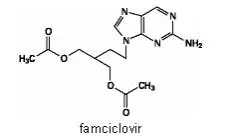
Drug Detail:Famvir (Famciclovir [ fam-sye-klo-veer ])
Drug Class: Purine nucleosides
Highlights of Prescribing Information
FAMVIR (famciclovir) tablets, for oral use
Initial U.S. Approval: 1994
Indications and Usage for Famvir
FAMVIR, a prodrug of penciclovir, is a nucleoside analog DNA polymerase inhibitor indicated for:
Immunocompetent Adult Patients (1.1)
- Herpes labialis (cold sores)
○ Treatment of recurrent episodes
- Genital herpes
○ Treatment of recurrent episodes
○ Suppressive therapy of recurrent episodes
- Herpes zoster (shingles)
Human Immunodeficiency Virus (HIV)-Infected Adult Patients (1.2)
- Treatment of recurrent episodes of orolabial or genital herpes
Limitation of Use
The efficacy and safety of FAMVIR have not been established for:
- Patients less than 18 years of age
- Immunocompromised patients other than for the treatment of recurrent episodes of orolabial or genital herpes in HIV-infected patients
- Black and African American patients with recurrent genital herpes
Famvir Dosage and Administration
| Immunocompetent Adult Patients (2.1) | |
| Herpes labialis (cold sores) | 1500 mg as a single dose |
| Genital herpes Treatment of recurrent episodes Suppressive therapy |
1000 mg twice daily for 1 day 250 mg twice daily |
| Herpes zoster (shingles) | 500 mg every 8 hours for 7 days |
| HIV-Infected Adult Patients (2.2) | |
| Recurrent episodes of orolabial or genital herpes | 500 mg twice daily for 7 days |
Patients with renal impairment: Adjust dose based on creatinine clearance. (2.3)
Dosage Forms and Strengths
Tablets: 125 mg, 250 mg, 500 mg (3)
Contraindications
Known hypersensitivity to the product, its components, or Denavir® (penciclovir cream). (4)
Warnings and Precautions
Acute renal failure: May occur in patients with underlying renal disease who receive higher than recommended doses of FAMVIR for their level of renal function. Reduce dosage in patients with renal impairment. (2.3, 8.6)
Adverse Reactions/Side Effects
The most common adverse events reported in at least 1 indication by greater than 10% of adult patients are headache and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Probenecid: May increase penciclovir levels. Monitor for evidence of penciclovir toxicity. (7.2)
Use In Specific Populations
Nursing mothers: FAMVIR should not be used in nursing mothers unless the potential benefits outweigh the potential risks associated with treatment. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2016
Full Prescribing Information
1. Indications and Usage for Famvir
1.1 Immunocompetent Adult Patients
Herpes labialis (cold sores): FAMVIR is indicated for the treatment of recurrent herpes labialis.
Genital herpes:
Recurrent episodes: FAMVIR is indicated for the treatment of recurrent episodes of genital herpes. The efficacy of FAMVIR when initiated more than 6 hours after onset of symptoms or lesions has not been established.
Suppressive therapy: FAMVIR is indicated for chronic suppressive therapy of recurrent episodes of genital herpes. The efficacy and safety of FAMVIR for the suppression of recurrent genital herpes beyond 1 year have not been established.
Herpes zoster (shingles): FAMVIR is indicated for the treatment of herpes zoster. The efficacy of FAMVIR when initiated more than 72 hours after onset of rash has not been established.
1.2 HIV-Infected Adult Patients
Recurrent orolabial or genital herpes: FAMVIR is indicated for the treatment of recurrent episodes of orolabial or genital herpes in HIV-infected adults. The efficacy of FAMVIR when initiated more than 48 hours after onset of symptoms or lesions has not been established.
Limitation of Use
The efficacy and safety of FAMVIR have not been established for:
- Patients less than 18 years of age
- Patients with first episode of genital herpes
- Patients with ophthalmic zoster
- Immunocompromised patients other than for the treatment of recurrent orolabial or genital herpes in HIV-infected patients
- Black and African American patients with recurrent genital herpes
2. Famvir Dosage and Administration
FAMVIR may be taken with or without food.
2.3 Dosing Recommendation in Patients with Renal Impairment
Dosage recommendations for adult patients with renal impairment are provided in Table 1 [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
| Indication and Normal Dosage
Regimen | Creatinine Clearance
(mL/min) | Adjusted Dosage
Regimen Dose (mg) | Dosing Interval |
| Single-Day Dosing Regimens | |||
| Recurrent Genital Herpes 1000 mg every 12 hours for 1 day | ≥ 60 | 1000 | every 12 hours for 1 day |
| 40-59 | 500 | every 12 hours for 1 day | |
| 20-39 | 500 | single dose | |
| < 20 | 250 | single dose | |
| HD* | 250 | single dose following dialysis |
|
| Recurrent Herpes Labialis 1500 mg single dose | ≥ 60 | 1500 | single dose |
| 40-59 | 750 | single dose | |
| 20-39 | 500 | single dose | |
| < 20 | 250 | single dose | |
| HD* | 250 | single dose following dialysis |
|
| Multiple-Day Dosing Regimens | |||
| Herpes Zoster 500 mg every 8 hours | ≥ 60 | 500 | every 8 hours |
| 40-59 | 500 | every 12 hours | |
| 20-39 | 500 | every 24 hours | |
| < 20 | 250 | every 24 hours | |
| HD* | 250 | following each dialysis | |
| Suppression of Recurrent Genital Herpes 250 mg every 12 hours | ≥ 40 | 250 | every 12 hours |
| 20-39 | 125 | every 12 hours | |
| < 20 | 125 | every 24 hours | |
| HD* | 125 | following each dialysis | |
| Recurrent Orolabial or Genital Herpes in HIV-Infected Patients 500 mg every 12 hours | ≥ 40 | 500 | every 12 hours |
| 20-39 | 500 | every 24 hours | |
| < 20 | 250 | every 24 hours | |
| HD* | 250 | following each dialysis | |
*Hemodialysis
3. Dosage Forms and Strengths
FAMVIR tablets are available in 3 strengths:
- 125 mg: White, round film-coated, biconvex, beveled edges, debossed with “FAMVIR” on one side and “125” on the other side
- 250 mg: White, round film-coated, biconvex, beveled edges, debossed with “FAMVIR” on one side and “250” on the other side
- 500 mg: White, oval film-coated, biconvex, debossed with “FAMVIR” on one side and “500” on the other side
4. Contraindications
FAMVIR is contraindicated in patients with known hypersensitivity to the product, its components, or Denavir® (penciclovir cream).
6. Adverse Reactions/Side Effects
Acute renal failure is discussed in greater detail in other sections of the label [see Warnings and Precautions (5)].
The most common adverse events reported in at least 1 indication by greater than 10% of adult patients treated with FAMVIR are headache and nausea.
6.1 Clinical Trials Experience in Adult Patients
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Immunocompetent patients: The safety of FAMVIR has been evaluated in active- and placebo-controlled clinical studies involving 816 FAMVIR-treated patients with herpes zoster (FAMVIR, 250 mg three times daily to 750 mg three times daily); 163 FAMVIR-treated patients with recurrent genital herpes (FAMVIR, 1000 mg twice daily); 1,197 patients with recurrent genital herpes treated with FAMVIR as suppressive therapy (125 mg once daily to 250 mg three times daily) of which 570 patients received FAMVIR (open-labeled and/or double-blind) for at least 10 months; and 447 FAMVIR-treated patients with herpes labialis (FAMVIR, 1500 mg once daily or 750 mg twice daily). Table 2 lists selected adverse events.
| Incidence | |||||||||
| Herpes Zoster† | Recurrent
Genital Herpes‡ | Genital Herpes-
Suppression§ | Herpes Labialis‡ | ||||||
| FAMVIR | Placebo | FAMVIR | Placebo | FAMVIR | Placebo | FAMVIR | Placebo | ||
| (n=273) | (n=146) | (n=163) | (n=166) | (n=458) | (n=63) | (n=447) | (n=254) | ||
| Events | % | % | % | % | % | % | % | % | |
| Nervous System | |||||||||
| Headache | 22.7 | 17.8 | 13.5 | 5.4 | 39.3 | 42.9 | 8.5 | 6.7 | |
| Paresthesia | 2.6 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | |
| Migraine | 0.7 | 0.7 | 0.6 | 0.6 | 3.1 | 0.0 | 0.2 | 0.0 | |
| Gastrointestinal | |||||||||
| Nausea | 12.5 | 11.6 | 2.5 | 3.6 | 7.2 | 9.5 | 2.2 | 3.9 | |
| Diarrhea | 7.7 | 4.8 | 4.9 | 1.2 | 9.0 | 9.5 | 1.6 | 0.8 | |
| Vomiting | 4.8 | 3.4 | 1.2 | 0.6 | 3.1 | 1.6 | 0.7 | 0.0 | |
| Flatulence | 1.5 | 0.7 | 0.6 | 0.0 | 4.8 | 1.6 | 0.2 | 0.0 | |
| Abdominal Pain | 1.1 | 3.4 | 0.0 | 1.2 | 7.9 | 7.9 | 0.2 | 0.4 | |
| Body as a Whole | |||||||||
| Fatigue | 4.4 | 3.4 | 0.6 | 0.0 | 4.8 | 3.2 | 1.6 | 0.4 | |
| Skin and Appendages | |||||||||
| Pruritus | 3.7 | 2.7 | 0.0 | 0.6 | 2.2 | 0.0 | 0.0 | 0.0 | |
| Rash | 0.4 | 0.7 | 0.0 | 0.0 | 3.3 | 1.6 | 0.0 | 0.0 | |
| Reproductive (Female) | |||||||||
| Dysmenorrhea | 0.0 | 0.7 | 1.8 | 0.6 | 7.6 | 6.3 | 0.4 | 0.0 | |
*Patients may have entered into more than one clinical trial.
†7 days of treatment
‡1 day of treatment
§daily treatment
Table 3 lists selected laboratory abnormalities in genital herpes suppression trials.
| Parameter | FAMVIR
(n=660)† % | Placebo
(n=210)† % |
| Anemia (<0.8 x NRL) | 0.1 | 0.0 |
| Leukopenia (<0.75 x NRL) | 1.3 | 0.9 |
| Neutropenia (<0.8 x NRL) | 3.2 | 1.5 |
| AST (SGOT) (>2 x NRH) | 2.3 | 1.2 |
| ALT (SGPT) (>2 x NRH) | 3.2 | 1.5 |
| Total Bilirubin (>1.5 x NRH) | 1.9 | 1.2 |
| Serum Creatinine (>1.5 x NRH) | 0.2 | 0.3 |
| Amylase (>1.5 x NRH) | 1.5 | 1.9 |
| Lipase (>1.5 x NRH) | 4.9 | 4.7 |
*Percentage of patients with laboratory abnormalities that were increased or decreased from baseline and were outside of specified ranges.
†n values represent the minimum number of patients assessed for each laboratory parameter.
NRH=Normal Range High.
NRL=Normal Range Low.
HIV-infected patients: In HIV-infected patients, the most frequently reported adverse events for FAMVIR (500 mg twice daily; n=150) and acyclovir (400 mg, 5x/day; n=143), respectively, were headache (17% vs. 15%), nausea (11% vs. 13%), diarrhea (7% vs. 11%), vomiting (5% vs. 4%), fatigue (4% vs. 2%), and abdominal pain (3% vs. 6%).
7. Drug Interactions
7.2 Potential for Other Drugs to Affect Penciclovir
No clinically significant alterations in penciclovir pharmacokinetics were observed following single-dose administration of 500 mg famciclovir after pretreatment with multiple doses of allopurinol, cimetidine, theophylline, zidovudine, promethazine, when given shortly after an antacid (magnesium and aluminum hydroxide), or concomitantly with emtricitabine. No clinically significant effect on penciclovir pharmacokinetics was observed following multiple-dose (three times daily) administration of famciclovir (500 mg) with multiple doses of digoxin.
Concurrent use with probenecid or other drugs significantly eliminated by active renal tubular secretion may result in increased plasma concentrations of penciclovir.
The conversion of 6-deoxy penciclovir to penciclovir is catalyzed by aldehyde oxidase. Interactions with other drugs metabolized by this enzyme and/or inhibiting this enzyme could potentially occur. Clinical interaction studies of famciclovir with cimetidine and promethazine, in vitro inhibitors of aldehyde oxidase, did not show relevant effects on the formation of penciclovir. Raloxifene, a potent aldehyde oxidase inhibitor in vitro, could decrease the formation of penciclovir. However, a clinical drug-drug interaction study to determine the magnitude of interaction between penciclovir and raloxifene has not been conducted.
8. Use In Specific Populations
8.3 Nursing Mothers
It is not known whether famciclovir (prodrug) or penciclovir (active drug) are excreted in human milk. Following oral administration of famciclovir to lactating rats, penciclovir was excreted in breast milk at concentrations higher than those seen in the plasma. There are no data on the safety of FAMVIR in infants. FAMVIR should not be used in nursing mothers unless the potential benefits are considered to outweigh the potential risks associated with treatment.
8.6 Patients with Renal Impairment
Apparent plasma clearance, renal clearance, and the plasma-elimination rate constant of penciclovir decreased linearly with reductions in renal function. After the administration of a single 500 mg famciclovir oral dose (n=27) to healthy volunteers and to volunteers with varying degrees of renal impairment (CLCR ranged from 6.4 to 138.8 mL/min), the following results were obtained (Table 4):
| Parameter
(mean ± S.D.) | CLCR† ≥60
(mL/min) (n=15) | CLCR 40-59
(mL/min) (n=5) | CLCR 20-39
(mL/min) (n=4) | CLCR <20
(mL/min) (n=3) |
| CLCR (mL/min) | 88.1 ± 20.6 | 49.3 ± 5.9 | 26.5 ± 5.3 | 12.7 ± 5.9 |
| CLR (L/hr) | 30.1 ± 10.6 | 13.0 ± 1.3‡ | 4.2 ± 0.9 | 1.6 ± 1.0 |
| CL/F§ (L/hr) | 66.9 ± 27.5 | 27.3 ± 2.8 | 12.8 ± 1.3 | 5.8 ± 2.8 |
| Half-life (hr) | 2.3 ± 0.5 | 3.4 ± 0.7 | 6.2 ± 1.6 | 13.4 ± 10.2 |
† CLCR is measured creatinine clearance.
‡ n=4.
§ CL/F consists of bioavailability factor and famciclovir to penciclovir conversion factor.
In a multiple-dose study of famciclovir conducted in subjects with varying degrees of renal impairment (n=18), the pharmacokinetics of penciclovir were comparable to those after single doses.
A dosage adjustment is recommended for patients with renal impairment [see Dosage and Administration (2.3)].
| FAMVIR
famciclovir tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| FAMVIR
famciclovir tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| FAMVIR
famciclovir tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |