substituted for titrated components (2.3)
- When used as initial therapy: Initiate with 5/160 mg, then titrate upwards as necessary to a maximum of 10/320 mg once daily (2.4)
Dosage Forms and Strengths
Tablets (amlodipine/valsartan mg): 5/160, 10/160, 5/320, 10/320 (3)
Contraindications
Known hypersensitivity to any component;
Do not coadminister aliskiren with Exforge in patients with diabetes (4)
Warnings and Precautions
- Hypotension: Correct volume depletion prior to initiation (5.2)
- Increased angina and/or myocardial infarction (5.3)
- Monitor renal function and potassium in susceptible patients (5.4, 5.5)
Adverse Reactions/Side Effects
In placebo-controlled clinical trials, discontinuation due to side effects occurred in 1.8% of patients in the Exforge-treated patients and 2.1% in the placebo-treated group. The most common reasons for discontinuation of therapy with Exforge were peripheral edema and vertigo. The adverse experiences that occurred in clinical trials (≥ 2% of patients) at a higher incidence than placebo included peripheral edema, nasopharyngitis, upper respiratory tract infection, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- If simvastatin is coadministered with amlodipine, do not exceed doses greater than 20 mg daily of simvastatin (7)
- Non-Steroidal Anti-Inflammatory Drug (NSAID) use may lead to increased risk of renal impairment and loss of anti-hypertensive effect (7)
- Dual inhibition of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia (7)
- Lithium: Increases in serum lithium level and lithium toxicity (7)
Use In Specific Populations
Lactation: Breastfeeding is not recommended (8.2)
Geriatric Patients: Not recommended for initial therapy (8.5)
Hepatic Impairment: Not recommended for initial therapy (8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2021
Full Prescribing Information
1. Indications and Usage for Exforge
1.1 Hypertension
Exforge (amlodipine and valsartan) is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including amlodipine and the angiotensin II receptor blocker (ARB) class to which valsartan principally belongs. There are no controlled trials demonstrating risk reduction with Exforge.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Exforge (amlodipine and valsartan) is indicated for the treatment of hypertension.
Exforge may be used in patients whose blood pressure is not adequately controlled on either monotherapy.
Exforge may also be used as initial therapy in patients who are likely to need multiple drugs to achieve their blood pressure goals.
The choice of Exforge as initial therapy for hypertension should be based on an assessment of potential benefits and risks including whether the patient is likely to tolerate the lowest dose of Exforge.
Patients with stage 2 hypertension (moderate or severe) are at a relatively higher risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and should be shaped by considerations such as baseline blood pressure, the target goal and the incremental likelihood of achieving goal with a combination compared to monotherapy. Individual blood pressure goals may vary based upon the patient’s risk.
Data from the high-dose multifactorial study [see Clinical Studies (14)] provide estimates of the probability of reaching a blood pressure goal with Exforge compared to amlodipine or valsartan monotherapy. The figures below provide estimates of the likelihood of achieving systolic or diastolic blood pressure control with Exforge 10/320 mg, based upon baseline systolic or diastolic blood pressure. The curve of each treatment group was estimated by logistic regression modeling. The estimated likelihood at the right tail of each curve is less reliable due to small numbers of subjects with high baseline blood pressures.
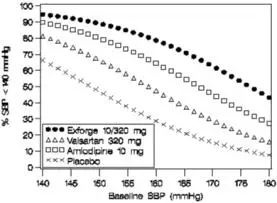 Figure 1: Probability of Achieving Systolic Blood Pressure <140 mmHg at Week 8 | 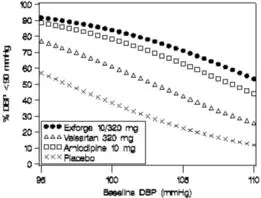 Figure 2: Probability of Achieving Diastolic Blood Pressure <90 mmHg at Week 8 |
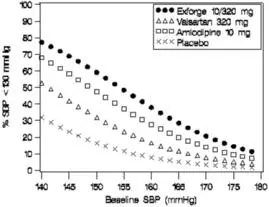 Figure 3: Probability of Achieving Systolic Blood Pressure <130 mmHg at Week 8 | 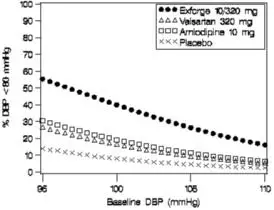 Figure 4: Probability of Achieving Diastolic Blood Pressure <80 mmHg at Week 8 |
For example, a patient with a baseline blood pressure of 160/100 mmHg has about a 67% likelihood of achieving a goal of < 140 mmHg (systolic) and 80% likelihood of achieving < 90 mmHg (diastolic) on amlodipine alone, and the likelihood of achieving these goals on valsartan alone is about 47% (systolic) or 62% (diastolic). The likelihood of achieving these goals on Exforge rises to about 80% (systolic) or 85% (diastolic). The likelihood of achieving these goals on placebo is about 28% (systolic) or 37% (diastolic).
2. Exforge Dosage and Administration
2.1 General Considerations
Dose once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of one 10/320 mg tablet once daily as needed to control blood pressure. The majority of the antihypertensive effect is attained within 2 weeks after initiation of therapy or a change in dose.
Exforge may be administered with other antihypertensive agents.
2.2 Add-on Therapy
A patient whose blood pressure is not adequately controlled with amlodipine (or another dihydropyridine calcium-channel blocker) alone or with valsartan (or another ARB) alone may be switched to combination therapy with Exforge.
A patient who experiences dose-limiting adverse reactions on either component alone may be switched to Exforge containing a lower dose of that component in combination with the other to achieve similar blood pressure reductions. The clinical response to Exforge should be subsequently evaluated and if blood pressure remains uncontrolled after 3 to 4 weeks of therapy, the dose may be titrated up to a maximum of 10/320 mg.
3. Dosage Forms and Strengths
Exforge (amlodipine and valsartan) tablets are available as follows:
5/160 mg tablets, debossed with NVR/ECE (side 1/side 2)
10/160 mg tablets, debossed with NVR/UIC
5/320 mg tablets, debossed with NVR/CSF
10/320 mg tablets, debossed with NVR/LUF
4. Contraindications
Do not use in patients with known hypersensitivity to any component.
Do not coadminister aliskiren with Exforge in patients with diabetes [see Drug Interactions (7)].
5. Warnings and Precautions
5.1 Fetal Toxicity
Exforge can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Exforge as soon as possible [see Use in Specific Populations (8.1)].
5.2 Hypotension
Excessive hypotension was seen in 0.4% of patients with uncomplicated hypertension treated with Exforge in placebo-controlled studies. In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients receiving high doses of diuretics, symptomatic hypotension may occur in patients receiving angiotensin receptor blockers. Volume depletion should be corrected prior to administration of Exforge. Treatment with Exforge should start under close medical supervision.
Initiate therapy cautiously in patients with heart failure or recent myocardial infarction and in patients undergoing surgery or dialysis. Patients with heart failure or post-myocardial infarction patients given valsartan commonly have some reduction in blood pressure, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed. In controlled trials in heart failure patients, the incidence of hypotension in valsartan-treated patients was 5.5% compared to 1.8% in placebo-treated patients. In the Valsartan in Acute Myocardial Infarction Trial (VALIANT), hypotension in post-myocardial infarction patients led to permanent discontinuation of therapy in 1.4% of valsartan-treated patients and 0.8% of captopril-treated patients.
Since the vasodilation induced by amlodipine is gradual in onset, acute hypotension has rarely been reported after oral administration. Nonetheless, caution, as with any other peripheral vasodilator, should be exercised when administering amlodipine, particularly in patients with severe aortic stenosis.
If excessive hypotension occurs with Exforge, place the patient in a supine position and, if necessary, give intravenous normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.3 Risk of Myocardial Infarction or Increased Angina
Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease.
5.4 Impaired Renal Function
Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on Exforge. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on Exforge [see Drug Interactions (7)].
5.5 Hyperkalemia
Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Monitor serum electrolytes periodically.
Some patients with heart failure have developed increases in potassium with valsartan therapy. These effects are usually minor and transient, and they are more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of Exforge may be required [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Studies with Exforge:
Exforge has been evaluated for safety in over 2600 patients with hypertension; over 1440 of these patients were treated for at least 6 months and over 540 of these patients were treated for at least 1 year. Adverse reactions have generally been mild and transient in nature and have only infrequently required discontinuation of therapy.
The hazards [see Warnings and Precautions (5)] of valsartan are generally independent of dose; those of amlodipine are a mixture of dose-dependent phenomena (primarily peripheral edema) and dose-independent phenomena, the former much more common than the latter.
The overall frequency of adverse reactions was neither dose-related nor related to gender, age, or race. In placebo-controlled clinical trials, discontinuation due to side effects occurred in 1.8% of patients in the Exforge-treated patients and 2.1% in the placebo-treated group. The most common reasons for discontinuation of therapy with Exforge were peripheral edema (0.4%), and vertigo (0.2%).
The adverse reactions that occurred in placebo-controlled clinical trials in at least 2% of patients treated with Exforge but at a higher incidence in amlodipine/valsartan patients (n=1437) than placebo (n=337) included peripheral edema (5.4% vs 3.0%), nasopharyngitis (4.3% vs 1.8%), upper respiratory tract infection (2.9% vs 2.1%) and dizziness (2.1% vs 0.9%).
Orthostatic events (orthostatic hypotension and postural dizziness) were seen in less than 1% of patients.
Studies with Valsartan:
Diovan® has been evaluated for safety in more than 4000 hypertensive patients in clinical trials. In trials in which valsartan was compared to an angiotensin-converting enzyme (ACE) inhibitor with or without placebo, the incidence of dry cough was significantly greater in the ACE inhibitor group (7.9%) than in the groups who received valsartan (2.6%) or placebo (1.5%). In a 129-patient trial limited to patients who had had dry cough when they had previously received ACE inhibitors, the incidences of cough in patients who received valsartan, HCTZ, or lisinopril were 20%, 19%, and 69% respectively (p<0.001).
Clinical Lab Test Findings:
Creatinine: In heart failure patients, greater than 50% increases in creatinine were observed in 3.9% of valsartan-treated patients compared to 0.9% of placebo-treated patients. In post-myocardial infarction patients, doubling of serum creatinine was observed in 4.2% of valsartan-treated patients and 3.4% of captopril-treated patients.
Blood Urea Nitrogen (BUN): In hypertensive patients, greater than 50% increases in BUN were observed in 5.5% of Exforge-treated patients compared to 4.7% of placebo-treated patients. In heart failure patients, greater than 50% increases in BUN were observed in 16.6% of valsartan-treated patients compared to 6.3% of placebo-treated patients [see Warnings and Precautions (5.4)].
Neutropenia: Neutropenia was observed in 1.9% of patients treated with Diovan and 0.8% of patients treated with placebo.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported in postmarketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Amlodipine: Gynecomastia has been reported infrequently and a causal relationship is uncertain. Jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine.
Valsartan: The following additional adverse reactions have been reported in postmarketing experience with valsartan:
Hypersensitivity: Angioedema has been reported. Some of these patients previously experienced angioedema with other drugs including ACE inhibitors. Diovan should not be re-administered to patients who have had angioedema.
Digestive: Elevated liver enzymes and reports of hepatitis
Musculoskeletal: Rhabdomyolysis
Renal: Impaired renal function, renal failure
Dermatologic: Alopecia, bullous dermatitis
Blood and Lymphatic: Thrombocytopenia
Vascular: Vasculitis
7. Drug Interactions
No drug interaction studies have been conducted with Exforge and other drugs, although studies have been conducted with the individual amlodipine and valsartan components.
Amlodipine
Impact of Other Drugs on Amlodipine
CYP3A Inhibitors
Coadministration with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is coadministered with CYP3A inhibitors to determine the need for dose adjustment [see Clinical Pharmacology (12.3)].
CYP3A Inducers
No information is available on the quantitative effects of CYP3A inducers on amlodipine. Blood pressure should be closely monitored when amlodipine is coadministered with CYP3A inducers (e.g. rifampicin, St. John's Wort).
Sildenafil
Monitor for hypotension when sildenafil is coadministered with amlodipine [see Clinical Pharmacology (12.2)].
Impact of Amlodipine on Other Drugs
Simvastatin
Coadministration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily [see Clinical Pharmacology (12.3)].
Immunosuppressants
Amlodipine may increase the systemic exposure of cyclosporine or tacrolimus when coadministered. Frequent monitoring of trough blood levels of cyclosporine and tacrolimus is recommended and adjust the dose when appropriate [see Clinical Pharmacology (12.3)].
Valsartan
Agents Increasing Serum Potassium: Concomitant use of valsartan with other agents that block the renin-angiotensin system, potassium-sparing diuretics (e.g., spironolactone, triamterene, amiloride), potassium supplements, salt substitutes containing potassium or other drugs that may increase potassium levels (e.g., heparin) may lead to increases in serum potassium and in heart failure patients to increases in serum creatinine. If co-medication is considered necessary, monitoring of serum potassium is advisable.
Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists, including valsartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving valsartan and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including valsartan, may be attenuated by NSAIDs including selective COX-2 inhibitors.
Dual Blockade of the Renin-Angiotensin System (RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on valsartan and other agents that affect the RAS.
Do not coadminister aliskiren with Exforge in patients with diabetes. Avoid use of aliskiren with Exforge in patients with renal impairment (GFR <60 mL/min).
Lithium: Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists. Monitor serum lithium levels during concomitant use.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Exforge can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Published reports include cases of anhydramnios and oligohydramnios in pregnant women treated with valsartan (see Clinical Considerations).
When pregnancy is detected, discontinue EXFORGE as soon as possible.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension and death.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. If oligohydramnios is observed, consider alternative drug treatment. Closely observe neonates with histories of in utero exposure to Exforge for hypotension, oliguria, and hyperkalemia. In neonates with a history of in utero exposure to Exforge, if oliguria or hypotension occurs, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and replacing renal function.
Data
Animal Data
In rats, administered 20 mg/kg/day amlodipine plus 320 mg/kg/day valsartan, treatment-related maternal and fetal effects (developmental delays and alterations noted in the presence of significant maternal toxicity) were noted with the high dose combination. This corresponds to dose multiples of 9 and 19.5 times, respectively, the maximum recommended human dose (MRHD) of 10 mg/day for amlodipine and 320 mg/day for valsartan (based on body surface area and considering a 60 kg patient).
8.2 Lactation
Risk Summary
There is limited information regarding the presence of Exforge in human milk, the effects on the breastfed infant, or the effects on milk production. Valsartan is present in rat milk. Limited published studies report that amlodipine is present in human milk. Because of the potential for serious adverse reactions in breastfed infants, advise a nursing woman that breastfeeding is not recommended during treatment with Exforge.
Data
Valsartan was detected in the milk of lactating rats 15 minutes after oral administration of a 3 mg/kg dose.
8.4 Pediatric Use
Safety and effectiveness of Exforge in pediatric patients have not been established.
8.5 Geriatric Use
In controlled clinical trials, 323 (22.5%) hypertensive patients treated with Exforge were ≥ 65 years and 79 (5.5%) were ≥ 75 years. No overall differences in the efficacy or safety of Exforge was observed in this patient population, but greater sensitivity of some older individuals cannot be ruled out.
Amlodipine: The recommended starting dose of amlodipine 2.5 mg is not an available strength with Exforge.
Clinical studies of amlodipine besylate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. Elderly patients have decreased clearance of amlodipine with a resulting increase of area under the curve (AUC) of approximately 40% to 60%.
Valsartan: In the controlled clinical trials of valsartan, 1214 (36.2%) of hypertensive patients treated with valsartan were ≥ 65 years and 265 (7.9%) were ≥ 75 years. No overall difference in the efficacy or safety of valsartan was observed in this patient population, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Safety and effectiveness of Exforge in patients with severe renal impairment (CrCl < 30 mL/min) have not been established. No dose adjustment is required in patients with mild (CrCl 60 to 90 mL/min) or moderate (CrCl 30 to 60 mL/min) renal impairment.
8.7 Hepatic Impairment
Amlodipine
Exposure to amlodipine is increased in patients with hepatic insufficiency [see Clinical Pharmacology (12.3)]. The recommended initial dose of amlodipine in patients with hepatic impairment is 2.5 mg, which is not an available strength with Exforge.
Valsartan
No dose adjustment is necessary for patients with mild-to-moderate disease. No dosing recommendations can be provided for patients with severe liver disease.
| EXFORGE
amlodipine besylate and valsartan tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| EXFORGE
amlodipine besylate and valsartan tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| EXFORGE
amlodipine besylate and valsartan tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| EXFORGE
amlodipine besylate and valsartan tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |