Drug Detail:Fabior (Tazarotene topical [ ta-zar-oh-teen ])
Drug Class: Topical acne agents
Highlights of Prescribing Information
FABIOR (tazarotene) Foam, 0 .1%, for topical use
Initial U.S. Approval: 1997
Indications and Usage for Fabior Foam
- FABIOR Foam is a retinoid indicated for the topical treatment of acne vulgaris in patients 12 years of age or older. (1)
Fabior Foam Dosage and Administration
- Apply a thin layer to the entire affected areas of the face and/or upper trunk once daily in the evening. Avoid the eyes, lips, and mucous membranes. Wash hands after application. (2)
Dosage Forms and Strengths
- 0.1%, foam. (3)
Contraindications
- Pregnancy. (4, 8.1)
Warnings and Precautions
- Fetal Risk: FABIOR Foam contains tazarotene, which is a teratogenic substance. FABIOR Foam is contraindicated in pregnancy. Females of childbearing potential should have a negative pregnancy test within 2 weeks prior to initiating treatment and use an effective method of contraception during treatment. (5.1)
- Local Irritation: Use with caution in patients with a history of local tolerability reactions or local hypersensitivity. (5.2)
- Potential Irritant Effect with Concomitant Topical Medications: Use with caution because a cumulative irritant effect may occur. (5.3)
- Photosensitivity and Risk for Sunburn: Avoid exposure to sunlight, sunlamps, and weather extremes. Wear sunscreen daily. (5.4)
- Contents are flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. (5.5)
Adverse Reactions/Side Effects
- Most common adverse reactions reported at an incidence ≥6% are application site irritation, application site dryness, application site erythema, and application site exfoliation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
- Avoid concomitant dermatologic medications and cosmetics that have a strong drying effect. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2023
Full Prescribing Information
1. Indications and Usage for Fabior Foam
FABIOR® (tazarotene) Foam, 0.1% is indicated for the topical treatment of acne vulgaris in patients 12 years of age or older.
2. Fabior Foam Dosage and Administration
FABIOR Foam is for topical use only. FABIOR Foam is not for oral, ophthalmic, or intravaginal use.
FABIOR Foam should be applied once daily in the evening after washing with a mild cleanser and fully drying the affected area. Dispense a small amount of foam into the palm of the hand. Using fingertips, apply only enough foam to lightly cover the entire affected areas of the face and/or upper trunk with a thin layer; gently massage the foam into the skin until the foam disappears. Avoid the eyes, lips, and mucous membranes. Wash hands after application.
Patients may use moisturizer as needed.
If undue irritation (redness, peeling, or discomfort) occurs, patients should reduce frequency of application or temporarily interrupt treatment. Treatment may be resumed once irritation subsides. Treatment should be discontinued if irritation persists.
4. Contraindications
FABIOR Foam is contraindicated in pregnancy.
FABIOR Foam may cause fetal harm when administered to a pregnant woman. Tazarotene elicits teratogenic and developmental effects associated with retinoids after topical or systemic administration in rats and rabbits [see Use in Specific Populations (8.1)].
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, treatment should be discontinued and the patient apprised of the potential hazard to the fetus [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
5. Warnings and Precautions
5.1 Fetal Risk
Systemic exposure to tazarotenic acid is dependent upon the extent of the body surface area treated. In patients treated topically over sufficient body surface area, exposure could be in the same order of magnitude as in orally treated animals. Tazarotene is a teratogenic substance, and it is not known what level of exposure is required for teratogenicity in humans [see Clinical Pharmacology (12)].
There were 5 reported pregnancies in subjects who participated in clinical trials for topical tazarotene foam. One of the subjects was found to have been treated with topical tazarotene for 25 days, 2 were treated with vehicle foam, and the other 2 did not receive either tazarotene foam or vehicle foam. The subjects were discontinued from the trials when their pregnancy was reported. The one pregnant woman who was inadvertently exposed to topical tazarotene during the clinical trial delivered a full-term healthy infant.
5.2 Local Irritation
FABIOR Foam should be used with caution in patients with a history of local tolerability reactions or local hypersensitivity. Retinoids should not be used on abraded or eczematous skin, as they may cause severe irritation. Contact with the mouth, eyes, and mucous membranes should be avoided. In case of accidental contact, rinse well with water.
Some individuals may experience skin redness, peeling, burning or excessive pruritus. If these effects occur, the medication should either be discontinued until the integrity of the skin is restored, or the dosing should be reduced to an interval the patient can tolerate. However, efficacy at reduced frequency of application has not been established.
Weather extremes, such as wind or cold, may be more irritating to patients using FABIOR Foam.
5.3 Potential Irritant Effect with Concomitant Topical Medications
Concomitant topical acne therapy should be used with caution because a cumulative irritant effect may occur. If irritancy or dermatitis occurs, reduce frequency of application or temporarily interrupt treatment and resume once the irritation subsides. Treatment should be discontinued if the irritation persists.
5.4 Photosensitivity and Risk for Sunburn
Because of heightened burning susceptibility, exposure to sunlight (including sunlamps) should be avoided. Patients must be warned to use sunscreens and protective clothing when using FABIOR Foam. Patients with sunburn should be advised not to use FABIOR Foam until fully recovered. Patients who may have considerable sun exposure due to their occupation and those patients with inherent sensitivity to sunlight should exercise particular caution when using FABIOR Foam and ensure that the precautions are observed [see FDA-approved patient labeling]. Due to the potential for photosensitivity resulting in greater risk for sunburn, FABIOR Foam should be used with caution in patients with a personal or family history of skin cancer.
FABIOR Foam should be administered with caution if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the increased possibility of augmented photosensitivity.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data reflect exposure to FABIOR Foam in 744 subjects with acne vulgaris. Subjects were aged 12 to 45 years and were treated once daily in the evening for 12 weeks. Adverse reactions reported in ≥ 1% of subjects treated with FABIOR Foam are presented in Table 1. Most adverse reactions were mild to moderate in severity. Severe adverse reactions represented 3.0% of the subjects treated. Overall, 2.7% (20/744) of subjects discontinued FABIOR Foam because of local skin reactions.
| FABIOR Foam N = 744 | Vehicle Foam N = 741 |
|
|---|---|---|
| Patients with any adverse reaction, n (%) | 163 (22) | 19 (3) |
| Application site irritation | 107 (14) | 9 (1) |
| Application site dryness | 50 (7) | 8 (1) |
| Application site erythema | 48 (6) | 3 (<1) |
| Application site exfoliation | 44 (6) | 3 (<1) |
| Application site pain | 9 (1) | 0 |
| Application site photosensitivity (including sunburn) | 8 (1) | 3 (<1) |
| Application site pruritus | 7 (1) | 3 (<1) |
| Application site dermatitis | 6 (1) | 1 (<1) |
Additional adverse reactions that were reported in <1% of subjects treated with FABIOR Foam included application site reactions (including discoloration, discomfort, edema, rash, and swelling), dermatitis, impetigo, and pruritus.
Local skin reactions, dryness, erythema, and peeling actively assessed by the investigator and burning/stinging and itching reported by the subject were evaluated at baseline, during treatment, and end of treatment. During the 12 weeks of treatment, each local skin reaction peaked at Week 2 and gradually reduced thereafter with the continued use of FABIOR Foam.
7. Drug Interactions
No formal drug-drug interaction studies were conducted with FABIOR Foam.
Concomitant dermatologic medications and cosmetics that have a strong drying effect should be avoided. It is recommended to postpone treatment until the effects of these products subside before use of FABIOR Foam is started.
Concomitant use with oxidizing agents, such as benzoyl peroxide, may cause degradation of tazarotene and may reduce the clinical efficacy of tazarotene. If combination therapy is required, they should be applied at different times of the day (e.g., one in the morning and the other in the evening).
The impact of tazarotene on the pharmacokinetics of progestin-only oral contraceptives (i.e., minipills) has not been evaluated.
In a trial of 27 healthy female subjects between the ages of 20 to 55 years receiving a combination oral contraceptive tablet containing 1 mg norethindrone and 35 mcg ethinyl estradiol, concomitant use of tazarotene did not affect the pharmacokinetics of norethindrone and ethinyl estradiol over a complete cycle.
8. Use In Specific Populations
8.1 Pregnancy
FABIOR Foam is contraindicated in pregnancy [see Contraindications (4)].
There are no adequate and well-controlled studies with FABIOR Foam in pregnant women. FABIOR Foam is contraindicated in females who are or may become pregnant [see Contraindications (4)]. Females of child-bearing potential should be warned of the potential risk and use adequate birth-control measures when FABIOR Foam is used. The possibility that a female of child-bearing potential is pregnant at the time of institution of therapy should be considered. A negative serum or urine result for pregnancy test having a sensitivity down to at least 25 mIU/mL for hCG should be obtained within 2 weeks prior to therapy with FABIOR Foam, which should begin during a normal menstrual period for females of childbearing potential.
In rats, tazarotene 0.05% gel administered topically during gestation days 6 through 17 at 0.25 mg/kg/day resulted in reduced fetal body weights and reduced skeletal ossification. Rabbits dosed topically with 0.25 mg/kg/day tazarotene gel during gestation days 6 through 18 were noted with single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies.
Systemic exposure (AUC) to tazarotenic acid at topical doses of 0.25 mg/kg/day tazarotene in a gel formulation in rats and rabbits were 15 and 166 times, respectively, the AUC in acne patients treated with 2 mg/cm2 of FABIOR Foam 0.1% over a 15% body surface area.
As with other retinoids, when tazarotene was administered orally to experimental animals, developmental delays were seen in rats, and teratogenic effects and post-implantation loss were observed in rats and rabbits at doses 13 and 325 times, respectively, the AUC to tazarotenic acid in acne patients treated with 2 mg/cm2 of FABIOR Foam 0.1% over a 15% body surface area.
In female rats orally administered 2 mg/kg/day tazarotene from 15 days before mating through gestation day 7, a number of classic developmental effects of retinoids were observed including decreased number of implantation sites, decreased litter size, decreased numbers of live fetuses, and decreased fetal body weights. A low incidence of retinoid-related malformations was also observed. AUC in rats was 42 times the AUC in acne patients treated with 2 mg/cm2 of FABIOR Foam 0.1% over a 15% body surface area.
8.3 Nursing Mothers
After single topical doses of 14C-tazarotene to the skin of lactating rats, radioactivity was detected in milk, suggesting that there would be transfer of drug-related material to the offspring via milk. It is not known whether this drug is excreted in human milk. The safe use of FABIOR Foam during lactation has not been established. A decision should be made whether to discontinue breastfeeding or to discontinue therapy with FABIOR Foam taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
10. Overdosage
Excessive topical application of FABIOR Foam may lead to marked redness, peeling, or discomfort. [see Warnings and Precautions (5.2)]. Management of accidental ingestion or excessive application to the skin should be as clinically indicated.
11. Fabior Foam Description
FABIOR (tazarotene) Foam, 0.1% contains the compound tazarotene, a member of the acetylenic class of retinoids. It is for topical use only.
Chemically, tazarotene is ethyl 6-[(4,4-dimethylthiochroman-6-yl)ethynyl]nicotinate. The structural formula is represented below:
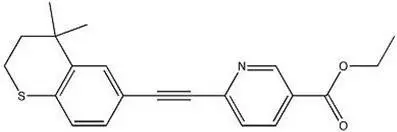
Molecular Formula: C21H21NO2S Molecular Weight: 351.46
Tazarotene is a pale yellow to yellow substance. FABIOR Foam contains tazarotene, 1 mg/g, in aqueous-based white to off-white foam vehicle consisting of butylated hydroxytoluene, ceteareth-12, citric acid anhydrous, diisopropyl adipate, light mineral oil, potassium citrate monohydrate, potassium sorbate, purified water, and sorbic acid. FABIOR Foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant.
12. Fabior Foam - Clinical Pharmacology
12.1 Mechanism of Action
Tazarotene is a retinoid prodrug that is converted to its active form, the cognate carboxylic acid of tazarotene, by rapid deesterification in animals and man. Tazarotenic acid binds to all 3 members of the retinoic acid receptor (RAR) family: RARα, RARβ, and RARγ but shows relative selectivity for RARβ and RARγ and may modify gene expression. The clinical significance of these findings is unknown.
The mechanism of tazarotene action in acne vulgaris is not defined. However, the basis of tazarotene's therapeutic effect in acne may be due to its anti-hyperproliferative, normalizing-of-differentiation and anti-inflammatory effects. Tazarotene inhibited corneocyte accumulation in rhino mouse skin and cross- linked envelope formation in cultured human keratinocytes. The clinical significance of these findings is unknown.
12.3 Pharmacokinetics
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. Tazarotenic acid was highly bound to plasma proteins (greater than 99%). Tazarotene and tazarotenic acid were metabolized to sulfoxides, sulfones, and other polar metabolites which were eliminated through urinary and fecal pathways.
Systemic exposure following topical application of FABIOR Foam 0.1% was evaluated in one trial. Patients aged 15 years and older with moderate-to-severe acne applied approximately 3.7 grams of FABIOR Foam 0.1% (N = 13) to approximately 15% body surface area (face, upper chest, upper back, and shoulders) once daily for 22 days. On Day 22, the mean (±SD) tazarotenic acid Cmax was 0.43 (±0.19) ng/mL, the AUC0-24h was 6.98 (±3.56) ng∙h/mL, and the half-life was 21.7 (±15.7) hours. The median Tmax was 6 hours (range: 4.4 to 12 hours). The AUC0-24h for tazarotenic acid was approximately 50-fold higher compared with the parent compound tazarotene. The mean (±SD) half-life of tazarotene was 8.1 (±3.7) hours.
Accumulation was observed upon repeated once-daily dosing as the tazarotenic acid predose concentrations were measurable in the majority of subjects. Steady state was attained within 22 days of daily application. Once-daily dosing resulted in little to no accumulation of tazarotene as predose concentrations were mostly below the quantitation limit throughout the study.
14. Clinical Studies
In 2 multi-center, randomized, double-blind, vehicle-controlled trials, a total of 1,485 subjects with moderate-to-severe acne vulgaris were randomized 1:1 to FABIOR Foam or vehicle applied once daily for 12 weeks. Acne severity was evaluated using lesion counts and the 6-point Investigator's Global Assessment (IGA) scale (see Table 2). At baseline, 80% of subjects were graded as "moderate" or Grade 3 and 20% were graded as "severe" or Grade 4 on the IGA scale. At baseline, subjects had an average of 79.8 total lesions of which the mean number of inflammatory lesions was 31.9 and the mean number of non-inflammatory lesions was 47.8. Subjects ranged in age from 12 to 45 years, with 860 (58%) subjects aged 12 to 17 years; 428 (29%) subjects aged 18 to 25 years; 143 (10%) subjects aged 26 to 35 years and 54 (4%) subjects aged 36 to 45 years. Subjects enrolled in the trials by race were white (77%), black (15%), Asian (4%), and other (4%). Hispanics comprised 18% of the population. An equal number of males (49%) and females (51%) were enrolled. Treatment success was defined as a score of "clear" (Grade 0) or "almost clear" (Grade 1) and at least 2-grade improvement from the baseline score to Week 12.
| Grade | Description | |
|---|---|---|
| 0 | Clear | Clear skin with no inflammatory or non-inflammatory lesions. |
| 1 | Almost clear | Rare non-inflammatory lesions with no more than rare papules. |
| 2 | Mild | Greater than Grade 1, some non-inflammatory lesions with no more than a few inflammatory lesions (papules/pustules only, no nodular lesions). |
| 3 | Moderate | Greater than Grade 2, up to many non-inflammatory lesions and may have some inflammatory lesions, but no more than one small nodular lesion. |
| 4 | Severe | Greater than Grade 3, up to many non-inflammatory and inflammatory lesions, but no more than a few nodular lesions. |
| 5 | Very severe | Many non-inflammatory and inflammatory lesions and more than a few nodular lesions. May have cystic lesions. |
Absolute and percent reductions in lesion counts and the IGA scale after 12 weeks of treatment in these 2 trials are shown in Table 3. Each trial needed to have a statistically significant reduction in 2 out of 3 lesion counts at Week 12.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| FABIOR Foam N = 371 | Vehicle Foam N = 372 | FABIOR Foam N = 373 | Vehicle Foam N = 369 |
|
| Inflammatory Lesions | ||||
| Mean absolute reduction from Baseline | 18.0 | 14.0 | 18.0 | 15.0 |
| Mean percent reduction from Baseline | 58% | 45% | 55% | 45% |
| Non-inflammatory Lesions | ||||
| Mean absolute reduction from Baseline | 28.0 | 17.0 | 26.0 | 18.0 |
| Mean percent reduction from Baseline | 55% | 33% | 57% | 41% |
| Total Lesions | ||||
| Mean absolute reduction from Baseline | 46.0 | 31.0 | 43.0 | 33.0 |
| Mean percent reduction from Baseline | 56% | 39% | 56% | 43% |
| Investigator's Global Assessment (IGA), n (%) | ||||
| Minimum 2-grade improvement and IGA of 0 or 1 | 107 (29%) | 60 (16%) | 103 (28%) | 49 (13%) |
17. Patient Counseling Information
See FDA-approved patient labeling (Patient Information).
Inform the patient of the following:
- Fetal risk associated with FABIOR Foam for females of childbearing potential. Advise patients to use an effective method of contraception during treatment to avoid pregnancy. Advise the patient to stop medication if she becomes pregnant and call her doctor.
- If undue irritation (redness, peeling, or discomfort) occurs, reduce frequency of application or temporarily interrupt treatment. Treatment may be resumed once irritation subsides.
- Do not place FABIOR Foam in the freezer.
- Avoid exposure of the treated areas to either natural or artificial sunlight, including tanning beds and sun lamps.
- Avoid contact with the eyes. If FABIOR Foam gets in or near their eyes, to rinse thoroughly with water.
- Wash their hands after applying FABIOR Foam.
- Avoid fire, flame, or smoking during and immediately following application since FABIOR Foam is flammable.
- Keep out of the reach of children.
- Not for ophthalmic, oral, or intravaginal use.
Patient Information FABIOR® (fab' ee ore) (tazarotene)Foam
IMPORTANT: For skin use only. Do not get FABIOR Foam in your eyes, mouth, or vagina.
Read the Patient Information that comes with FABIOR Foam before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or treatment.
What is FABIOR Foam?
FABIOR Foam is a prescription medicine used on the skin (topical) to treat acne in people 12 years and older.
It is not known if FABIOR Foam is safe and effective in children under 12 years of age.
Who should not use FABIOR Foam?
Do not use FABIOR Foam if you are pregnant or plan to become pregnant. FABIOR Foam may harm your unborn baby, if used during pregnancy.
If you are a female who can become pregnant:
- Use an effective method of birth control during treatment with FABIOR Foam. Talk with your doctor about birth control methods that are right for you during treatment with FABIOR Foam.
- Your doctor should do a blood or urine pregnancy test within 2 weeks before you begin to use FABIOR Foam to be sure you are not pregnant.
- If you have menstrual periods, begin using FABIOR Foam during a normal menstrual period to help assure that you are not pregnant when you begin use.
Stop using FABIOR Foam and call your doctor right away if you become pregnant during treatment with FABIOR Foam.
What should I tell my doctor before using FABIOR Foam?
Before you use FABIOR Foam, tell your doctor if you:
- or a family member have or had skin cancer.
- have eczema.
- have had a reaction to topical products in the past.
- have any condition that makes you sensitive to light.
- have any other medical conditions.
- are pregnant or plan to become pregnant. See "Who should not use FABIOR Foam?"
- are breastfeeding or plan to breastfeed. It is not known if tazarotene passes into your breast milk. You and your doctor should decide if you will use FABIOR Foam or breastfeed. You should not do both. Talk to your doctor about the best way to feed your baby if you use FABIOR Foam.
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins, and herbal supplements.
Especially tell your doctor if you:
- use other medicines or products that make your skin dry
- take other medicines that may increase your sensitivity to sunlight
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I use FABIOR Foam?
- Use FABIOR Foam exactly as your doctor tells you to. Do not use more FABIOR Foam than prescribed and do not use it more often than your doctor tells you to.
- If you are a female and have menstrual periods, begin using FABIOR Foam during a normal menstrual period to help assure that you are not pregnant when you begin use. See "Who should not use FABIOR Foam?"
- FABIOR Foam is flammable. Avoid fire, flame, and smoking during and right after you apply FABIOR Foam.
- Gently clean the affected area (face and/or upper trunk) with a mild cleanser and dry completely before using FABIOR Foam.
- Apply FABIOR Foam one time each day, before going to bed, to the affected areas (face and/or upper trunk) where you have acne lesions. Use enough foam to cover the entire affected area with a thin film of FABIOR Foam.
- Keep FABIOR Foam away from your eyes, mouth, and vagina. If FABIOR Foam comes into contact with your eyes, rinse them well with water.
- Wash your hands after applying FABIOR Foam.
- If you use too much FABIOR Foam, you may get redness, peeling, or skin irritation in the treated area. Call your doctor if this happens, or if you accidentally swallow FABIOR Foam.
- Follow your doctor's directions for other routine skin care and the use of make-up.
- You may also use a moisturizer as needed.
Instructions for applying FABIOR Foam
1. Shake the FABIOR Foam can before use.
2. Remove cap from can. See Figure A.
3. Hold the FABIOR Foam can upright at a slight angle and press the nozzle. See Figure B.
4. Dispense a small amount of FABIOR Foam into the palm of your hand. See Figure C.
5. Use the fingertips of your other hand to apply enough FABIOR Foam to cover the affected area with a thin layer. Gently rub the foam into the affected area until it disappears into the skin. See Figure D.
6. Wash hands after applying FABIOR Foam. See Figure E.
Avoid getting FABIOR Foam in your eyes, mouth, or vagina.
What should I avoid while using FABIOR Foam?
- Avoid using abrasive soaps or cleansers that might dry or irritate your skin, unless your doctor tells you it is ok.
- Avoid sunlight. FABIOR Foam can make your skin sensitive to sunlight and the light from sunlamps or tanning beds. You could get a sunburn. Use sunscreen and protective clothing during the day if you must be in sunlight.
- Avoid using FABIOR Foam if you have a sunburn. If you have a sunburn, wait until it is fully healed before using FABIOR Foam.
- Talk to your doctor before using FABIOR Foam if you are sensitive to sunlight, take medications that increase your sensitivity to sunlight, or you must spend a lot of time in the sun for your job.
- Avoid weather extremes, such as wind and cold, because they may irritate your skin more while you are using FABIOR Foam.
What are the possible side effects of FABIOR Foam?
FABIOR Foam may harm your unborn baby, if used during pregnancy.
- Do not use FABIOR Foam during pregnancy. See "Who should not use FABIOR Foam?"
The most common side effects of FABIOR Foam are:
- burning or stinging
- dry skin
- red skin
- peeling or flaking skin
Sometimes these symptoms can become severe and may be uncomfortable. Tell your doctor if these side effects become uncomfortable for you. Your doctor may tell you to stop using FABIOR Foam until your skin heals and your symptoms improve, or to use FABIOR Foam less often to help you tolerate it better.
These are not all the possible side effects of FABIOR Foam. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088.
You may also report side effects to Mayne Pharma at 1-844-825-8500.
How should I s tore FABIOR Foam?
- Store FABIOR Foam at room temperature, between 68°F to 77°F (20°C to 25°C).
- Store FABIOR Foam upright.
- Do not freeze FABIOR Foam.
- FABIOR Foam is flammable. Keep the can away from fire and heat. Do not spray FABIOR Foam near fire or direct heat.
- Do not puncture the can or throw it into a fire, even if the can is empty.
Keep FABIOR Foam and all medicines out of the reach of children.
General Information about FABIOR Foam
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use FABIOR Foam for a condition for which it was not prescribed. Do not give FABIOR Foam to other people even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about FABIOR Foam. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about FABIOR Foam that is written for health professionals.
What are the ingredients in FABIOR Foam?
Active ingredient: tazarotene
Inactive ingredients: butylated hydroxytoluene, ceteareth-12, citric acid anhydrous, diisopropyl adipate, light mineral oil, potassium citrate monohydrate, potassium sorbate, purified water, and sorbic acid. The foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant.
This Patient Information has been approved by the U.S. Food and Drug Administration
FABIOR is a registered trademark of Mayne Pharma LLC.
©2016 Mayne Pharma. All rights reserved.
Distributed by:
Mayne Pharma
Raleigh, NC 27609
02/2023
| FABIOR
tazarotene aerosol, foam |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Mayne Pharma (867220261) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224526 | ANALYSIS(51862-295) , MANUFACTURE(51862-295) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224591 | PACK(51862-295) , LABEL(51862-295) | |




 Figure A
Figure A Figure B
Figure B Figure C
Figure C Figure D
Figure D Figure E
Figure E
