Drug Detail:Exjade (Deferasirox [ de-fer-a-sir-ox ])
Drug Class: Chelating agents
Highlights of Prescribing Information
EXJADE® (deferasirox) tablets, for oral suspension
Initial U.S. Approval: 2005
WARNING: RENAL FAILURE, HEPATIC FAILURE, and GASTROINTESTINAL HEMORRHAGE
See full prescribing information for complete boxed warning.
Exjade may cause:
-
acute kidney injury, including acute renal failure requiring dialysis and renal tubular toxicity including Fanconi syndrome (5.1)
-
hepatic toxicity, including failure (5.2)
- gastrointestinal hemorrhage (5.3)
Exjade therapy requires close patient monitoring, including laboratory tests of renal and hepatic function. (5)
Recent Major Changes
|
Indications and Usage, Limitations of Use (1.3) |
7/2019 |
Indications and Usage for Exjade
Exjade is an iron chelator indicated for the treatment of chronic iron overload due to blood transfusions in patients 2 years of age and older. (1.1)
Exjade is indicated for the treatment of chronic iron overload in patients 10 years of age and older with non-transfusion-dependent thalassemia (NTDT) syndromes, and with a liver iron (Fe) concentration (LIC) of at least 5 mg Fe per gram of dry weight and a serum ferritin greater than 300 mcg/L. (1.2)
Limitations of Use:
The safety and efficacy of Exjade when administered with other iron chelation therapy have not been established. (1.3)
Exjade Dosage and Administration
- Transfusional Iron Overload: Initial dose for patients with estimated glomerular filtration rate (eGFR) greater than 60 mL/min/1.73 m2 is 20 mg per kg body weight once daily, as oral suspension. Calculate dose to the nearest whole tablet. (2.1)
- NTDT Syndromes: Initial dose for patients with eGFR greater than 60 mL/min/1.73 m2 is 10 mg per kg body weight once daily, as oral suspension. Calculate dose to the nearest whole tablet. (2.2)
Dosage Forms and Strengths
Tablets for oral suspension: 125 mg, 250 mg, 500 mg. (3)
Contraindications
- Estimated GFR less than 40 mL/min/1.73 m2. (4)
- Patients with poor performance status. (4)
- Patients with high-risk myelodysplastic syndrome (MDS). (4)
- Patients with advanced malignancies. (4)
- Patients with platelet counts less than 50 x 109/L. (4)
- Known hypersensitivity to deferasirox or any component of Exjade. (4)
Warnings and Precautions
- Acute Kidney Injury: Measure serum creatinine in duplicate before starting therapy. Monitor renal function during Exjade therapy and reduce dose or interrupt therapy for toxicity. (2.1, 2.4, 5.1)
- Hepatic Toxicity: Monitor hepatic function. Reduce dose or interrupt therapy for toxicity. (5.2)
- Fatal and Nonfatal Gastrointestinal Bleeding, Ulceration, and Irritation: Risk may be greater in patients who are taking Exjade in combination with drugs that have known ulcerogenic or hemorrhagic potential. (5.3)
- Bone Marrow Suppression: Neutropenia, agranulocytosis, worsening anemia, and thrombocytopenia, including fatal events; monitor blood counts during Exjade therapy. Interrupt therapy for toxicity. (5.4)
- Age-related Risk of Toxicity: Monitor elderly and pediatric patients closely for toxicity. (5.5)
- Hypersensitivity Reactions: Discontinue Exjade for severe reactions and institute medical intervention. (5.7)
- Severe Skin Reactions, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Discontinue Exjade. (5.8)
Adverse Reactions/Side Effects
In patients with transfusional iron overload, the most frequently occurring (greater than 5%) adverse reactions are diarrhea, vomiting, nausea, abdominal pain, skin rashes, and increases in serum creatinine. In Exjade-treated patients with NTDT syndromes, the most frequently occurring (greater than 5%) adverse reactions are diarrhea, rash, and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Do not take Exjade with aluminum-containing antacid preparations. (7.1)
- Exjade increases the exposure of the CYP2C8 substrate repaglinide. Consider repaglinide dose reduction and monitor blood glucose levels. (7.3)
- Avoid the use of Exjade with CYP1A2 substrate theophylline. (7.4)
- Deferasirox increases exposure of busulfan. Monitor plasma concentrations of busulfan when coadministered with deferasirox to allow dose adjustment of busulfan as needed. (7.7)
Use In Specific Populations
Lactation: Advise women not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2020
Related/similar drugs
deferasirox, Jadenu, deferiprone, Ferriprox, Jadenu SprinkleFull Prescribing Information
1. Indications and Usage for Exjade
1.1 Treatment of Chronic Iron Overload Due to Blood Transfusions (Transfusional Iron Overload)
Exjade is indicated for the treatment of chronic iron overload due to blood transfusions (transfusional hemosiderosis) in patients 2 years of age and older.
1.2 Treatment of Chronic Iron Overload in Non-Transfusion-Dependent Thalassemia Syndromes
Exjade is indicated for the treatment of chronic iron overload in patients 10 years of age and older with non-transfusion-dependent thalassemia (NTDT) syndromes and with a liver iron concentration (LIC) of at least 5 milligrams of iron per gram of liver dry weight (mg Fe/g dw) and a serum ferritin greater than 300 mcg/L.
2. Exjade Dosage and Administration
2.1 Transfusional Iron Overload
Exjade therapy should only be considered when a patient has evidence of chronic transfusional iron overload. The evidence should include the transfusion of at least 100 mL/kg of packed red blood cells (e.g., at least 20 units of packed red blood cells for a 40 kg person or more in individuals weighing more than 40 kg), and a serum ferritin consistently greater than 1,000 mcg/L.
Prior to starting therapy or increasing dose, evaluate:
- Serum ferritin level
- Baseline renal function:
- Obtain serum creatinine in duplicate (due to variations in measurements) to establish accurate baseline
- Calculate the estimated glomerular filtration rate (eGFR). Use a prediction equation appropriate for adult patients (e.g., CKD-EPI, MDRD method) and in pediatric patients (e.g., Schwartz equations).
- Obtain urinalyses and serum electrolytes to evaluate renal tubular function [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
- Serum transaminases and bilirubin [see Dosage and Administration (2.4), Warnings and Precautions (5.2)]
- Baseline auditory and ophthalmic examinations [see Warnings and Precautions (5.10)]
Initiating Therapy:
The recommended initial dose of Exjade for patients 2 years of age and older with eGFR greater than 60 mL/min/1.73 m2 is 20 mg per kg body weight orally, once daily. Calculate doses (mg per kg per day) to the nearest whole tablet.
During Therapy:
- Monitor serum ferritin monthly and adjust the dose of Exjade, if necessary, every 3-6 months based on serum ferritin trends.
- Use the minimum effective dose to achieve a trend of decreasing ferritin.
- Make dose adjustments in steps of 5 or 10 mg per kg and tailor adjustments to the individual patient’s response and therapeutic goals.
- In patients not adequately controlled with doses of 30 mg per kg (e.g., serum ferritin levels persistently above 2,500 mcg/L and not showing a decreasing trend over time), doses of up to 40 mg per kg may be considered. Doses above 40 mg per kg are not recommended [see Warnings and Precautions (5.6)].
- Adjust dose based on serum ferritin levels
- If the serum ferritin falls below 1,000 mcg/L at 2 consecutive visits, consider dose reduction, especially if the dose is greater than 25 mg/kg/day [see Adverse Reactions (6.1)].
- If the serum ferritin falls below 500 mcg/L, interrupt Exjade to minimize the risk of overchelation, and continue monthly monitoring [see Warnings and Precautions (5.6)].
- Evaluate the need for ongoing chelation therapy for patients whose conditions no longer require regular blood transfusions.
- Use the minimum effective dose to maintain iron burden in the target range [see Warnings and Precautions (5.6)].
- Monitor blood counts, liver function, renal function and ferritin monthly [see Warnings and Precautions (5.1, 5.2, 5.4)].
- Interrupt Exjade for pediatric patients who have acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake, and monitor more frequently. Resume therapy as appropriate, based on assessments of renal function, when oral intake and volume status are normal [see Dosage and Administration (2.4, 2.5), Warnings and Precautions (5.1), Use in Specific Populations (8.4), Clinical Pharmacology (12.3)].
2.2 Iron Overload in Non-Transfusion-Dependent Thalassemia Syndromes
Exjade therapy should only be considered when a patient with NTDT syndrome has an LIC of at least 5 mg Fe/g dw and a serum ferritin greater than 300 mcg/L.
Prior to starting therapy, obtain:
- LIC by liver biopsy or by an FDA-cleared or approved method for identifying patients for treatment with deferasirox therapy
- Serum ferritin level on at least 2 measurements 1-month apart [see Clinical Studies (14)]
- Baseline renal function:
- Obtain serum creatinine in duplicate (due to variations in measurements) to establish accurate baseline
- Calculate eGFR. Use a prediction equation appropriate for adult patients (e.g., CKD-EPI, MDRD method) and in pediatric patients (e.g., Schwartz equations).
- Obtain urinalyses and serum electrolytes to evaluate renal tubular function [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
- Serum transaminases and bilirubin [see Dosage and Administration (2.4), Warnings and Precautions (5.2)]
- Baseline auditory and ophthalmic examinations [see Warnings and Precautions (5.10)]
Initiating Therapy:
- The recommended initial dose of Exjade for patients with eGFR greater than 60 mL/min/1.73 m2 is 10 mg per kg body weight orally once daily. Calculate doses (mg per kg per day) to the nearest whole tablet.
- If the baseline LIC is greater than 15 mg Fe/g dw, consider increasing the dose to 20 mg/kg/day after 4 weeks.
During Therapy:
- Monitor serum ferritin monthly to assess the patient’s response to therapy and to minimize the risk of overchelation [see Warnings and Precautions (5.6)]. Interrupt treatment when serum ferritin is less than 300 mcg/L and obtain an LIC to determine whether the LIC has fallen to less than 3 mg Fe/g dw.
- Use the minimum effective dose to achieve a trend of decreasing ferritin.
- Monitor LIC every 6 months.
- After 6 months of therapy, if the LIC remains greater than 7 mg Fe/g dw, increase the dose of deferasirox to a maximum of 20 mg/kg/day. Do not exceed a maximum of 20 mg/kg/day.
- If after 6 months of therapy, the LIC is 3-7 mg Fe/g dw, continue treatment with deferasirox at no more than 10 mg/kg/day.
- When the LIC is less than 3 mg Fe/g dw, interrupt treatment with deferasirox and continue to monitor the LIC.
- Monitor blood counts, liver function, renal function and ferritin monthly [see Warnings and Precautions (5.1, 5.2, 5.4)].
- Increase monitoring frequency for pediatric patients who have acute illness, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake. Consider dose interruption until oral intake and volume status are normal [see Dosage and Administration (2.4, 2.5), Warnings and Precautions (5.1), Use in Specific Populations (8.4), Clinical Pharmacology (12.3)].
Restart treatment when the LIC rises again to more than 5 mg Fe/g dw.
2.3 Administration
Do not chew tablets or swallow them whole.
Take Exjade once daily on an empty stomach at least 30 minutes before food, preferably at the same time each day. Completely disperse tablets by stirring in water, orange juice, or apple juice until a fine suspension is obtained. Disperse doses of less than 1 g in 3.5 ounces of liquid and doses of 1 g or greater in 7 ounces of liquid. After swallowing the suspension, resuspend any residue in a small volume of liquid and swallow. Do not take Exjade with aluminum-containing antacid products [see Drug Interactions (7.1)].
2.4 Use in Patients With Baseline Hepatic or Renal Impairment
Patients with Baseline Hepatic Impairment
Mild (Child-Pugh A) Hepatic Impairment: No dose adjustment is necessary.
Moderate (Child-Pugh B) Hepatic Impairment: Reduce the starting dose by 50%.
Severe (Child-Pugh C) Hepatic Impairment: Avoid Exjade [see Warnings and Precautions (5.2), Use in Specific Populations (8.7)].
Patients with Baseline Renal Impairment
Do not use Exjade in adult or pediatric patients with eGFR less than 40 mL/min/1.73 m2 [see Dosage and Administration (2.5), Contraindications (4)].
For patients with renal impairment (eGFR 40-60 mL/min/1.73 m2), reduce the starting dose by 50% [see Use in Specific Populations (8.6)].
Exercise caution in pediatric patients with eGFR between 40 and 60 mL/min/1.73 m2. If treatment is needed, use the minimum effective dose and monitor renal function frequently. Individualize dose titration based on improvement in renal injury [see Use in Specific Populations (8.6)].
2.5 Dose Modifications for Decreases in Renal Function While on Exjade
Exjade is contraindicated in patients with eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)].
For decreases in renal function while receiving Exjade [see Warnings and Precautions (5.1)], modify the dose as follows:
Transfusional Iron Overload
Adults:
- If the serum creatinine increases by 33% or more above the average baseline measurement, repeat the serum creatinine within 1 week, and if still elevated by 33% or more, reduce the dose by 10 mg per kg.
Pediatric Patients (ages 2 years–17 years):
- Reduce the dose by 10 mg/kg/day if eGFR decreases by greater than 33% below the average baseline measurement and repeat the eGFR within 1 week.
- Interrupt Exjade for acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake, and monitor more frequently. Resume therapy as appropriate, based on assessments of renal function, when oral intake and volume status are normal. Avoid use of other nephrotoxic drugs [see Warnings and Precautions (5.1)].
- In the setting of decreased renal function, evaluate the risk benefit profile of continued Exjade use. Use the minimum effective Exjade dose and monitor renal function more frequently, by evaluating tubular and glomerular function. Titrate dosing based on renal injury. Consider dose reduction or interruption and less nephrotoxic therapies until improvement of renal function. If signs of renal tubular or glomerular injury occur in the presence of other risk factors such as volume depletion, reduce or interrupt Exjade to prevent severe and irreversible renal injury [see Warnings and Precautions (5.1)].
All Patients (regardless of age):
- Discontinue therapy for eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)].
Non-Transfusion-Dependent Thalassemia Syndromes
Adults:
- If the serum creatinine increases by 33% or more above the average baseline measurement, repeat the serum creatinine within 1 week, and if still elevated by 33% or more, interrupt therapy if the dose is 5 mg per kg, or reduce by 50% if the dose is 10 or 20 mg per kg.
Pediatric Patients (ages 10 years–17 years):
- Reduce the dose by 5 mg/kg/day if eGFR decreases by greater than 33% below the average baseline measurement and repeat the eGFR within 1 week.
- Increase monitoring frequency for pediatric patients who have acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake. Consider dose interruption until oral intake and volume status are normal. Avoid use of other nephrotoxic drugs [see Warnings and Precautions (5.1)].
- In the setting of decreased renal function, evaluate the risk benefit profile of continued Exjade use. Use the minimum effective Exjade dose and monitor renal function more frequently, by evaluating tubular and glomerular function. Titrate dosing based on renal injury. Consider dose reduction or interruption and less nephrotoxic therapies until improvement of renal function. If signs of renal tubular or glomerular injury occur in the presence of other risk factors such as volume depletion, reduce or interrupt Exjade to prevent severe and irreversible renal injury [see Warnings and Precautions (5.1)].
All Patients (regardless of age):
- Discontinue therapy for eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)].
2.6 Dose Modifications Based on Concomitant Medications
UDP-glucuronosyltransferases (UGT) Inducers
Concomitant use of UGT inducers decreases Exjade systemic exposure. Avoid the concomitant use of potent UGT inducers (e.g., rifampicin, phenytoin, phenobarbital, ritonavir) with Exjade. If you must administer Exjade with 1 of these agents, consider increasing the initial dose of Exjade by 50%, and monitor serum ferritin levels and clinical responses for further dose modification [see Dosage and Administration (2.1, 2.2), Drug Interactions (7.5)].
Bile Acid Sequestrants
Concomitant use of bile acid sequestrants decreases Exjade systemic exposure. Avoid the concomitant use of bile acid sequestrants (e.g., cholestyramine, colesevelam, colestipol) with Exjade. If you must administer Exjade with 1 of these agents, consider increasing the initial dose of Exjade by 50%, and monitor serum ferritin levels and clinical responses for further dose modification [see Dosage and Administration (2.1, 2.2), Drug Interactions (7.6)].
3. Dosage Forms and Strengths
- 125 mg tablets
Off-white, round, flat tablet with beveled edge and imprinted with “J” and “125” on one side and “NVR” on the other. - 250 mg tablets
Off-white, round, flat tablet with beveled edge and imprinted with “J” and “250” on one side and “NVR” on the other. - 500 mg tablets
Off-white, round, flat tablet with beveled edge and imprinted with “J” and “500” on one side and “NVR” on the other.
4. Contraindications
Exjade is contraindicated in patients with:
- Estimated GFR less than 40 mL/min/1.73 m2 [see Dosage and Administration (2.5), Warnings and Precautions (5.1)];
- Poor performance status; [see Warnings and Precautions (5.1, 5.3)]
- High-risk myelodysplastic syndromes; (this patient population was not studied and is not expected to benefit from chelation therapy)
- Advanced malignancies. [see Warnings and Precautions (5.1, 5.3)]
- Platelet counts less than 50 x 109/L [see Warnings and Precautions (5.3, 5.4)
- Known hypersensitivity to deferasirox or any component of Exjade [see Warnings and Precautions (5.7), Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Acute Kidney Injury, Including Acute Renal Failure Requiring Dialysis, and Renal Tubular Toxicity Including Fanconi Syndrome
Exjade is contraindicated in patients with eGFR less than 40 mL/min/1.73 m2. Exercise caution in pediatric patients with eGFR between 40 and 60 mL/minute/1.73 m2. If treatment is needed, use the minimum effective dose and monitor renal function frequently. Individualize dose titration based on improvement in renal injury [see Use in Specific Populations (8.6)]. For patients with renal impairment (eGFR 40–60 mL/min/1.73 m2), reduce the starting dose by 50% [see Dosage and Administration (2.4, 2.5), Use in Specific Populations (8.6)].
Exjade can cause acute kidney injury including renal failure requiring dialysis that has resulted in fatal outcomes. Based on postmarketing experience, most fatalities have occurred in patients with multiple comorbidities and who were in advanced stages of their hematological disorders. In the clinical trials, adult and pediatric Exjade-treated patients with no preexisting renal disease experienced dose-dependent mild, non-progressive increases in serum creatinine and proteinuria. Preexisting renal disease and concomitant use of other nephrotoxic drugs may increase the risk of acute kidney injury in adult and pediatric patients. Acute illnesses associated with volume depletion and overchelation may increase the risk of acute kidney injury in pediatric patients. In pediatric patients, small decreases in eGFR can result in increases in Exjade exposure, particularly in younger patients with body surface area typical of patients less than age 7 years. This can lead to a cycle of worsening renal function and further increases in Exjade exposure, unless the dose is reduced or interrupted. Renal tubular toxicity, including acquired Fanconi syndrome, has been reported in patients treated with Exjade, most commonly in pediatric patients with beta-thalassemia and serum ferritin levels less than 1,500 mcg/L [see Warnings and Precautions (5.6), Adverse Reactions (6.1, 6.2), Use in Specific Populations (8.4), Clinical Pharmacology (12.3)].
Evaluate renal glomerular and tubular function before initiating therapy or increasing the dose. Use prediction equations validated for use in adult and pediatric patients to estimate GFR. Obtain serum electrolytes and urinalysis in all patients to evaluate renal tubular function [see Dosage and Administration (2.1, 2.2)].
Monitor all patients for changes in eGFR and for renal tubular toxicity weekly during the first month after initiation or modification of therapy and at least monthly thereafter. Dose reduction or interruption may be considered if abnormalities occur in levels of markers of renal tubular function and/or as clinically indicated. Monitor serum ferritin monthly to evaluate for overchelation. Use the minimum dose to establish and maintain a low iron burden. Monitor renal function more frequently in patients with preexisting renal disease or decreased renal function. In pediatric patients, interrupt Exjade during acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake, and monitor renal function more frequently. Promptly correct fluid deficits to prevent renal injury. Resume therapy as appropriate, based on assessments of renal function, when oral intake and volume status are normal [see Dosage and Administration (2.5), Warnings and Precautions (5.6), Adverse Reactions (6.1, 6.2), Use in Specific Populations (8.4)].
5.2 Hepatic Toxicity and Failure
Exjade can cause hepatic injury, fatal in some patients. In Study 1, 4 patients (1.3%) discontinued Exjade because of hepatic toxicity (drug-induced hepatitis in 2 patients and increased serum transaminases in 2 additional patients). Hepatic toxicity appears to be more common in patients greater than 55 years of age. Hepatic failure was more common in patients with significant comorbidities, including liver cirrhosis and multi-organ failure [see Adverse Reactions (6.1)]. Acute liver injury and failure, including fatal outcomes, have occurred in pediatric Exjade-treated patients. Liver failure occurred in association with acute kidney injury in pediatric patients at risk for overchelation during a volume depleting event. Interrupt Exjade therapy when acute liver injury or acute kidney injury is suspected and during volume depletion. Monitor liver and renal function more frequently in pediatric patients who are receiving Exjade in the 20-40 mg/kg/day range and when iron burden is approaching normal. Use the minimum effective dose to achieve and maintain a low iron burden [see Dosage and Administration (2.5), Warnings and Precautions (5.6), Adverse Reactions (6.1)].
Measure transaminases [aspartate transaminase (AST) and alanine transaminase (ALT)] and bilirubin in all patients before the initiation of treatment, and every 2 weeks during the first month and at least monthly thereafter. Consider dose modifications or interruption of treatment for severe or persistent elevations.
Avoid the use of Exjade in patients with severe (Child-Pugh C) hepatic impairment. Reduce the starting dose in patients with moderate (Child-Pugh B) hepatic impairment [see Dosage and Administration (2.4), Use in Specific Populations (8.7)]. Patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment may be at higher risk for hepatic toxicity.
5.3 Gastrointestinal (GI) Ulceration, Hemorrhage, and Perforation
GI hemorrhage, including deaths, has been reported in Exjade-treated patients, especially in elderly patients who had advanced hematologic malignancies and/or low platelet counts. Nonfatal upper GI irritation, ulceration and hemorrhage have been reported in patients, including children and adolescents, receiving Exjade [see Adverse Reactions (6.1)]. Monitor for signs and symptoms of GI ulceration and hemorrhage during Exjade therapy and promptly initiate additional evaluation and treatment if a serious GI adverse reaction is suspected. The risk of GI hemorrhage may be increased when administering Exjade in combination with drugs that have ulcerogenic or hemorrhagic potential, such as nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, oral bisphosphonates, or anticoagulants. There have been reports of ulcers complicated with GI perforation (including fatal outcome) [see Adverse Reactions (6.2)].
5.4 Bone Marrow Suppression
Neutropenia, agranulocytosis, worsening anemia, and thrombocytopenia, including fatal events, have been reported in patients treated with Exjade. Preexisting hematologic disorders may increase this risk. Monitor blood counts in all patients. Interrupt treatment with Exjade in patients who develop cytopenias until the cause of the cytopenia has been determined. Exjade is contraindicated in patients with platelet counts below 50 x 109/L.
5.5 Age-Related Risk of Toxicity
Elderly Patients
Exjade has been associated with serious and fatal adverse reactions in the postmarketing setting among adults, predominantly in elderly patients. Monitor elderly patients treated with Exjade more frequently for toxicity [see Use in Specific Populations (8.5)].
Pediatric Patients
Exjade has been associated with serious and fatal adverse reactions in pediatric patients in the postmarketing setting. These events were frequently associated with volume depletion or with continued Exjade doses in the 20-40 mg/kg/day range when body iron burden was approaching or in the normal range. Interrupt Exjade in patients with volume depletion, and resume Exjade when renal function and fluid volume have normalized. Monitor liver and renal function more frequently during volume depletion and in patients receiving Exjade in the 20-40 mg/kg/day range when iron burden is approaching the normal range. Use the minimum effective dose to achieve and maintain a low iron burden [see Dosage and Administration (2.4), Warnings and Precautions (5.6), Use in Specific Populations (8.4)].
5.6 Overchelation
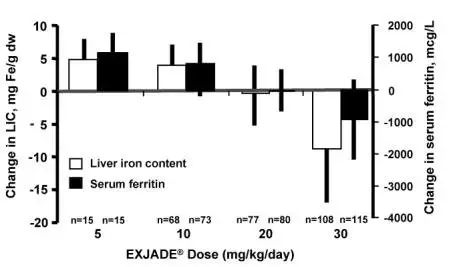
For patients with transfusional iron overload, measure serum ferritin monthly to assess the patient’s response to therapy and minimize the risk of overchelation. An analysis of pediatric patients treated with Exjade in pooled clinical trials (n = 158) found a higher rate of renal adverse reactions among patients receiving doses greater than 25 mg/kg/day while their serum ferritin values were less than 1,000 mcg/L. Consider dose reduction or closer monitoring of renal and hepatic function, and serum ferritin levels during these periods. Use the minimum effective dose to maintain a low-iron burden [see Adverse Reactions (6.1), Use in Specific Populations (8.4)].
If the serum ferritin falls below 1,000 mcg/L at 2 consecutive visits, consider dose reduction, especially if the dose is greater than 25 mg/kg/day [see Adverse Reactions (6.1)]. If the serum ferritin falls below 500 mcg/L, interrupt therapy with Exjade and continue monthly monitoring. Evaluate the need for ongoing chelation for patients whose conditions do not require regular blood transfusions. Use the minimum effective dose to maintain iron burden in the target range. Continued administration of Exjade in the 20-40 mg/kg/day range when the body iron burden is approaching or within the normal range has resulted in life-threatening adverse reactions [see Dosage and Administration (2.1)].
For patients with NTDT, measure LIC by liver biopsy or by using an FDA-cleared or approved method for monitoring patients receiving deferasirox therapy every 6 months on treatment. Interrupt Exjade administration when the LIC is less than 3 mg Fe/g dw. Measure serum ferritin monthly, and if the serum ferritin falls below 300 mcg/L, interrupt Exjade and obtain a confirmatory LIC [see Clinical Studies (14)].
5.7 Hypersensitivity
Exjade may cause serious hypersensitivity reactions (such as anaphylaxis and angioedema), with the onset of the reaction usually occurring within the first month of treatment [see Adverse Reactions (6.2)]. If reactions are severe, discontinue Exjade and institute appropriate medical intervention. Exjade is contraindicated in patients with known hypersensitivity to deferasirox products and should not be reintroduced in patients who have experienced previous hypersensitivity reactions on deferasirox products due to the risk of anaphylactic shock.
5.8 Severe Skin Reactions
Severe cutaneous adverse reactions (SCARs) including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS), which could be life-threatening or fatal have been reported during Exjade therapy [see Adverse Reactions (6.1, 6.2)]. Cases of erythema multiforme have been observed. Advise patients of the signs and symptoms of severe skin reactions, and closely monitor. If any severe skin reactions are suspected, discontinue Exjade immediately and do not reintroduce Exjade therapy.
5.9 Skin Rash
Rashes may occur during Exjade treatment [see Adverse Reactions (6.1)]. For rashes of mild to moderate severity, Exjade may be continued without dose adjustment, since the rash often resolves spontaneously. In severe cases, interrupt treatment with Exjade. Reintroduction at a lower dose with escalation may be considered after resolution of the rash.
5.10 Auditory and Ocular Abnormalities
Auditory disturbances (high frequency hearing loss, decreased hearing), and ocular disturbances (lens opacities, cataracts, elevations in intraocular pressure, and retinal disorders) were reported at a frequency of less than 1% with Exjade therapy in the clinical studies. The frequency of auditory adverse reactions was increased among pediatric patients who received Exjade doses greater than 25 mg/kg/day when serum ferritin was less than 1,000 mcg/L [see Warnings and Precautions (5.6)].
Perform auditory and ophthalmic testing (including slit-lamp examinations and dilated fundoscopy) before starting Exjade treatment and thereafter at regular intervals (every 12 months). If disturbances are noted, monitor more frequently. Consider dose reduction or interruption.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
- Acute Kidney Injury, Including Acute Renal Failure Requiring Dialysis, and Renal Tubular Toxicity Including Fanconi Syndrome [see Warnings and Precautions (5.1, 5.6)]
- Hepatic Toxicity and Failure [see Warnings and Precautions (5.2, 5.6)]
- GI Hemorrhage [see Warnings and Precautions (5.3)]
- Bone Marrow Suppression [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Warnings and Precautions (5.7)]
- Severe Skin Reactions [see Warnings and Precautions (5.8)]
- Skin Rash [see Warnings and Precautions (5.9)]
- Auditory and Ocular Abnormalities [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Transfusional Iron Overload
A total of 700 adult and pediatric patients were treated with Exjade (deferasirox) for 48 weeks in premarketing studies. These included 469 patients with beta-thalassemia, 99 with rare anemias, and 132 with sickle cell disease. Of these patients, 45% were male, 70% were Caucasian, and 292 patients were less than 16 years of age. In the sickle cell disease population, 89% of patients were black. Median treatment duration among the sickle cell patients was 51 weeks. Of the 700 patients treated, 469 (403 beta-thalassemia and 66 rare anemias) were entered into extensions of the original clinical protocols. In ongoing extension studies, median durations of treatment were 88-205 weeks.
Six hundred twenty-seven (627) patients with myelodysplastic syndrome (MDS) were enrolled across 5 uncontrolled trials. These studies varied in duration from 1 to 5 years. The discontinuation rate across studies in the first year was 46% (adverse events 20%, withdrawal of consent 10%, death 8%, other 4%, lab abnormalities 3%, and lack of efficacy 1%). Among 47 patients enrolled in the study of 5-year duration, 10 remained on Exjade at the completion of the study.
Table 1 displays adverse reactions occurring in greater than 5% of Exjade-treated beta-thalassemia patients (Study 1), sickle cell disease patients (Study 3), and patients with MDS (MDS pool). Abdominal pain, nausea, vomiting, diarrhea, skin rashes, and increases in serum creatinine were the most frequent adverse reactions reported with a suspected relationship to Exjade. Gastrointestinal symptoms, increases in serum creatinine, and skin rash were dose related.
| Abbreviation: MDS, myelodysplastic syndrome. aAdverse reaction frequencies are based on adverse events reported regardless of relationship to study drug. bIncludes ‘abdominal pain’, ‘abdominal pain lower’, and ‘abdominal pain upper’. cIncludes ‘blood creatinine increased’ and ‘blood creatinine abnormal’. See also Table 2. |
|||||
| Study 1 (Beta-thalassemia) | Study 3 (Sickle Cell Disease) | MDS Pool | |||
| Adverse Reactions | Exjade
N = 296 n (%) | Deferoxamine
N = 290 n (%) | Exjade
N = 132 n (%) | Deferoxamine
N = 63 n (%) | Exjade
N = 627 n (%) |
| Abdominal Painb | 63 (21) | 41 (14) | 37 (28) | 9 (14) | 145 (23) |
| Diarrhea | 35 (12) | 21 (7) | 26 (20) | 3 (5) | 297 (47) |
| Creatinine Increasedc | 33 (11) | 0 (0) | 9 (7) | 0 | 89 (14) |
| Nausea | 31 (11) | 14 (5) | 30 (23) | 7 (11) | 161 (26) |
| Vomiting | 30 (10) | 28 (10) | 28 (21) | 10 (16) | 83 (13) |
| Rash | 25 (8) | 9 (3) | 14 (11) | 3 (5) | 83 (13) |
In Study 1, a total of 113 (38%) patients treated with Exjade had increases in serum creatinine greater than 33% above baseline on 2 separate occasions (Table 2) and 25 (8%) patients required dose reductions. Increases in serum creatinine appeared to be dose related [see Warnings and Precautions (5.1)]. In this study, 17 (6%) patients treated with Exjade developed elevations in serum glutamic-pyruvic transaminase (SGPT)/ALT levels greater than 5 times the upper limit of normal (ULN) at 2 consecutive visits. Of these, 2 patients had liver biopsy proven drug-induced hepatitis and both discontinued Exjade therapy [see Warnings and Precautions (5.2)]. An additional 2 patients, who did not have elevations in SGPT/ALT greater than 5 times the ULN, discontinued Exjade because of increased SGPT/ALT. Increases in transaminases did not appear to be dose related. Adverse reactions that led to discontinuations included abnormal liver function tests (2 patients) and drug-induced hepatitis (2 patients), skin rash, glycosuria/proteinuria, Henoch Schönlein purpura, hyperactivity/insomnia, drug fever, and cataract (1 patient each).
In Study 3, a total of 48 (36%) patients treated with Exjade had increases in serum creatinine greater than 33% above baseline on 2 separate occasions (Table 2) [see Warnings and Precautions (5.1)]. Of the patients who experienced creatinine increases in Study 3, 8 Exjade-treated patients required dose reductions. In this study, 5 patients in the Exjade group developed elevations in SGPT/ALT levels greater than 5 times the ULN at 2 consecutive visits and 1 patient subsequently had Exjade permanently discontinued. Four additional patients discontinued Exjade due to adverse reactions with a suspected relationship to study drug, including diarrhea, pancreatitis associated with gallstones, atypical tuberculosis, and skin rash.
In the MDS pool, in the first year, a total of 229 (37%) patients treated with Exjade had increases in serum creatinine greater than 33% above baseline on 2 consecutive occasions (Table 2) and 8 (3.5%) patients permanently discontinued [see Warnings and Precautions (5.1)]. A total of 5 (0.8%) patients developed SGPT/ALT levels greater than 5 times the ULN at 2 consecutive visits. The most frequent adverse reactions that led to discontinuation included increases in serum creatinine, diarrhea, nausea, rash, and vomiting. Death was reported in the first year in 52 (8%) of patients [see Clinical Studies (14)].
| Abbreviations: ALT, alanine transaminase; MDS, myelodysplastic syndrome; SGPT, serum glutamic-pyruvic transaminase; ULN, upper limit of normal. | |||||
| Study 1 (Beta-thalassemia) | Study 3 (Sickle Cell Disease) | MDS Pool | |||
| Laboratory Parameter | Exjade
N = 296 n (%) | Deferoxamine
N = 290 n (%) | Exjade
N = 132 n (%) | Deferoxamine
N = 63 n (%) | Exjade
N = 627 n (%) |
| Serum Creatinine | |||||
| Creatinine increase > 33% at 2 consecutive post-baseline visits | 113 (38) | 41 (14) | 48 (36) | 14 (22) | 229 (37) |
| Creatinine increase > 33% and > ULN at 2 consecutive post-baseline visits | 7 (2) | 1 (0) | 3 (2) | 2 (3) | 126 (20) |
| SGPT/ALT | |||||
| SGPT/ALT > 5 x ULN at 2 post-baseline visits | 25 (8) | 7 (2) | 2 (2) | 0 | 9 (1) |
| SGPT/ALT > 5 x ULN at 2 consecutive post-baseline visits | 17 (6) | 5 (2) | 5 (4) | 0 | 5 (1) |
Non-Transfusion-Dependent Thalassemia Syndromes
In Study 5, 110 patients with NTDT received 1 year of treatment with Exjade 5 or 10 mg/kg/day and 56 patients received placebo in a double-blind, randomized trial. In Study 6, 130 of the patients who completed Study 5 were treated with open-label Exjade at 5, 10, or 20 mg/kg/day (depending on the baseline LIC) for 1 year [see Clinical Studies (14)]. In Study 7, 134 patients with NTDT of 10 years of age or older with iron overload, received Exjade for up to 5 years, at a starting dose of 10 mg/kg/day followed by dose adjustment at Week 4, and then approximately every 6 months thereafter based on LIC levels. Tables 3 and 4 display the frequency of adverse reactions in patients with NTDT. Adverse reactions with a suspected relationship to study drug were included in Table 3 if they occurred at ≥ 5% of patients in Study 5.
| Abbreviation: NTDT, non-transfusion-dependent thalassemia. a The occurrence of nausea, and rash are included for Study 6 and rash for Study 7 for consistency. There were no additional adverse reactions with a suspected relationship to study drug occurring in greater than 5% of patients in Study 6 and Study 7. |
||||
| Study 5 | Study 6 | Study 7 | ||
| Exjade | Placebo | Exjade | Exjade | |
| N = 110 | N = 56 | N = 130 | N = 134 | |
| n (%) | n (%) | n (%) | n (%) | |
| Any adverse reaction | 31 (28) | 9 (16) | 27 (21) | 50 (37) |
| Nausea | 7 (6) | 4 (7) | 2 (2)a | 7 (5) |
| Rash | 7 (6) | 1 (2) | 2 (2)a | 3 (2)a |
| Diarrhea | 5 (5) | 1 (2) | 7 (5) | 8 (6) |
In Study 5, 1 patient in the placebo 10 mg/kg/day group experienced an ALT increase to greater than 5 times ULN and greater than 2 times baseline (Table 4). Three Exjade-treated patients (all in the 10 mg/kg/day group) had 2 consecutive serum creatinine level increases greater than 33% from baseline and greater than ULN. Serum creatinine returned to normal in all 3 patients (in 1 spontaneously and in the other 2 after drug interruption). Two additional cases of ALT increase and 2 additional cases of serum creatinine increase were observed in the 1-year extension of Study 5. The number (%) of patients with NTDT with increase in serum creatinine or SGPT/ALT in Study 5, Study 6, and Study 7 are presented in Table 4 below.
| Abbreviations: ALT, alanine transaminase; NTDT, non-transfusion-dependent thalassemia; SGPT, serum glutamic-pyruvic transaminase; ULN, upper limit of normal. | ||||
| Study 5 | Study 6 | Study 7 | ||
| Exjade | Placebo | Exjade | Exjade | |
| N = 110 | N = 56 | N = 130 | N = 134 | |
| Laboratory Parameter | n (%) | n (%) | n (%) | n (%) |
| Serum creatinine (> 33% increase from baseline and > ULN at ≥ 2 consecutive post-baseline values) | 3 (3) | 0 | 2 (2) | 2 (2) |
| SGPT/ALT (> 5 x ULN and > 2 x baseline) | 1 (1) | 1 (2) | 2 (2) | 1 (1) |
Proteinuria
In clinical studies, urine protein was measured monthly. Intermittent proteinuria (urine protein/creatinine ratio greater than 0.6 mg/mg) occurred in 18.6% of Exjade-treated patients compared to 7.2% of deferoxamine-treated patients in Study 1 [see Warnings and Precautions (5.1)].
Other Adverse Reactions
In the population of more than 5,000 patients with transfusional iron overload who have been treated with Exjade during clinical trials, adverse reactions occurring in 0.1% to 1% of patients included gastritis, edema, sleep disorder, pigmentation disorder, dizziness, anxiety, maculopathy, cholelithiasis, pyrexia, fatigue, laryngeal pain, cataract, hearing loss, GI hemorrhage, gastric ulcer (including multiple ulcers), duodenal ulcer, renal tubular disorder (Fanconi Syndrome), and acute pancreatitis (with and without underlying biliary conditions). Adverse reactions occurring in 0.01% to 0.1% of patients included optic neuritis, esophagitis, erythema multiforme, and drug reaction with eosinophilia and systemic symptoms (DRESS). Adverse reactions, which most frequently led to dose interruption or dose adjustment during clinical trials were rash, GI disorders, infections, increased serum creatinine, and increased serum transaminases.
Pooled Analysis of Pediatric Clinical Trial Data
A nested case control analysis was conducted within a deferasirox tablets for oral suspension pediatric pooled clinical trial dataset to evaluate the effects of dose and serum ferritin level, separately and combined, on kidney function. Among 1213 children (aged 2 to 15 years) with transfusion-dependent thalassemia, 162 cases of acute kidney injury (eGFR < 90 mL/min/1.73 m2) and 621 matched-controls with normal kidney function (eGFR > 120 mL/min/1.73 m2) were identified. The primary findings were:
- A 26% increased risk of acute kidney injury was observed with each 5 mg/kg increase in daily Exjade dosage starting at 20 mg/kg/day (95% confidence interval (CI): 1.08-1.48).
- A 25% increased risk for acute kidney injury was observed with each 250 mcg/L decrease in serum ferritin starting at 1250 mcg/L (95% CI: 1.01-1.56).
- Among pediatric patients with a serum ferritin < 1,000 mcg/L, those who received Exjade dosage > 30 mg/kg/day, compared to those who received lower dosages, had a higher risk for acute kidney injury (Odds ratio (OR) = 4.47, 95% CI: 1.25-15.95), consistent with overchelation.
In addition, a cohort based analysis of ARs was conducted in the deferasirox tablets for oral suspension pediatric pooled clinical trial data. Pediatric patients who received Exjade dose > 25 mg/kg/day when their serum ferritin was < 1,000 mcg/L (n = 158) had a 6-fold greater rate of renal adverse reactions (incidence rate ration (IRR) = 6.00, 95% CI: 1.75-21.36) and a 2-fold greater rate of dose interruptions (IRR = 2.06, 95% CI: 1.33-3.17) compared to the time-period prior to meeting these simultaneous criteria. Adverse reaction of special interest (cytopenia, renal, hearing, and GI disorders) occurred 1.9-fold more frequently when these simultaneous criteria were met, compared to preceding time-periods (IRR = 1.91, 95% CI: 1.05-3.48) [see Warnings and Precautions (5.6)].
6.2 Postmarketing Experience
The following adverse reactions have been spontaneously reported during postapproval use of Exjade in the transfusional-iron overload setting. Because these reactions are reported voluntarily from a population of uncertain size, in which patients may have received concomitant medication, it is not always possible to reliably estimate frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome (SJS), hypersensitivity vasculitis, urticaria, alopecia, toxic epidermal necrolysis (TEN)
Immune System Disorders: hypersensitivity reactions (including anaphylactic reaction and angioedema)
Renal and Urinary Disorders: acute renal failure, tubulointerstitial nephritis
Hepatobiliary Disorders: hepatic failure
Gastrointestinal Disorders: GI perforation
Blood and Lymphatic System Disorders: worsening anemia
5-Year Pediatric Registry
In a 5-year observational study, 267 pediatric patients 2 to < 6 years of age (at enrollment) with transfusional hemosiderosis received deferasirox. Of the 242 patients who had pre- and post-baseline eGFR measurements, 116 (48%) patients had a decrease in eGFR of ≥ 33% observed at least once. Twenty-one (18%) of these 116 patients with decreased eGFR had a dose interruption, and 15 (13%) of these 116 patients had a dose decrease within 30 days. Adverse reactions leading to permanent discontinuation from the study included liver injury (n = 11), renal tubular disorder
(n = 1), proteinuria (n = 1), hematuria (n = 1), upper GI hemorrhage (n = 1), vomiting (n = 2), abdominal pain (n = 1), and hypokalemia (n = 1).
7. Drug Interactions
7.1 Aluminum-Containing Antacid Preparations
The concomitant administration of Exjade and aluminum-containing antacid preparations has not been formally studied. Although deferasirox has a lower affinity for aluminum than for iron, do not take Exjade with aluminum-containing antacid preparations due to the mechanism of action of Exjade.
7.2 Agents Metabolized by CYP3A4
Deferasirox may induce CYP3A4 resulting in a decrease in CYP3A4 substrate concentration when these drugs are coadministered. Closely monitor patients for signs of reduced effectiveness when deferasirox is administered with drugs metabolized by CYP3A4 (e.g., alfentanil, aprepitant, budesonide, buspirone, conivaptan, cyclosporine, darifenacin, darunavir, dasatinib, dihydroergotamine, dronedarone, eletriptan, eplerenone, ergotamine, everolimus, felodipine, fentanyl, hormonal contraceptive agents, indinavir, fluticasone, lopinavir, lovastatin, lurasidone, maraviroc, midazolam, nisoldipine, pimozide, quetiapine, quinidine, saquinavir, sildenafil, simvastatin, sirolimus, tacrolimus, tolvaptan, tipranavir, triazolam, ticagrelor, and vardenafil) [see Clinical Pharmacology (12.3)].
7.3 Agents Metabolized by CYP2C8
Deferasirox inhibits CYP2C8 resulting in an increase in CYP2C8 substrate (e.g., repaglinide and paclitaxel) concentration when these drugs are coadministered. If Exjade and repaglinide are used concomitantly, consider decreasing the dose of repaglinide and perform careful monitoring of blood glucose levels. Closely monitor patients for signs of exposure related toxicity when Exjade is coadministered with other CYP2C8 substrates [see Clinical Pharmacology (12.3)].
7.4 Agents Metabolized by CYP1A2
Deferasirox inhibits CYP1A2 resulting in an increase in CYP1A2 substrate (e.g., alosetron, caffeine, duloxetine, melatonin, ramelteon, tacrine, theophylline, tizanidine) concentration when these drugs are coadministered. An increase in theophylline plasma concentrations could lead to clinically significant theophylline-induced CNS or other adverse reactions. Avoid the concomitant use of theophylline or other CYP1A2 substrates with a narrow therapeutic index (e.g., tizanidine) with Exjade. Monitor theophylline concentrations and consider theophylline dose modification if you must coadminister theophylline with Exjade. Closely monitor patients for signs of exposure related toxicity when Exjade is coadministered with other drugs metabolized by CYP1A2 [see Clinical Pharmacology (12.3)].
7.5 Agents Inducing UDP-glucuronosyltransferase (UGT) Metabolism
Deferasirox is a substrate of UGT1A1 and to a lesser extent UGT1A3. The concomitant use of Exjade with potent UGT inducers (e.g., rifampicin, phenytoin, phenobarbital, ritonavir) may result in a decrease in Exjade efficacy due to a possible decrease in deferasirox concentration. Avoid the concomitant use of potent UGT inducers with Exjade. Consider increasing the initial dose of Exjade if you must coadminister these agents together [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no studies with the use of Exjade in pregnant women to inform drug-associated risks.
Administration of deferasirox to rats during pregnancy resulted in decreased offspring viability and an increase in renal anomalies in male offspring at doses that were about or less than the recommended human dose on an mg/m2 basis. No fetal effects were noted in pregnant rabbits at doses equivalent to the human recommended dose on an mg/m2 basis. Exjade should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. However, the background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
In embryo-fetal developmental studies, pregnant rats and rabbits received oral deferasirox during the period of organogenesis at doses up to 100 mg/kg/day in rats and 50 mg/kg/day in rabbits (1.2 times the maximum recommended human dose (MRHD) on an mg/m2 basis). These doses resulted in maternal toxicity but no fetal harm was observed.
In a prenatal and postnatal developmental study, pregnant rats received oral deferasirox daily from organogenesis through lactation day 20 at doses of 10, 30, and 90 mg/kg/day (0.1, 0.3, and 1.0 times the MRHD on an mg/m2 basis). Maternal toxicity, loss of litters, and decreased offspring viability occurred at 90 mg/kg/day (1.0 times the MRHD on a mg/m2 basis) and increases in renal anomalies in male offspring occurred at 30 mg/kg/day (0.3 times the MRHD on a mg/m2 basis).
8.2 Lactation
Risk Summary
No data are available regarding the presence of Exjade or its metabolites in human milk, the effects of the drug on the breastfed child, or the effects of the drug on milk production. Deferasirox and its metabolites were excreted in rat milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in a breastfeeding child from deferasirox and its metabolites, a decision should be made whether to discontinue breastfeeding or to discontinue the drug, taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Contraception
Counsel patients to use non-hormonal method(s) of contraception since Exjade can render hormonal contraceptives ineffective [see Drug Interactions (7.2)].
8.4 Pediatric Use
Transfusional Iron Overload
The safety and effectiveness of Exjade have been established in pediatric patients 2 years of age and older for the treatment of transfusional iron overload [see Dosage and Administration (2.1)].
Safety and effectiveness have not been established in pediatric patients less than 2 years of age for the treatment of transfusional iron overload.
Pediatric approval for treatment of transfusional iron overload was based on clinical studies of 292 pediatric patients 2 years to less than 16 years of age with various congenital and acquired anemias. Seventy percent of these patients had beta-thalassemia [see Indications and Usage (1), Dosage and Administration (2.1), Clinical Studies (14)]. In those clinical studies, 173 children (ages 2 to < 12 years) and 119 adolescents (ages 12 to < 17 years) were exposed to deferasirox.
A trial conducted in treatment-naïve pediatric patients, 2 to < 18 years of age with transfusional iron overload (NCT02435212) did not provide additional relevant information about the safety or effectiveness of the deferasirox granules dosage form (Jadenu Sprinkle) compared to the deferasirox oral tablets for suspension dosage form (Exjade).
Iron Overload in Non-Transfusion-Dependent Thalassemia Syndromes
The safety and effectiveness of Exjade have been established in patients 10 years of age and older for the treatment of chronic iron overload with non-transfusion-dependent thalassemia (NTDT) syndromes [see Dosage and Administration (2.2)].
Safety and effectiveness have not been established in patients less than 10 years of age with chronic iron overload in NTDT syndromes.
Pediatric approval for treatment of NTDT syndromes with liver iron (Fe) concentration (LIC) of at least 5 mg Fe per gram of dry weight and a serum ferritin greater than 300 mcg/L was based on 16 pediatric patients treated with Exjade therapy (10 years to less than 16 years of age) with chronic iron overload and NTDT. Use of Exjade in these age groups is supported by evidence from adequate and well-controlled studies of Exjade in adult and pediatric patients [see Indications and Usage (1.2), Dosage and Administration (2.2), Clinical Studies (14)].
In general, risk factors for deferasirox-associated kidney injury include preexisting renal disease, volume depletion, overchelation, and concomitant use of other nephrotoxic drugs. Acute kidney injury, and acute liver injury and failure has occurred in pediatric patients. In a pooled safety analysis, pediatric patients with higher Exjade exposures had a greater probability of renal toxicity and decreased renal function, resulting in increased deferasirox exposure and progressive renal toxicity/kidney injury. Higher rates of renal adverse reactions have been identified among pediatric patients receiving Exjade doses greater than 25 mg/kg/day when their serum ferritin values were less than 1,000 mcg/L [see Dosage and Administration (2.5), Warnings and Precautions (5.1, 5.6), Adverse Reactions (6.1, 6.2)].
Monitoring Recommendations for pediatric patients with Transfusional Iron Overload and NTDT
It is recommended that serum ferritin be monitored every month to assess the patient’s response to therapy and to minimize the risk of overchelation [see Warnings and Precautions (5.6)].
Monitor renal function by estimating GFR using an eGFR prediction equation appropriate for pediatric patients and evaluate renal tubular function. Monitor renal function more frequently in pediatric patients in the presence of renal toxicity risk factors, including episodes of dehydration, fever and acute illness that may result in volume depletion or decreased renal perfusion. Use the minimum effective dose [see Warnings and Precautions (5.1)].
Interrupt Exjade in pediatric patients with transfusional iron overload and consider dose interruption in pediatric patients with non-transfusion-dependent iron overload, for acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake, and monitor more frequently. Resume therapy as appropriate, based on assessments of renal function, when oral intake and volume status are normal. Evaluate the risk benefit profile of continued Exjade use in the setting of decreased renal function. Avoid use of other nephrotoxic drugs [see Dosage and Administration (2.5), Warnings and Precautions (5.1)].
Juvenile Animal Toxicity Data
Renal toxicity was observed in adult mice, rats, and marmoset monkeys administered deferasirox at therapeutic doses. In a neonatal and juvenile toxicity study in rats, deferasirox was administered orally from postpartum Day 7 through 70, which equates to a human age range of term neonate through adolescence. Increased renal toxicity was identified in juvenile rats compared to adult rats at a dose based on mg/m2 approximately 0.4 times the recommended dose of 20 mg/kg/day. A higher frequency of renal abnormalities was noted when deferasirox was administered to non-iron overloaded animals compared to iron overloaded animals.
8.5 Geriatric Use
Four hundred thirty-one (431) patients greater than or equal to 65 years of age were studied in clinical trials of Exjade in the transfusional iron overload setting. Two hundred twenty-five (225) of these patients were between 65 and 75 years of age while 206 were greater than or equal to 75 years of age. The majority of these patients had myelodysplastic syndrome (MDS) (n = 393). In these trials, elderly patients experienced a higher frequency of adverse reactions than younger patients. Monitor elderly patients for early signs or symptoms of adverse reactions that may require a dose adjustment. Elderly patients are at increased risk for toxicity due to the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.
In elderly patients, including those with MDS, individualize the decision to remove accumulated iron based on clinical circumstances and the anticipated clinical benefit and risks of Exjade therapy.
8.6 Renal Impairment
Exjade is contraindicated in patients with eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)]. For patients with renal impairment (eGFR 40–60 mL/min/1.73 m2), reduce the starting dose by 50% [see Dosage and Administration (2.4)]. Exercise caution in pediatric patients with eGFR between 40 and 60 mL/min/1.73 m2 [see Dosage and Administration (2.4)]. If treatment is needed, use the minimum effective dose with enhanced monitoring of glomerular and renal tubular function. Individualize dose titration based on improvement in renal injury [see Dosage and Administration (2.4, 2.5)].
Exjade can cause glomerular dysfunction, renal tubular toxicity, or both, and can result in acute renal failure. Monitor all patients closely for changes in eGFR and renal tubular dysfunction during Exjade treatment. If either develops, consider dose reduction, interruption or discontinuation of Exjade until glomerular or renal tubular function returns to baseline [see Dosage and Administration (2.4, 2.5), Warnings and Precautions (5.1)].
8.7 Hepatic Impairment
Avoid the use of Exjade in patients with severe (Child-Pugh C) hepatic impairment. For patients with moderate (Child-Pugh B) hepatic impairment, the starting dose should be reduced by 50%. Closely monitor patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment for efficacy and adverse reactions that may require dose titration [see Dosage and Administration (2.4), Warnings and Precautions (5.2)].
| EXJADE
deferasirox tablet, for suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| EXJADE
deferasirox tablet, for suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| EXJADE
deferasirox tablet, for suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |