Drug Detail:Farxiga (Dapagliflozin)
Drug Class: SGLT-2 inhibitors
Highlights of Prescribing Information
FARXIGA® (dapagliflozin) tablets, for oral use
Initial U.S. Approval: 2014
Indications and Usage for Farxiga
FARXIGA is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated:
- •
- As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
- •
- To reduce the risk of hospitalization for heart failure in adults with type 2 diabetes mellitus and either established cardiovascular disease or multiple cardiovascular risk factors. (1)
- •
- To reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure with reduced ejection fraction (NYHA class II-IV). (1)
- •
- To reduce the risk of sustained eGFR decline, end stage kidney disease, cardiovascular death, and hospitalization for heart failure in adults with chronic kidney disease at risk of progression. (1)
Limitations of use:
- •
- Not for treatment of type 1 diabetes mellitus. It may increase the risk of diabetic ketoacidosis in these patients. (1)
- •
- Not recommended for use to improve glycemic control in adults with type 2 diabetes mellitus with an eGFR less than 45 mL/min/1.73 m2. FARXIGA is likely to be ineffective in this setting based upon its mechanism of action. (1)
- •
- Not recommended for the treatment of chronic kidney disease in patients with polycystic kidney disease or patients requiring or with a recent history of immunosuppressive therapy for the treatment of kidney disease. FARXIGA is not expected to be effective in these populations. (1)
Farxiga Dosage and Administration
- •
- Assess volume status and correct volume depletion before initiating. (2.1)
|
eGFR
|
Recommended Dose |
|
eGFR 45 or greater |
To improve glycemic control, the recommended starting dose is 5 mg orally once daily. Dose can be increased to 10 mg orally once daily for additional glycemic control. For all other indications, the recommended starting dose is 10 mg orally once daily. |
|
eGFR 25 to less than 45 |
10 mg orally once daily |
|
eGFR less than 25 |
Initiation is not recommended, however patients may continue 10 mg orally once daily to reduce the risk of eGFR decline, ESKD, CV death and hHF. |
|
On dialysis |
Contraindicated |
Dosage Forms and Strengths
- •
- Tablets: 5 mg and 10 mg (3)
Contraindications
- •
- History of serious hypersensitivity reaction to FARXIGA. (4)
- •
- Patients on dialysis. (4)
Warnings and Precautions
- •
- Ketoacidosis in Patients with Diabetes Mellitus: Assess patients who present with signs and symptoms of metabolic acidosis for ketoacidosis regardless of blood glucose level. If suspected, discontinue FARXIGA, evaluate and treat promptly. Before initiating FARXIGA, consider risk factors for ketoacidosis. Patients on FARXIGA may require monitoring and temporary discontinuation of therapy in clinical situations known to predispose to ketoacidosis. (5.1)
- •
- Volume depletion: Before initiating FARXIGA, assess volume status and renal function in the elderly, patients with renal impairment or low systolic blood pressure, and in patients on diuretics. Monitor for signs and symptoms during therapy. (5.2, 6.1)
- •
- Urosepsis and Pyelonephritis: Evaluate for signs and symptoms of urinary tract infections and treat promptly, if indicated. (5.3)
- •
- Hypoglycemia: Consider a lower dose of insulin or the insulin secretagogue to reduce the risk of hypoglycemia when used in combination with FARXIGA. (5.4)
- •
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene): Serious, life-threatening cases have occurred in patients with diabetes, both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment. (5.5)
- •
- Genital Mycotic Infections: Monitor and treat if indicated. (5.6)
Adverse Reactions/Side Effects
- •
- Most common adverse reactions (5% or greater incidence) were female genital mycotic infections, nasopharyngitis, and urinary tract infections. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See full prescribing information for information on drug interactions and interference of FARXIGA with laboratory tests. (7)
Use In Specific Populations
- •
- Pregnancy: Advise females of the potential risk to a fetus especially during the second and third trimesters. (8.1)
- •
- Lactation: Not recommended when breastfeeding. (8.2)
- •
- Geriatrics: Higher incidence of adverse reactions related to hypotension. (5.2, 8.5)
- •
- Renal Impairment: Higher incidence of adverse reactions related to volume depletion. (5.2, 8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2023
Related/similar drugs
lisinopril, metoprolol, metformin, furosemide, carvedilol, spironolactone, warfarinFull Prescribing Information
1. Indications and Usage for Farxiga
FARXIGA (dapagliflozin) is indicated:
- •
- As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- •
- To reduce the risk of hospitalization for heart failure in adults with type 2 diabetes mellitus and either established cardiovascular disease or multiple cardiovascular risk factors.
- •
- To reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure (NYHA class II-IV) with reduced ejection fraction.
- •
- To reduce the risk of sustained eGFR decline, end-stage kidney disease, cardiovascular death, and hospitalization for heart failure in adults with chronic kidney disease at risk of progression.
Limitations of Use
- •
- FARXIGA is not recommended for patients with type 1 diabetes mellitus. It may increase the risk of diabetic ketoacidosis in these patients [see Warnings and Precautions (5.1)].
- •
- FARXIGA is not recommended for use to improve glycemic control in adults with type 2 diabetes mellitus with an eGFR less than 45 mL/min/1.73 m2. FARXIGA is likely to be ineffective in this setting based upon its mechanism of action.
- •
- FARXIGA is not recommended for the treatment of chronic kidney disease in patients with polycystic kidney disease or patients requiring or with a recent history of immunosuppressive therapy for kidney disease. FARXIGA is not expected to be effective in these populations.
2. Farxiga Dosage and Administration
2.1 Prior to Initiation of FARXIGA
Assess renal function prior to initiation of FARXIGA therapy and then as clinically indicated [see Warnings and Precautions (5.2)].
Assess volume status and, if necessary, correct volume depletion prior to initiation of FARXIGA [see Warnings and Precautions (5.2) and Use in Specific Populations (8.5, 8.6)].
2.2 Recommended Dosage
See Table 1 for dosage recommendations based on estimated glomerular filtration rate (eGFR).
|
|
|
eGFR
|
Recommended Dose |
|
eGFR 45 or greater |
To improve glycemic control, the recommended starting dose is 5 mg orally once daily. Dose can be increased to 10 mg orally once daily for additional glycemic control*. For all other indications, the recommended starting dose is 10 mg orally once daily. |
|
eGFR 25 to less than 45 |
10 mg orally once daily*. |
|
eGFR less than 25 |
Initiation is not recommended, however patients may continue 10 mg orally once daily to reduce the risk of eGFR decline, ESKD, CV death and hHF. |
|
On dialysis |
Contraindicated. |
|
hHF: hospitalization for heart failure, CV: Cardiovascular, ESKD: End Stage Kidney Disease. |
|
3. Dosage Forms and Strengths
- •
- FARXIGA 5 mg tablets are yellow, biconvex, round, film-coated tablets with “5” engraved on one side and “1427” engraved on the other side.
- •
- FARXIGA 10 mg tablets are yellow, biconvex, diamond-shaped, film-coated tablets with “10” engraved on one side and “1428” engraved on the other side.
4. Contraindications
- •
- History of a serious hypersensitivity reaction to FARXIGA, such as anaphylactic reactions or angioedema [see Adverse Reactions (6.1)].
- •
- Patients on dialysis [see Use in Specific Populations (8.6)].
5. Warnings and Precautions
5.1 Ketoacidosis in Patients with Diabetes Mellitus
Reports of ketoacidosis, a serious life-threatening condition requiring urgent hospitalization have been identified in patients with type 1 and type 2 diabetes mellitus receiving sodium-glucose cotransporter 2 (SGLT2) inhibitors, including FARXIGA [see Adverse Reactions (6.1)]. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was increased in patients who received SGLT2 inhibitors compared to patients who received placebo. Fatal cases of ketoacidosis have been reported in patients taking FARXIGA. FARXIGA is not indicated for the treatment of patients with type 1 diabetes mellitus [see Indications and Usage (1)].
Patients treated with FARXIGA who present with signs and symptoms consistent with severe metabolic acidosis should be assessed for ketoacidosis regardless of presenting blood glucose levels as ketoacidosis associated with FARXIGA may be present even if blood glucose levels are less than 250 mg/dL. If ketoacidosis is suspected, FARXIGA should be discontinued, the patient should be evaluated, and prompt treatment should be instituted. Treatment of ketoacidosis may require insulin, fluid, and carbohydrate replacement.
In many of the postmarketing reports, and particularly in patients with type 1 diabetes, the presence of ketoacidosis was not immediately recognized, and the institution of treatment was delayed because the presenting blood glucose levels were below those typically expected for diabetic ketoacidosis (often less than 250 mg/dL). Signs and symptoms at presentation were consistent with dehydration and severe metabolic acidosis and included nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. In some but not all cases, factors predisposing to ketoacidosis, such as insulin dose reduction, acute febrile illness, reduced caloric intake, surgery, pancreatic disorders suggesting insulin deficiency (e.g., type 1 diabetes, history of pancreatitis or pancreatic surgery), and alcohol abuse were identified.
Before initiating FARXIGA, consider factors in the patient history that may predispose to ketoacidosis, including pancreatic insulin deficiency from any cause, caloric restriction, and alcohol abuse.
For patients who undergo scheduled surgery, consider temporarily discontinuing FARXIGA for at least 3 days prior to surgery [see Clinical Pharmacology (12.2, 12.3)].
Consider monitoring for ketoacidosis and temporarily discontinuing FARXIGA in other clinical situations known to predispose to ketoacidosis (e.g., prolonged fasting due to acute illness or post-surgery). Ensure risk factors for ketoacidosis are resolved prior to restarting FARXIGA.
Educate patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue FARXIGA and seek medical attention immediately if signs and symptoms occur.
5.2 Volume Depletion
FARXIGA can cause intravascular volume depletion which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine. There have been post-marketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including FARXIGA. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2), elderly patients, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating FARXIGA in patients with one or more of these characteristics, assess volume status and renal function. Monitor for signs and symptoms of hypotension, and renal function after initiating therapy.
5.3 Urosepsis and Pyelonephritis
Serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization have been reported in patients receiving SGLT2 inhibitors, including FARXIGA. Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6)].
5.4 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Insulin and insulin secretagogues are known to cause hypoglycemia. FARXIGA may increase the risk of hypoglycemia when combined with insulin or an insulin secretagogue [see Adverse Reactions (6.1)]. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when these agents are used in combination with FARXIGA.
5.5 Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier’s Gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors, including FARXIGA. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with FARXIGA presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue FARXIGA, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
6. Adverse Reactions/Side Effects
The following important adverse reactions are described below and elsewhere in the labeling:
- •
- Ketoacidosis in Patients with Diabetes Mellitus [see Warnings and Precautions (5.1)]
- •
- Volume Depletion [see Warnings and Precautions (5.2)]
- •
- Urosepsis and Pyelonephritis [see Warnings and Precautions (5.3)]
- •
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues [see Warnings and Precautions (5.4)]
- •
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) [see Warnings and Precautions (5.5)]
- •
- Genital Mycotic Infections [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
FARXIGA has been evaluated in clinical trials in patients with type 2 diabetes mellitus, in patients with heart failure, and in patients with chronic kidney disease. The overall safety profile of FARXIGA was consistent across the studied indications. Severe hypoglycemia and diabetic ketoacidosis (DKA) were observed only in patients with diabetes mellitus.
Clinical Trials in Patients with Type 2 Diabetes Mellitus
Pool of 12 Placebo-Controlled Studies for FARXIGA 5 and 10 mg for Glycemic Control
The data in Table 2 is derived from 12 glycemic control placebo-controlled studies in patients with type 2 diabetes mellitus ranging from 12 to 24 weeks. In 4 studies FARXIGA was used as monotherapy, and in 8 studies FARXIGA was used as add-on to background antidiabetic therapy or as combination therapy with metformin [see Clinical Studies (14.1)].
These data reflect exposure of 2338 patients to FARXIGA with a mean exposure duration of 21 weeks. Patients received placebo (N=1393), FARXIGA 5 mg (N=1145), or FARXIGA 10 mg (N=1193) once daily. The mean age of the population was 55 years and 2% were older than 75 years of age. Fifty percent (50%) of the population were male; 81% were White, 14% were Asian, and 3% were Black or African American. At baseline, the population had diabetes for an average of 6 years, had a mean hemoglobin A1c (HbA1c) of 8.3%, and 21% had established microvascular complications of diabetes. Baseline renal function was normal or mildly impaired in 92% of patients and moderately impaired in 8% of patients (mean eGFR 86 mL/min/1.73 m2).
Table 2 shows common adverse reactions associated with the use of FARXIGA. These adverse reactions were not present at baseline, occurred more commonly on FARXIGA than on placebo, and occurred in at least 2% of patients treated with either FARXIGA 5 mg or FARXIGA 10 mg.
| Adverse Reaction | % of Patients | ||
|---|---|---|---|
| Pool of 12 Placebo-Controlled Studies | |||
| Placebo
N=1393 | FARXIGA 5 mg
N=1145 | FARXIGA 10 mg
N=1193 |
|
|
|||
|
Female genital mycotic infections* |
1.5 |
8.4 |
6.9 |
|
Nasopharyngitis |
6.2 |
6.6 |
6.3 |
|
Urinary tract infections† |
3.7 |
5.7 |
4.3 |
|
Back pain |
3.2 |
3.1 |
4.2 |
|
Increased urination‡ |
1.7 |
2.9 |
3.8 |
|
Male genital mycotic infections§ |
0.3 |
2.8 |
2.7 |
|
Nausea |
2.4 |
2.8 |
2.5 |
|
Influenza |
2.3 |
2.7 |
2.3 |
|
Dyslipidemia |
1.5 |
2.1 |
2.5 |
|
Constipation |
1.5 |
2.2 |
1.9 |
|
Discomfort with urination |
0.7 |
1.6 |
2.1 |
|
Pain in extremity |
1.4 |
2.0 |
1.7 |
Pool of 13 Placebo-Controlled Studies for FARXIGA 10 mg for Glycemic Control
FARXIGA 10 mg was also evaluated in a larger glycemic control placebo-controlled study pool in patients with type 2 diabetes mellitus. This pool combined 13 placebo-controlled studies, including 3 monotherapy studies, 9 add-on to background antidiabetic therapy studies, and an initial combination with metformin study. Across these 13 studies, 2360 patients were treated once daily with FARXIGA 10 mg for a mean duration of exposure of 22 weeks. The mean age of the population was 59 years and 4% were older than 75 years. Fifty-eight percent (58%) of the population were male; 84% were White, 9% were Asian, and 3% were Black or African American. At baseline, the population had diabetes for an average of 9 years, had a mean HbA1c of 8.2%, and 30% had established microvascular disease. Baseline renal function was normal or mildly impaired in 88% of patients and moderately impaired in 11% of patients (mean eGFR 82 mL/min/1.73 m2).
Volume Depletion
FARXIGA causes an osmotic diuresis, which may lead to a reduction in intravascular volume. Adverse reactions related to volume depletion (including reports of dehydration, hypovolemia, orthostatic hypotension, or hypotension) in patients with type 2 diabetes mellitus for the 12-study and 13-study, short-term, placebo-controlled pools and for the DECLARE study are shown in Table 3 [see Warnings and Precautions (5.2)].
|
|||||||
|
Pool of 12 Placebo-Controlled
|
Pool of 13 Placebo-Controlled
|
DECLARE Study |
|||||
|
Placebo |
FARXIGA
|
FARXIGA
|
Placebo |
FARXIGA
|
Placebo |
FARXIGA
|
|
|
Overall population N (%) |
N=1393 |
N=1145 |
N=1193 |
N=2295 |
N=2360 |
N=8569 |
N=8574 |
|
Patient Subgroup n (%) |
|||||||
|
Patients on loop diuretics |
n=55 |
n=40 |
n=31 |
n=267 |
n=236 |
n=934 |
n=866 |
|
Patients with moderate renal impairment with eGFR ≥30 and <60 mL/min/1.73 m2 |
n=107 |
n=107 |
n=89 |
n=268 |
n=265 |
n=658 |
n=604 |
|
Patients ≥65 years of age |
n=276 |
n=216 |
n=204 |
n=711 |
n=665 |
n=3950 |
n=3948 |
Hypoglycemia
The frequency of hypoglycemia by study in patients with type 2 diabetes mellitus [see Clinical Studies (14.1)] is shown in Table 4. Hypoglycemia was more frequent when FARXIGA was added to sulfonylurea or insulin [see Warnings and Precautions (5.4)].
| Placebo/Active Control | FARXIGA
5 mg | FARXIGA
10 mg |
|
|---|---|---|---|
|
|||
|
Monotherapy (24 weeks) |
N=75 |
N=64 |
N=70 |
|
Severe [n (%)] |
0 |
0 |
0 |
|
Glucose <54 mg/dL [n (%)] |
0 |
0 |
0 |
|
Add-on to Metformin (24 weeks) |
N=137 |
N=137 |
N=135 |
|
Severe [n (%)] |
0 |
0 |
0 |
|
Glucose <54 mg/dL [n (%)] |
0 |
0 |
0 |
|
Add-on to Glimepiride (24 weeks) |
N=146 |
N=145 |
N=151 |
|
Severe [n (%)] |
0 |
0 |
0 |
|
Glucose <54 mg/dL [n (%)] |
1 (0.7) |
3 (2.1) |
5 (3.3) |
|
Add-on to Metformin and a Sulfonylurea (24 Weeks) |
N=109 |
- |
N=109 |
|
Severe [n (%)] |
0 |
- |
0 |
|
Glucose <54 mg/dL [n (%)] |
3 (2.8) |
- |
7 (6.4) |
|
Add-on to Pioglitazone (24 weeks) |
N=139 |
N=141 |
N=140 |
|
Severe [n (%)] |
0 |
0 |
0 |
|
Glucose <54 mg/dL [n (%)] |
0 |
1 (0.7) |
0 |
|
Add-on to DPP4 inhibitor (24 weeks) |
N=226 |
– |
N=225 |
|
Severe [n (%)] |
0 |
– |
1 (0.4) |
|
Glucose <54 mg/dL [n (%)] |
1 (0.4) |
– |
1 (0.4) |
|
Add-on to Insulin with or without other OADs‡ (24 weeks) |
N=197 |
N=212 |
N=196 |
|
Severe [n (%)] |
1 (0.5) |
2 (0.9) |
2 (1.0) |
|
Glucose <54 mg/dL [n (%)] |
43 (21.8) |
55 (25.9) |
45 (23.0) |
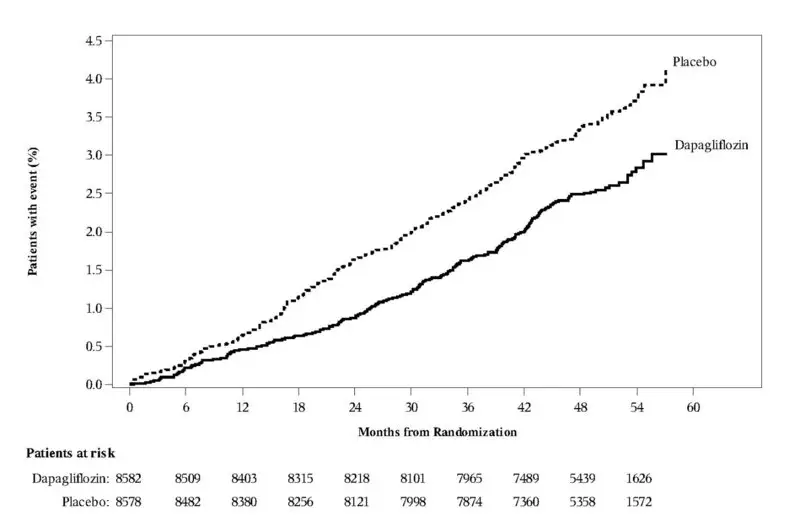
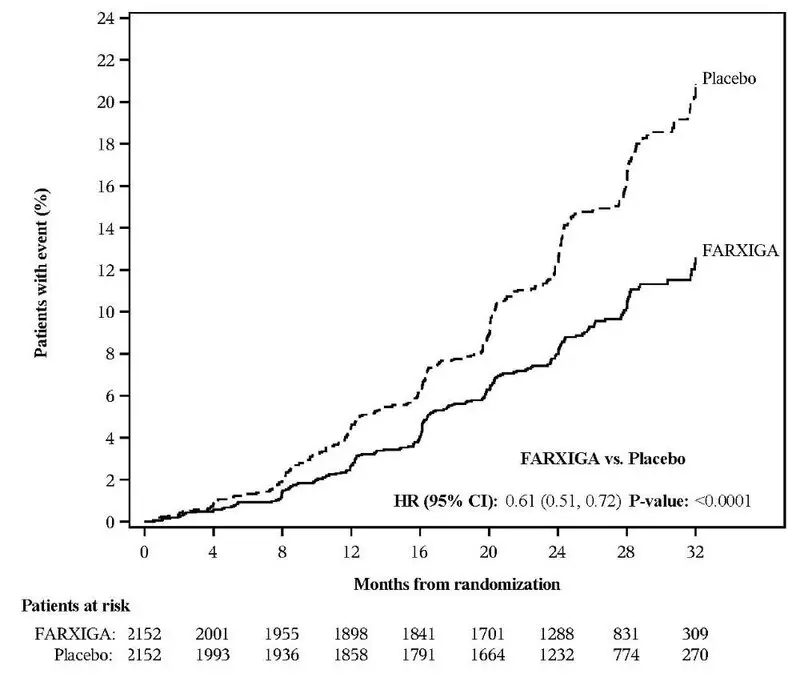
In the DECLARE study [see Clinical Studies (14.2)], severe events of hypoglycemia were reported in 58 (0.7%) out of 8574 patients treated with FARXIGA and 83 (1.0%) out of 8569 patients treated with placebo.
Genital Mycotic Infections
In the glycemic control trials, genital mycotic infections were more frequent with FARXIGA treatment. Genital mycotic infections were reported in 0.9% of patients on placebo, 5.7% on FARXIGA 5 mg, and 4.8% on FARXIGA 10 mg, in the 12-study placebo-controlled pool. Discontinuation from study due to genital infection occurred in 0% of placebo-treated patients and 0.2% of patients treated with FARXIGA 10 mg. Infections were more frequently reported in females than in males (see Table 2). The most frequently reported genital mycotic infections were vulvovaginal mycotic infections in females and balanitis in males. Patients with a history of genital mycotic infections were more likely to have a genital mycotic infection during the study than those with no prior history (10.0%, 23.1%, and 25.0% versus 0.8%, 5.9%, and 5.0% on placebo, FARXIGA 5 mg, and FARXIGA 10 mg, respectively). In the DECLARE study [see Clinical Studies (14.2)], serious genital mycotic infections were reported in <0.1% of patients treated with FARXIGA and <0.1% of patients treated with placebo. Genital mycotic infections that caused study drug discontinuation were reported in 0.9% of patients treated with FARXIGA and <0.1% of patients treated with placebo.
Hypersensitivity Reactions
Hypersensitivity reactions (e.g., angioedema, urticaria, hypersensitivity) were reported with FARXIGA treatment. In glycemic control studies, serious anaphylactic reactions and severe cutaneous adverse reactions and angioedema were reported in 0.2% of comparator-treated patients and 0.3% of FARXIGA-treated patients. If hypersensitivity reactions occur, discontinue use of FARXIGA; treat per standard of care and monitor until signs and symptoms resolve.
Ketoacidosis in Patients with Diabetes Mellitus
In the DECLARE study [see Warnings and Precautions (5.1) and Clinical Studies (14.2)], events of diabetic ketoacidosis (DKA) were reported in 27 out of 8574 patients in the FARXIGA-treated group and 12 out of 8569 patients in the placebo group. The events were evenly distributed over the study period.
Laboratory Tests
Increases in Serum Creatinine and Decreases in eGFR
Initiation of SGLT2 inhibitors, including FARXIGA causes a small increase in serum creatinine and decrease in eGFR. These changes in serum creatinine and eGFR generally occur within two weeks of starting therapy and then stabilize regardless of baseline kidney function. Changes that do not fit this pattern should prompt further evaluation to exclude the possibility of acute kidney injury [see Warnings and Precautions (5.2)]. In two studies that included patients with type 2 diabetes mellitus with moderate renal impairment, the acute effect on eGFR reversed after treatment discontinuation, suggesting acute hemodynamic changes may play a role in the renal function changes observed with FARXIGA.
Increase in Hematocrit
In the pool of 13 placebo-controlled studies of glycemic control, increases from baseline in mean hematocrit values were observed in FARXIGA-treated patients starting at Week 1 and continuing up to Week 16, when the maximum mean difference from baseline was observed. At Week 24, the mean changes from baseline in hematocrit were −0.33% in the placebo group and 2.30% in the FARXIGA 10 mg group. By Week 24, hematocrit values >55% were reported in 0.4% of placebo-treated patients and 1.3% of FARXIGA 10 mg-treated patients.
Increase in Low-Density Lipoprotein Cholesterol
In the pool of 13 placebo-controlled studies of glycemic control, changes from baseline in mean lipid values were reported in FARXIGA-treated patients compared to placebo-treated patients. Mean percent changes from baseline at Week 24 were 0.0% versus 2.5% for total cholesterol, and -1.0% versus 2.9% for LDL cholesterol in the placebo and FARXIGA 10 mg groups, respectively. In the DECLARE study [see Clinical Studies (14.2)], mean changes from baseline after 4 years were 0.4 mg/dL versus -4.1 mg/dL for total cholesterol, and -2.5 mg/dL versus -4.4 mg/dL for LDL cholesterol, in FARXIGA-treated and the placebo groups, respectively.
Decrease in Serum Bicarbonate
In a study of concomitant therapy of FARXIGA 10 mg with exenatide extended-release (on a background of metformin), four patients (1.7%) on concomitant therapy had a serum bicarbonate value of less than or equal to 13 mEq/L compared to one each (0.4%) in the FARXIGA and exenatide-extended release treatment groups [see Warnings and Precautions (5.1)].
DAPA-HF Heart Failure Study
No new adverse reactions were identified in the DAPA-HF heart failure study.
DAPA-CKD Chronic Kidney Disease Study
No new adverse reactions were identified in the DAPA-CKD study in patients with chronic kidney disease.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of FARXIGA in patients with diabetes mellitus. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Ketoacidosis
- •
- Acute Kidney Injury
- •
- Urosepsis and Pyelonephritis
- •
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
- •
- Rash
7. Drug Interactions
|
Insulin or Insulin Secretagogues |
|
|
Clinical Impact |
The risk of hypoglycemia may be increased when FARXIGA is used concomitantly with insulin or insulin secretagogues (e.g., sulfonylurea) [see Warnings and Precautions (5.4)]. |
|
Intervention |
Concomitant use may require lower doses of insulin or the insulin secretagogue to reduce the risk of hypoglycemia. |
|
Lithium |
|
|
Clinical Impact |
Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. |
|
Intervention |
Monitor serum lithium concentration more frequently during FARXIGA initiation and dosage changes. |
|
Positive Urine Glucose Test |
|
|
Clinical Impact |
SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. |
|
Intervention |
Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
|
Interference with 1,5-anhydroglucitol (1,5-AG) Assay |
|
|
Clinical Impact |
Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. |
|
Intervention |
Monitoring glycemic control with 1,5-AG assay is not recommended. Use alternative methods to monitor glycemic control. |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on animal data showing adverse renal effects, FARXIGA is not recommended during the second and third trimesters of pregnancy.
Limited data with FARXIGA in pregnant women are not sufficient to determine drug-associated risk for major birth defects or miscarriage. There are risks to the mother and fetus associated with poorly controlled diabetes and untreated heart failure in pregnancy (see Clinical Considerations).
In animal studies, adverse renal pelvic and tubule dilatations, that were not fully reversible, were observed in rats when dapagliflozin was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy, at all doses tested; the lowest of which provided an exposure 15-times the 10 mg clinical dose (see Data).
The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational diabetes with a HbA1c greater than 7% and has been reported to be as high as 20 to 25% in women with HbA1c greater than 10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Animal Data
Dapagliflozin dosed directly to juvenile rats from postnatal day (PND) 21 until PND 90 at doses of 1, 15, or 75 mg/kg/day, increased kidney weights and increased the incidence of renal pelvic and tubular dilatations at all dose levels. Exposure at the lowest dose tested was 15-times the 10 mg clinical dose (based on AUC). The renal pelvic and tubular dilatations observed in juvenile animals did not fully reverse within a 1-month recovery period.
In a prenatal and postnatal development study, dapagliflozin was administered to maternal rats from gestation day 6 through lactation day 21 at doses of 1, 15, or 75 mg/kg/day, and pups were indirectly exposed in utero and throughout lactation. Increased incidence or severity of renal pelvic dilatation was observed in 21-day-old pups offspring of treated dams at 75 mg/kg/day (maternal and pup dapagliflozin exposures were 1415-times and 137-times, respectively, the human values at the 10 mg clinical dose, based on AUC). Dose-related reductions in pup body weights were observed at greater or equal to 29-times the 10 mg clinical dose (based on AUC). No adverse effects on developmental endpoints were noted at 1 mg/kg/day (19-times the 10 mg clinical dose, based on AUC). These outcomes occurred with drug exposure during periods of renal development in rats that corresponds to the late second and third trimester of human development.
In embryofetal development studies in rats and rabbits, dapagliflozin was administered throughout organogenesis, corresponding to the first trimester of human pregnancy. In rats, dapagliflozin was neither embryolethal nor teratogenic at doses up to 75 mg/kg/day (1441-times the 10 mg clinical dose, based on AUC). Dose related effects on the rat fetus (structural abnormalities and reduced body weight) occurred only at higher dosages, equal to or greater than 150 mg/kg (more than 2344-times the 10 mg clinical dose, based on AUC), which were associated with maternal toxicity. No developmental toxicities were observed in rabbits at doses up to 180 mg/kg/day (1191-times the 10 mg clinical dose, based on AUC).
8.2 Lactation
Risk Summary
There is no information regarding the presence of dapagliflozin in human milk, the effects on the breastfed infant, or the effects on milk production. Dapagliflozin is present in the milk of lactating rats (see Data). However, due to species-specific differences in lactation physiology, the clinical relevance of these data are not clear. Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney.
Because of the potential for serious adverse reactions in breastfed infants, advise women that use of FARXIGA is not recommended while breastfeeding.
Data
Dapagliflozin was present in rat milk at a milk/plasma ratio of 0.49, indicating that dapagliflozin and its metabolites are transferred into milk at a concentration that is approximately 50% of that in maternal plasma. Juvenile rats directly exposed to dapagliflozin showed risk to the developing kidney (renal pelvic and tubular dilatations) during maturation.
8.4 Pediatric Use
Safety and effectiveness of FARXIGA in pediatric patients under 18 years of age have not been established.
8.5 Geriatric Use
No FARXIGA dosage change is recommended based on age.
A total of 1424 (24%) of the 5936 FARXIGA-treated patients were 65 years and older and 207 (3.5%) patients were 75 years and older in a pool of 21 double-blind, controlled, clinical studies assessing the efficacy of FARXIGA in improving glycemic control in type 2 diabetes mellitus. After controlling for level of renal function (eGFR), efficacy was similar for patients under age 65 years and those 65 years and older. In patients ≥65 years of age, a higher proportion of patients treated with FARXIGA for glycemic control had adverse reactions of hypotension [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
In both the DAPA-HF and DAPA-CKD studies, safety and efficacy were similar for patients age 65 years and younger and those older than 65. In the DAPA-HF study, 2714 (57%) out of 4744 patients with HFrEF were older than 65 years. In the DAPA-CKD study, 1818 (42%) out of 4304 patients with CKD were older than 65 years.
8.6 Renal Impairment
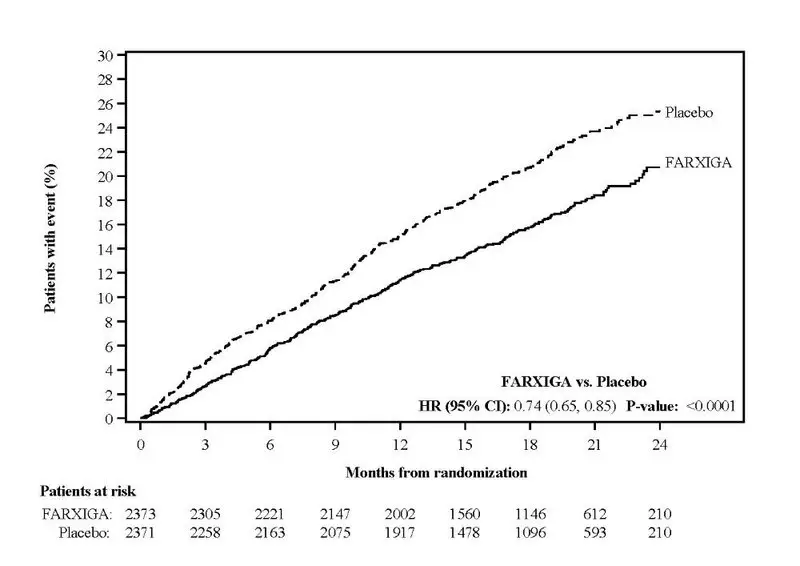
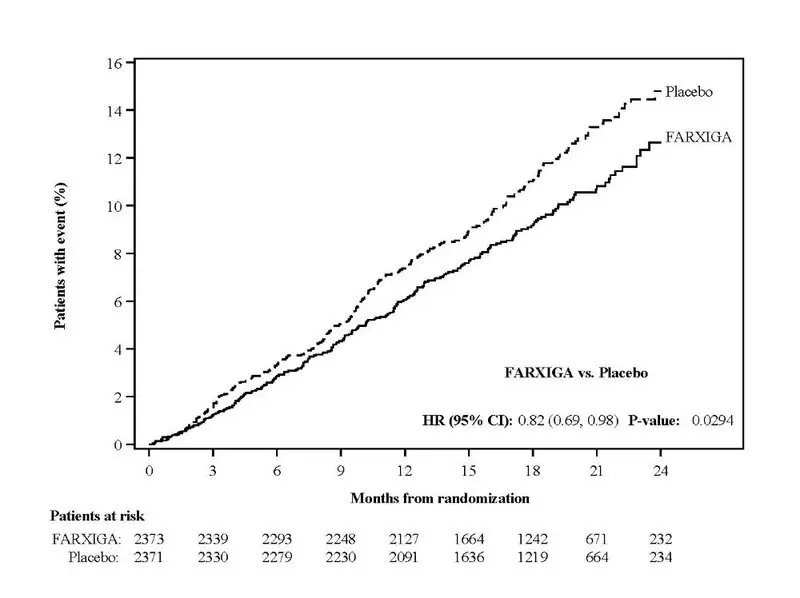
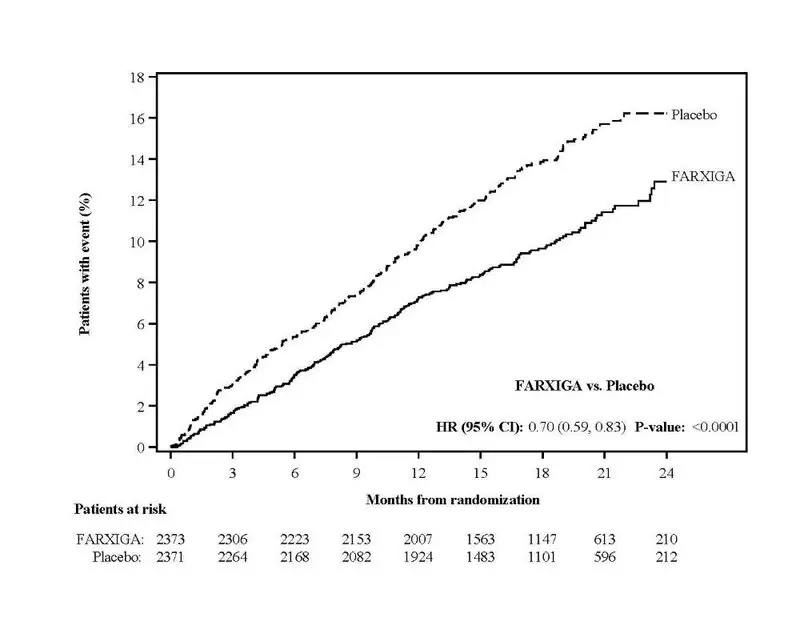
FARXIGA was evaluated in 4304 patients with chronic kidney disease (eGFR 25 to 75 mL/min/1.73 m2) in the DAPA-CKD study. FARXIGA was also evaluated in 1926 patients with an eGFR of 30 to 60 mL/min/1.73 m2 in the DAPA-HF study. The safety profile of FARXIGA across eGFR subgroups in these studies was consistent with the known safety profile [see Adverse Reactions (6.1) and Clinical Studies (14.3 and 14.4)].
FARXIGA was evaluated in two glycemic control studies that included patients with type 2 diabetes mellitus with moderate renal impairment (an eGFR of 45 to less than 60 mL/min/1.73 m2 [see Clinical Studies (14.1)], and an eGFR of 30 to less than 60 mL/min/1.73 m2, respectively). Patients with diabetes and renal impairment using FARXIGA may be more likely to experience hypotension and may be at higher risk for acute kidney injury secondary to volume depletion. In the study of patients with an eGFR 30 to less than 60 mL/min/1.73 m2, 13 patients receiving FARXIGA experienced bone fractures compared to none receiving placebo. Use of FARXIGA for glycemic control in patients without established CV disease or CV risk factors is not recommended when eGFR is less than 45 mL/min/1.73 m2 [see Dosage and Administration (2.2)].
Efficacy and safety studies with FARXIGA did not enroll patients with an eGFR less than 25 mL/min/1.73 m2. FARXIGA is contraindicated in patients on dialysis.
8.7 Hepatic Impairment
No dose adjustment is recommended for patients with mild, moderate, or severe hepatic impairment. However, the benefit-risk for the use of dapagliflozin in patients with severe hepatic impairment should be individually assessed since the safety and efficacy of dapagliflozin have not been specifically studied in this population [see Clinical Pharmacology (12.3)].
10. Overdosage
There were no reports of overdose during the clinical development program for FARXIGA.
In the event of an overdose, contact the Poison Control Center. It is also reasonable to employ supportive measures as dictated by the patient’s clinical status. The removal of dapagliflozin by hemodialysis has not been studied.
11. Farxiga Description
Dapagliflozin is described chemically as D-glucitol, 1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-, (1S)-, compounded with (2S)-1,2-propanediol, hydrate (1:1:1). The empirical formula is C21H25ClO6•C3H8O2•H2O and the molecular weight is 502.98. The structural formula is:
FARXIGA is available as a film-coated tablet for oral administration containing the equivalent of 5 mg dapagliflozin as dapagliflozin propanediol or the equivalent of 10 mg dapagliflozin as dapagliflozin propanediol, and the following inactive ingredients: microcrystalline cellulose, anhydrous lactose, crospovidone, silicon dioxide, and magnesium stearate. In addition, the film coating contains the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and yellow iron oxide.
12. Farxiga - Clinical Pharmacology
12.1 Mechanism of Action
Sodium-glucose cotransporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Dapagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and thereby promotes urinary glucose excretion. Dapagliflozin also reduces sodium reabsorption and increases the delivery of sodium to the distal tubule. This may influence several physiological functions including, but not restricted to, lowering both pre- and afterload of the heart and downregulation of sympathetic activity, and decreased intraglomerular pressure which is believed to be mediated by increased tubuloglomerular feedback.
12.2 Pharmacodynamics
General
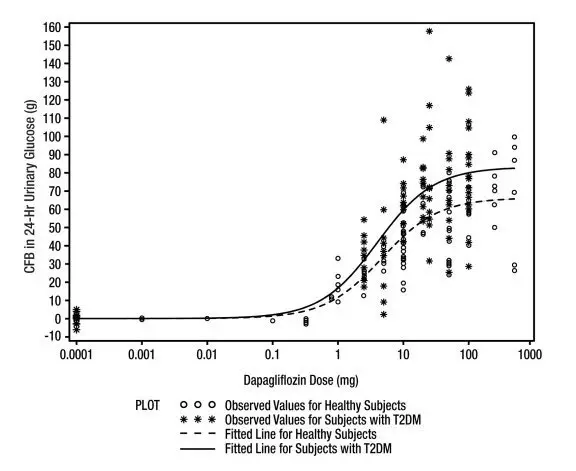
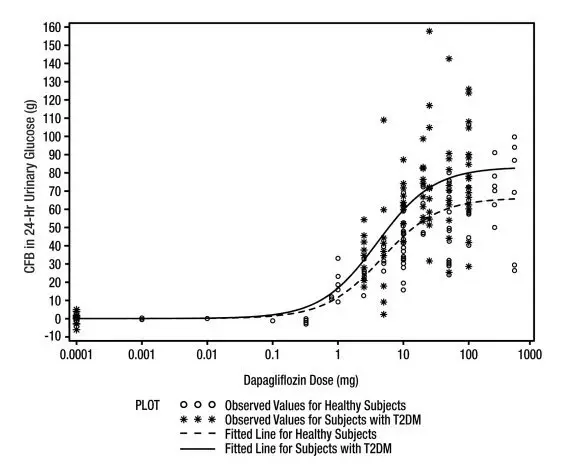
Increases in the amount of glucose excreted in the urine were observed in healthy subjects and in patients with type 2 diabetes mellitus following the administration of dapagliflozin (see Figure 1). Dapagliflozin doses of 5 or 10 mg per day in patients with type 2 diabetes mellitus for 12 weeks resulted in excretion of approximately 70 grams of glucose in the urine per day at Week 12. A near maximum glucose excretion was observed at the dapagliflozin daily dose of 20 mg. This urinary glucose excretion with dapagliflozin also results in increases in urinary volume [see Adverse Reactions (6.1)]. After discontinuation of dapagliflozin, on average, the elevation in urinary glucose excretion approaches baseline by about 3 days for the 10 mg dose.
Figure 1: Scatter Plot and Fitted Line of Change from Baseline in 24-Hour Urinary Glucose Amount versus Dapagliflozin Dose in Healthy Subjects and Subjects with Type 2 Diabetes Mellitus (T2DM) (Semi-Log Plot)
Cardiac Electrophysiology
Dapagliflozin was not associated with clinically meaningful prolongation of QTc interval at daily doses up to 150 mg (15-times the recommended maximum dose) in a study of healthy subjects. In addition, no clinically meaningful effect on QTc interval was observed following single doses of up to 500 mg (50-times the recommended maximum dose) of dapagliflozin in healthy subjects.
12.3 Pharmacokinetics
Absorption
Following oral administration of dapagliflozin, the maximum plasma concentration (Cmax) is usually attained within 2 hours under fasting state. The Cmax and AUC values increase dose proportionally with increase in dapagliflozin dose in the therapeutic dose range. The absolute oral bioavailability of dapagliflozin following the administration of a 10 mg dose is 78%. Administration of dapagliflozin with a high-fat meal decreases its Cmax by up to 50% and prolongs Tmax by approximately 1 hour, but does not alter AUC as compared with the fasted state. These changes are not considered to be clinically meaningful and dapagliflozin can be administered with or without food.
Distribution
Dapagliflozin is approximately 91% protein bound. Protein binding is not altered in patients with renal or hepatic impairment.
Metabolism
The metabolism of dapagliflozin is primarily mediated by UGT1A9; CYP-mediated metabolism is a minor clearance pathway in humans. Dapagliflozin is extensively metabolized, primarily to yield dapagliflozin 3-O-glucuronide, which is an inactive metabolite. Dapagliflozin 3-O-glucuronide accounted for 61% of a 50 mg [14C]-dapagliflozin dose and is the predominant drug-related component in human plasma.
Elimination
Dapagliflozin and related metabolites are primarily eliminated via the renal pathway. Following a single 50 mg dose of [14C]-dapagliflozin, 75% and 21% total radioactivity is excreted in urine and feces, respectively. In urine, less than 2% of the dose is excreted as parent drug. In feces, approximately 15% of the dose is excreted as parent drug. The mean plasma terminal half-life (t½) for dapagliflozin is approximately 12.9 hours following a single oral dose of FARXIGA 10 mg.
Specific Populations
Renal Impairment
At steady-state (20 mg once daily dapagliflozin for 7 days), patients with type 2 diabetes with mild, moderate, or severe renal impairment (as determined by eGFR) had geometric mean systemic exposures of dapagliflozin that were 45%, 100%, and 200% higher, respectively, as compared to patients with type 2 diabetes mellitus with normal renal function. There was no meaningful difference in exposure between patients with chronic kidney disease with and without type 2 diabetes. Higher systemic exposure of dapagliflozin in patients with type 2 diabetes mellitus with renal impairment did not result in a correspondingly higher 24-hour urinary glucose excretion. The steady-state 24-hour urinary glucose excretion in patients with type 2 diabetes mellitus and mild, moderate, and severe renal impairment was 42%, 80%, and 90% lower, respectively, than in patients with type 2 diabetes mellitus with normal renal function.
The impact of hemodialysis on dapagliflozin exposure is not known [see Dosage and Administration (2.2), Warnings and Precautions (5.2), Use in Specific Populations (8.6), and Clinical Studies (14)].
Hepatic Impairment
In subjects with mild and moderate hepatic impairment (Child-Pugh classes A and B), mean Cmax and AUC of dapagliflozin were up to 12% and 36% higher, respectively, as compared to healthy matched control subjects following single-dose administration of 10 mg dapagliflozin. These differences were not considered to be clinically meaningful. In patients with severe hepatic impairment (Child-Pugh class C), mean Cmax and AUC of dapagliflozin were up to 40% and 67% higher, respectively, as compared to healthy matched controls [see Use in Specific Populations (8.7)].
Drug Interactions
In Vitro Assessment of Drug Interactions
In in vitro studies, dapagliflozin and dapagliflozin 3-O-glucuronide neither inhibited CYP 1A2, 2C9, 2C19, 2D6, or 3A4, nor induced CYP 1A2, 2B6, or 3A4. Dapagliflozin is a weak substrate of the P-glycoprotein (P-gp) active transporter, and dapagliflozin 3-O-glucuronide is a substrate for the OAT3 active transporter. Dapagliflozin or dapagliflozin 3-O-glucuronide did not meaningfully inhibit P-gp, OCT2, OAT1, or OAT3 active transporters. Overall, dapagliflozin is unlikely to affect the pharmacokinetics of concurrently administered medications that are P-gp, OCT2, OAT1, or OAT3 substrates.
Effects of Other Drugs on Dapagliflozin
Table 6 shows the effect of coadministered drugs on the pharmacokinetics of dapagliflozin. No dose adjustments are recommended for dapagliflozin.
| Coadministered Drug
(Dose Regimen)* | Dapagliflozin
(Dose Regimen)* | Effect on Dapagliflozin Exposure
(% Change [90% CI]) |
|
|---|---|---|---|
| Cmax | AUC† | ||
|
|||
|
No dosing adjustments required for the following: |
|||
|
Oral Antidiabetic Agents |
|||
|
Metformin (1000 mg) |
20 mg |
↔ |
↔ |
|
Pioglitazone (45 mg) |
50 mg |
↔ |
↔ |
|
Sitagliptin (100 mg) |
20 mg |
↔ |
↔ |
|
Glimepiride (4 mg) |
20 mg |
↔ |
↔ |
|
Voglibose (0.2 mg three times daily) |
10 mg |
↔ |
↔ |
|
Other Medications |
|||
|
Hydrochlorothiazide (25 mg) |
50 mg |
↔ |
↔ |
|
Bumetanide (1 mg) |
10 mg once daily |
↔ |
↔ |
|
Valsartan (320 mg) |
20 mg |
↓12% |
↔ |
|
Simvastatin (40 mg) |
20 mg |
↔ |
↔ |
|
Anti-infective Agent |
|||
|
Rifampin (600 mg once daily for 6 days) |
10 mg |
↓7% |
↓22% |
|
Nonsteroidal Anti-inflammatory Agent |
|||
|
Mefenamic Acid (loading dose of 500 mg followed by 14 doses of 250 mg every 6 hours) |
10 mg |
↑13% |
↑51% |
|
↔ = no change (geometric mean ratio of test: reference within 0.80 to 1.25); ↓ or ↑ = parameter was lower or higher, respectively, with coadministration compared to dapagliflozin administered alone (geometric mean ratio of test: reference was lower than 0.80 or higher than 1.25) |
|||
Effects of Dapagliflozin on Other Drugs
Table 7 shows the effect of dapagliflozin on other coadministered drugs. Dapagliflozin did not meaningfully affect the pharmacokinetics of the coadministered drugs.
|
|||
|
Coadministered Drug
|
Dapagliflozin
|
Effect on Coadministered Drug Exposure
|
|
|
Cmax |
AUC† |
||
|
No dosing adjustments required for the following: |
|||
|
Oral Antidiabetic Agents |
|||
|
Metformin (1000 mg) |
20 mg |
↔ |
↔ |
|
Pioglitazone (45 mg) |
50 mg |
↓7% |
↔ |
|
Sitagliptin (100 mg) |
20 mg |
↔ |
↔ |
|
Glimepiride (4 mg) |
20 mg |
↔ |
↑13% |
|
Other Medications |
|||
|
Hydrochlorothiazide (25 mg) |
50 mg |
↔ |
↔ |
|
Bumetanide (1 mg) |
10 mg once daily |
↑13% |
↑13% |
|
Valsartan (320 mg) |
20 mg |
↓6% |
↑5% |
|
Simvastatin (40 mg) |
20 mg |
↔ |
↑19% |
|
Digoxin (0.25 mg) |
20 mg loading dose |
↔ |
↔ |
|
Warfarin (25 mg) |
20 mg loading dose |
↔ |
↔ |
|
↔ = no change (geometric mean ratio of test: reference within 0.80 to 1.25); ↓ or ↑ = parameter was lower or higher, respectively, with coadministration compared to the other medicine administered alone (geometric mean ratio of test: reference was lower than 0.80 or higher than 1.25). |
|||
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dapagliflozin did not induce tumors in either mice or rats at any of the doses evaluated in 2-year carcinogenicity studies. Oral doses in mice consisted of 5, 15, and 40 mg/kg/day in males and 2, 10, and 20 mg/kg/day in females, and oral doses in rats were 0.5, 2, and 10 mg/kg/day for both males and females. The highest doses evaluated in mice were approximately 72-times (males) and 105-times (females) the clinical dose of 10 mg per day, based on AUC exposure. In rats, the highest dose was approximately 131-times (males) and 186-times (females) the clinical dose of 10 mg per day, based on AUC exposure.
Dapagliflozin was negative in the Ames mutagenicity assay and was positive in a series of in vitro clastogenicity assays in the presence of S9 activation and at concentrations greater than or equal to 100 μg/mL. Dapagliflozin was negative for clastogenicity in a series of in vivo studies evaluating micronuclei or DNA repair in rats at exposure multiples greater than 2100-times the clinical dose.
There was no carcinogenicity or mutagenicity signal in animal studies, suggesting that dapagliflozin does not represent a genotoxic risk to humans.
Dapagliflozin had no effects on mating, fertility, or early embryonic development in treated male or female rats at exposure multiples less than or equal to 1708-times and 998-times the maximum recommended human dose in males and females, respectively.
14. Clinical Studies
14.1 Glycemic Control in Patients with Type 2 Diabetes Mellitus
Overview of Clinical Studies of FARXIGA for Type 2 Diabetes Mellitus
FARXIGA has been studied as monotherapy, in combination with metformin, pioglitazone, sulfonylurea (glimepiride), sitagliptin (with or without metformin), metformin plus a sulfonylurea, or insulin (with or without other oral antidiabetic therapy), compared to a sulfonylurea (glipizide), and in combination with a GLP-1 receptor agonist (exenatide extended-release) added-on to metformin. FARXIGA has also been studied in patients with type 2 diabetes mellitus and moderate renal impairment.
Treatment with FARXIGA as monotherapy and in combination with metformin, glimepiride, pioglitazone, sitagliptin, or insulin produced statistically significant improvements in mean change from baseline at Week 24 in HbA1c compared to control. Reductions in HbA1c were seen across subgroups including gender, age, race, duration of disease, and baseline body mass index (BMI).
Monotherapy
A total of 840 treatment-naive patients with inadequately controlled type 2 diabetes mellitus participated in 2 placebo-controlled studies to evaluate the safety and efficacy of monotherapy with FARXIGA.
In 1 monotherapy study, a total of 558 treatment-naive patients with inadequately controlled diabetes participated in a 24-week study (NCT00528372). Following a 2-week diet and exercise placebo lead-in period, 485 patients with HbA1c ≥7% and ≤10% were randomized to FARXIGA 5 mg or FARXIGA 10 mg once daily in either the morning (QAM, main cohort) or evening (QPM), or placebo.
At Week 24, treatment with FARXIGA 10 mg QAM provided significant improvements in HbA1c and the fasting plasma glucose (FPG) compared with placebo (see Table 8).
| Efficacy Parameter | FARXIGA
10 mg N=70† | FARXIGA
5 mg N=64† | Placebo
N=75† |
|---|---|---|---|
|
|||
|
HbA1c (%) |
|||
|
Baseline (mean) |
8.0 |
7.8 |
7.8 |
|
Change from baseline (adjusted mean‡) |
−0.9 |
−0.8 |
−0.2 |
|
Difference from placebo (adjusted mean‡) |
−0.7§
|
−0.5 | |
|
Percent of patients achieving HbA1c <7% |
50.8%¶ |
44.2%¶ |
31.6% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
166.6 |
157.2 |
159.9 |
|
Change from baseline (adjusted mean‡) |
−28.8 |
−24.1 |
−4.1 |
|
Difference from placebo (adjusted mean‡) |
−24.7§
|
−19.9 | |
Initial Combination Therapy with Metformin XR
A total of 1236 treatment-naive patients with inadequately controlled type 2 diabetes mellitus (HbA1c ≥7.5% and ≤12%) participated in 2 active-controlled studies of 24-week duration to evaluate initial therapy with FARXIGA 5 mg (NCT00643851) or 10 mg (NCT00859898) in combination with metformin extended-release (XR) formulation.
In 1 study, 638 patients randomized to 1 of 3 treatment arms following a 1-week lead-in period received: FARXIGA 10 mg plus metformin XR (up to 2000 mg per day), FARXIGA 10 mg plus placebo, or metformin XR (up to 2000 mg per day) plus placebo. Metformin XR dose was up-titrated weekly in 500 mg increments, as tolerated, with a median dose achieved of 2000 mg.
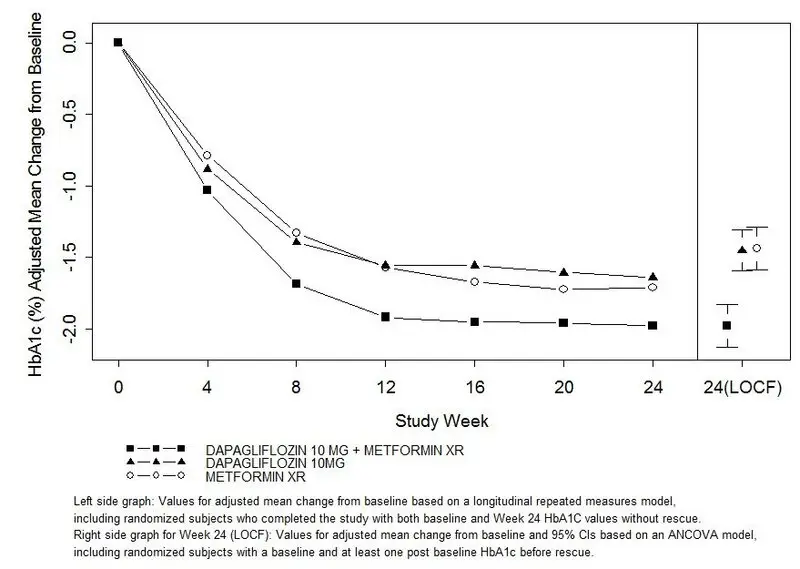
The combination treatment of FARXIGA 10 mg plus metformin XR provided statistically significant improvements in HbA1c and FPG compared with either of the monotherapy treatments and statistically significant reduction in body weight compared with metformin XR alone (see Table 9 and Figure 2). FARXIGA 10 mg as monotherapy also provided statistically significant improvements in FPG and statistically significant reduction in body weight compared with metformin alone and was noninferior to metformin XR monotherapy in lowering HbA1c.
| Efficacy Parameter | FARXIGA
10 mg + Metformin XR | FARXIGA
10 mg | Metformin
XR |
|---|---|---|---|
| N=211† | N=219† | N=208† | |
|
|||
|
HbA1c (%) |
|||
|
Baseline (mean) |
9.1 |
9.0 |
9.0 |
|
Change from baseline (adjusted mean‡) |
−2.0 |
−1.5 |
−1.4 |
|
Difference from FARXIGA (adjusted mean‡) |
−0.5§
| ||
|
Difference from metformin XR (adjusted mean‡) |
−0.5§
|
0.0¶
| |
|
Percent of patients achieving HbA1c <7% |
46.6%# |
31.7% |
35.2% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
189.6 |
197.5 |
189.9 |
|
Change from baseline (adjusted mean‡) |
−60.4 |
−46.4 |
−34.8 |
|
Difference from FARXIGA (adjusted mean‡) |
−13.9§
| ||
|
Difference from metformin XR (adjusted mean‡) |
−25.5§
|
−11.6#
| |
|
Body Weight (kg) |
|||
|
Baseline (mean) |
88.6 |
88.5 |
87.2 |
|
Change from baseline (adjusted mean‡) |
−3.3 |
−2.7 |
−1.4 |
|
Difference from metformin XR (adjusted mean‡) |
−2.0§
|
−1.4§ (−2.0, −0.7) | |
Figure 2: Adjusted Mean Change from Baseline Over Time in HbA1c (%) in a 24-Week Active-Controlled Study of FARXIGA Initial Combination Therapy with Metformin XR
In a second study, 603 patients were randomized to 1 of 3 treatment arms following a 1-week lead-in period: FARXIGA 5 mg plus metformin XR (up to 2000 mg per day), FARXIGA 5 mg plus placebo, or metformin XR (up to 2000 mg per day) plus placebo. Metformin XR dose was up-titrated weekly in 500 mg increments, as tolerated, with a median dose achieved of 2000 mg.
The combination treatment of FARXIGA 5 mg plus metformin XR provided statistically significant improvements in HbA1c and FPG compared with either of the monotherapy treatments and statistically significant reduction in body weight compared with metformin XR alone (see Table 10).
| Efficacy Parameter | FARXIGA
5 mg + Metformin XR | FARXIGA
5 mg | Metformin
XR |
|---|---|---|---|
| N=194† | N=203† | N=201† | |
|
|||
|
HbA1c (%) |
|||
|
Baseline (mean) |
9.2 |
9.1 |
9.1 |
|
Change from baseline (adjusted mean‡) |
−2.1 |
−1.2 |
−1.4 |
|
Difference from FARXIGA (adjusted mean‡) |
−0.9§
| ||
|
Difference from metformin XR (adjusted mean‡) |
−0.7§
| ||
|
Percent of patients achieving HbA1c <7% |
52.4%¶ |
22.5% |
34.6% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
193.4 |
190.8 |
196.7 |
|
Change from baseline (adjusted mean‡) |
-61.0 |
-42.0 |
-33.6 |
|
Difference from FARXIGA (adjusted mean‡) |
-19.1§
| ||
|
Difference from metformin XR (adjusted mean‡) |
-27.5§
| ||
|
Body Weight (kg) |
|||
|
Baseline (mean) |
84.2 |
86.2 |
85.8 |
|
Change from baseline (adjusted mean‡) |
-2.7 |
-2.6 |
-1.3 |
|
Difference from metformin XR (adjusted mean‡) |
-1.4§
| ||
Add-On to Metformin
A total of 546 patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c ≥7% and ≤10%) participated in a 24-week, placebo-controlled study to evaluate FARXIGA in combination with metformin (NCT00528879). Patients on metformin at a dose of at least 1500 mg per day were randomized after completing a 2-week, single-blind, placebo lead-in period. Following the lead-in period, eligible patients were randomized to FARXIGA 5 mg, FARXIGA 10 mg, or placebo in addition to their current dose of metformin.
As add-on treatment to metformin, FARXIGA 10 mg provided statistically significant improvements in HbA1c and FPG, and statistically significant reduction in body weight compared with placebo at Week 24 (see Table 11 and Figure 3). Statistically significant (p <0.05 for both doses) mean changes from baseline in systolic blood pressure relative to placebo plus metformin were -4.5 mmHg and -5.3 mmHg with FARXIGA 5 mg and 10 mg plus metformin, respectively.
| Efficacy Parameter | FARXIGA 10 mg
+ Metformin N=135† | FARXIGA 5 mg
+ Metformin N=137† | Placebo
+ Metformin N=137† |
|---|---|---|---|
|
|||
|
HbA1c (%) |
|||
|
Baseline (mean) |
7.9 |
8.2 |
8.1 |
|
Change from baseline (adjusted mean‡) |
-0.8 |
-0.7 |
-0.3 |
|
Difference from placebo (adjusted mean‡) |
-0.5§ (-0.7, -0.3) |
-0.4§ (-0.6, -0.2) | |
|
Percent of patients achieving HbA1c <7% |
40.6%¶ |
37.5%¶ |
25.9% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
156.0 |
169.2 |
165.6 |
|
Change from baseline at Week 24 (adjusted mean‡) |
-23.5 |
-21.5 |
-6.0 |
|
Difference from placebo (adjusted mean‡) |
-17.5§
|
-15.5§
| |
|
Change from baseline at Week 1 (adjusted mean‡) |
-16.5§
|
-12.0§
|
1.2 |
|
Body Weight (kg) |
|||
|
Baseline (mean) |
86.3 |
84.7 |
87.7 |
|
Change from baseline (adjusted mean‡) |
-2.9 |
-3.0 |
-0.9 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-2.0§
|
-2.2§ (-2.8, -1.5) | |
Figure 3: Adjusted Mean Change from Baseline Over Time in HbA1c (%) in a 24-Week Placebo-Controlled Study of FARXIGA in Combination with Metformin
Active Glipizide-Controlled Study Add-On to Metformin
A total of 816 patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c >6.5% and ≤10%) were randomized in a 52-week, glipizide-controlled, noninferiority study to evaluate FARXIGA as add-on therapy to metformin (NCT00660907). Patients on metformin at a dose of at least 1500 mg per day were randomized following a 2-week placebo lead-in period to glipizide or dapagliflozin (5 mg or 2.5 mg, respectively) and were up-titrated over 18 weeks to optimal glycemic effect (FPG <110 mg/dL, <6.1 mmol/L) or to the highest dose level (up to glipizide 20 mg and FARXIGA 10 mg) as tolerated by patients. Thereafter, doses were kept constant, except for down-titration to prevent hypoglycemia.
At the end of the titration period, 87% of patients treated with FARXIGA had been titrated to the maximum study dose (10 mg) versus 73% treated with glipizide (20 mg). FARXIGA led to a similar mean reduction in HbA1c from baseline at Week 52 (LOCF), compared with glipizide, thus demonstrating noninferiority (see Table 12). FARXIGA treatment led to a statistically significant mean reduction in body weight from baseline at Week 52 (LOCF) compared with a mean increase in body weight in the glipizide group. Statistically significant (p<0.0001) mean change from baseline in systolic blood pressure relative to glipizide plus metformin was -5.0 mmHg with FARXIGA plus metformin.
| Efficacy Parameter | FARXIGA
+ Metformin N=400† | Glipizide
+ Metformin N=401† |
|---|---|---|
|
||
|
HbA1c (%) |
||
|
Baseline (mean) |
7.7 |
7.7 |
|
Change from baseline (adjusted mean‡) |
-0.5 |
-0.5 |
|
Difference from glipizide + metformin (adjusted mean‡) |
0.0§
| |
|
Body Weight (kg) |
||
|
Baseline (mean) |
88.4 |
87.6 |
|
Change from baseline (adjusted mean‡) |
-3.2 |
1.4 |
|
Difference from glipizide + metformin (adjusted mean‡) |
-4.7¶
| |
Add-On Combination Therapy with Other Antidiabetic Agents
Add-On Combination Therapy with a Sulfonylurea
A total of 597 patients with type 2 diabetes mellitus and inadequate glycemic control (HbA1c ≥7% and ≤10%) were randomized in this 24-week, placebo-controlled study to evaluate FARXIGA in combination with glimepiride (a sulfonylurea) (NCT00680745).
Patients on at least half the maximum recommended dose of glimepiride as monotherapy (4 mg) for at least 8 weeks lead-in were randomized to FARXIGA 5 mg, FARXIGA 10 mg, or placebo in addition to glimepiride 4 mg per day. Down-titration of glimepiride to 2 mg or 0 mg was allowed for hypoglycemia during the treatment period; no up-titration of glimepiride was allowed.
In combination with glimepiride, FARXIGA 10 mg provided statistically significant improvement in HbA1c, FPG, and 2-hour PPG, and statistically significant reduction in body weight compared with placebo plus glimepiride at Week 24 (see Table 13). Statistically significant (p<0.05 for both doses) mean changes from baseline in systolic blood pressure relative to placebo plus glimepiride were -2.8 mmHg and -3.8 mmHg with FARXIGA 5 mg and 10 mg plus glimepiride, respectively.
Add-on Combination Therapy with Metformin and a Sulfonylurea
A total of 218 patients with type 2 diabetes mellitus and inadequate glycemic control (HbA1c ≥7% and ≤10.5%) participated in a 24-week, placebo-controlled study to evaluate FARXIGA in combination with metformin and a sulfonylurea (NCT01392677). Patients on a stable dose of metformin (immediate- or extended-release formulations) ≥1500 mg/day plus maximum tolerated dose, which must be at least half the maximum dose, of a sulfonylurea for at least 8 weeks prior to enrollment were randomized after an 8-week placebo lead-in period to FARXIGA 10 mg or placebo. Dose-titration of FARXIGA or metformin was not permitted during the 24-week treatment period. Down-titration of the sulfonylurea was permitted to prevent hypoglycemia, but no up-titration was permitted. As add-on treatment to combined metformin and a sulfonylurea, treatment with FARXIGA 10 mg provided statistically significant improvements in HbA1c and FPG and statistically significant reduction in body weight compared with placebo at Week 24 (Table 13). A statistically significant (p <0.05) mean change from baseline in systolic blood pressure relative to placebo in combination with metformin and a sulfonylurea was -3.8 mmHg with FARXIGA 10 mg in combination with metformin and a sulfonylurea at Week 8.
Add-On Combination Therapy with a Thiazolidinedione
A total of 420 patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c ≥7% and ≤10.5%) participated in a 24-week, placebo-controlled study to evaluate FARXIGA in combination with pioglitazone (a thiazolidinedione [TZD]) alone (NCT00683878). Patients on a stable dose of pioglitazone of 45 mg per day (or 30 mg per day, if 45 mg per day was not tolerated) for 12 weeks were randomized after a 2-week lead-in period to 5 or 10 mg of FARXIGA or placebo in addition to their current dose of pioglitazone. Dose titration of FARXIGA or pioglitazone was not permitted during the study.
In combination with pioglitazone, treatment with FARXIGA 10 mg provided statistically significant improvements in HbA1c, 2-hour PPG, FPG, the proportion of patients achieving HbA1c <7%, and a statistically significant reduction in body weight compared with the placebo plus pioglitazone treatment groups (see Table 13) at Week 24. A statistically significant (p <0.05) mean change from baseline in systolic blood pressure relative to placebo in combination with pioglitazone was -4.5 mmHg with FARXIGA 10 mg in combination with pioglitazone.
Add-On Combination Therapy with a DPP4 Inhibitor
A total of 452 patients with type 2 diabetes mellitus who were drug naive, or who were treated at entry with metformin or a DPP4 inhibitor alone or in combination, and had inadequate glycemic control (HbA1c ≥7.0% and ≤10.0% at randomization), participated in a 24-week, placebo-controlled study to evaluate FARXIGA in combination with sitagliptin (a DPP4 inhibitor) with or without metformin (NCT00984867).
Eligible patients were stratified based on the presence or absence of background metformin (≥1500 mg per day), and within each stratum were randomized to either FARXIGA 10 mg plus sitagliptin 100 mg once daily, or placebo plus sitagliptin 100 mg once daily. Endpoints were tested for FARXIGA 10 mg versus placebo for the total study group (sitagliptin with and without metformin) and for each stratum (sitagliptin alone or sitagliptin with metformin). Thirty-seven percent (37%) of patients were drug naive, 32% were on metformin alone, 13% were on a DPP4 inhibitor alone, and 18% were on a DPP4 inhibitor plus metformin. Dose titration of FARXIGA, sitagliptin, or metformin was not permitted during the study.
In combination with sitagliptin (with or without metformin), FARXIGA 10 mg provided statistically significant improvements in HbA1c, FPG, and a statistically significant reduction in body weight compared with the placebo plus sitagliptin (with or without metformin) group at Week 24 (see Table 13). These improvements were also seen in the stratum of patients who received FARXIGA 10 mg plus sitagliptin alone (placebo-corrected mean change for HbA1c -0.56%; n=110) compared with placebo plus sitagliptin alone (n=111), and the stratum of patients who received FARXIGA 10 mg plus sitagliptin and metformin (placebo-corrected mean change for HbA1c -0.40; n=113) compared with placebo plus sitagliptin with metformin (n=113).
Add-On Combination Therapy with Insulin
A total of 808 patients with type 2 diabetes mellitus who had inadequate glycemic control (HbA1c ≥7.5% and ≤10.5%) were randomized in a 24-week, placebo-controlled study to evaluate FARXIGA as add-on therapy to insulin (NCT00673231). Patients on a stable insulin regimen, with a mean dose of at least 30 IU of injectable insulin per day, for a period of at least 8 weeks prior to enrollment and on a maximum of 2 oral antidiabetic medications (OADs), including metformin, were randomized after completing a 2-week enrollment period to receive either FARXIGA 5 mg, FARXIGA 10 mg, or placebo in addition to their current dose of insulin and other OADs, if applicable. Patients were stratified according to the presence or absence of background OADs. Up- or down-titration of insulin was only permitted during the treatment phase in patients who failed to meet specific glycemic goals. Dose modifications of blinded study medication or OAD(s) were not allowed during the treatment phase, with the exception of decreasing OAD(s) where there were concerns over hypoglycemia after cessation of insulin therapy.
In this study, 50% of patients were on insulin monotherapy at baseline, while 50% were on 1 or 2 OADs in addition to insulin. At Week 24, FARXIGA 10 mg dose provided statistically significant improvement in HbA1c and reduction in mean insulin dose, and a statistically significant reduction in body weight compared with placebo in combination with insulin, with or without up to 2 OADs (see Table 13); the effect of FARXIGA on HbA1c was similar in patients treated with insulin alone and patients treated with insulin plus OAD. Statistically significant (p<0.05) mean change from baseline in systolic blood pressure relative to placebo in combination with insulin was -3.0 mmHg with FARXIGA 10 mg in combination with insulin.
At Week 24, FARXIGA 5 mg (-5.7 IU, difference from placebo) and 10 mg (-6.2 IU, difference from placebo) once daily resulted in a statistically significant reduction in mean daily insulin dose (p<0.0001 for both doses) compared to placebo in combination with insulin, and a statistically significantly higher proportion of patients on FARXIGA 10 mg (19.6%) reduced their insulin dose by at least 10% compared to placebo (11.0%).
| Efficacy Parameter | FARXIGA 10 mg | FARXIGA 5 mg | Placebo
|
|---|---|---|---|
|
|||
|
In Combination with Sulfonylurea (Glimepiride) |
|||
|
Intent-to-Treat Population |
N=151† |
N=142† |
N=145† |
|
HbA1c (%) |
|||
|
Baseline (mean) |
8.1 |
8.1 |
8.2 |
|
Change from baseline (adjusted mean‡) |
-0.8 |
-0.6 |
-0.1 |
|
Difference from placebo (adjusted mean‡) |
-0.7§ (-0.9, -0.5) |
-0.5§ (-0.7, -0.3) | |
|
Percent of patients achieving HbA1c <7% adjusted for baseline |
31.7%§ |
30.3%§ |
13.0% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
172.4 |
174.5 |
172.7 |
|
Change from baseline (adjusted mean‡) |
-28.5 |
-21.2 |
-2.0 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-26.5§ (-33.5, -19.5) |
-19.3§ (-26.3, -12.2) | |
|
2-hour PPG¶ (mg/dL) |
|||
|
Baseline (mean) |
329.6 |
322.8 |
324.1 |
|
Change from baseline (adjusted mean‡) |
-60.6 |
–54.5 |
-11.5 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-49.1§ (-64.1, -34.1) |
-43.0§ (–58.4, -27.5) | |
|
Body Weight (kg) |
|||
|
Baseline (mean) |
80.6 |
81.0 |
80.9 |
|
Change from baseline (adjusted mean‡) |
-2.3 |
-1.6 |
-0.7 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-1.5§ (-2.2, -0.9) |
-0.8§ (-1.5, -0.2) | |
|
In Combination with Metformin and a Sulfonylurea |
|||
|
Intent-to-Treat Population |
N=108† |
- |
N=108† |
|
HbA1c (%) |
|||
|
Baseline (mean) |
8.08 |
- |
8.24 |
|
Change from baseline (adjusted mean‡#) |
-0.86 |
- |
-0.17 |
|
Difference from placebo (adjusted mean‡#) (95% CI) |
-0.69§ (-0.89, -0.49) |
- | |
|
Percent of patients achieving HbA1c <7% |
31.8%§ |
- |
11.1% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
167.4 |
- |
180.3 |
|
Change from baseline (adjusted mean‡) |
-34.2 |
- |
-0.8 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-33.5§ (-43.1, -23.8) |
- | |
|
Body Weight (kg) |
|||
|
Baseline (mean) |
88.57 |
- |
90.07 |
|
Change from baseline (adjusted mean‡) |
-2.65 |
- |
-0.58 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-2.07§ (-2.79, -1.35) |
- | |
|
In Combination with Thiazolidinedione (Pioglitazone) |
|||
|
Intent-to-Treat Population |
N=140Þ |
N=141Þ |
N=139Þ |
|
HbA1c (%) |
|||
|
Baseline (mean) |
8.4 |
8.4 |
8.3 |
|
Change from baseline (adjusted mean‡) |
-1.0 |
-0.8 |
-0.4 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-0.6§ (-0.8, -0.3) |
-0.4§ (-0.6, -0.2) | |
|
Percent of patients achieving HbA1c <7% |
38.8%ß |
32.5%ß |
22.4% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
164.9 |
168.3 |
160.7 |
|
Change from baseline (adjusted mean‡) |
-29.6 |
-24.9 |
-5.5 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-24.1§ (-32.2, -16.1) |
-19.5§ (-27.5, -11.4) | |
|
2-hour PPG¶ (mg/dL) |
|||
|
Baseline (mean) |
308.0 |
284.8 |
293.6 |
|
Change from baseline (adjusted mean‡) |
-67.5 |
-65.1 |
-14.1 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-53.3§ (-71.1, -35.6) |
-51.0§ (-68.7, -33.2) | |
|
Body Weight (kg) |
|||
|
Baseline (mean) |
84.8 |
87.8 |
86.4 |
|
Change from baseline (adjusted mean‡) |
-0.1 |
0.1 |
1.6 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-1.8§ (-2.6, -1.0) |
-1.6§ (-2.3, -0.8) | |
|
In Combination with DPP4 Inhibitor (Sitagliptin) with or without Metformin |
|||
|
Intent-to-Treat Population |
N=223† |
– |
N=224† |
|
HbA1c (%) |
|||
|
Baseline (mean) |
7.90 |
– |
7.97 |
|
Change from baseline (adjusted mean‡) |
-0.45 |
– |
0.04 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-0.48§ (-0.62, -0.34) |
– | |
|
Patients with HbA1c decrease ≥0.7% (adjusted percent) |
35.4% |
– |
16.6% |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
161.7 |
– |
163.1 |
|
Change from baseline at Week 24 (adjusted mean‡) |
-24.1 |
– |
3.8 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-27.9§ (-34.5, -21.4) |
– | |
|
Body Weight (kg) |
|||
|
Baseline (mean) |
91.02 |
– |
89.23 |
|
Change from baseline (adjusted mean‡) |
-2.14 |
– |
-0.26 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-1.89§ (-2.37, -1.40) |
– | |
|
In Combination with Insulin with or without up to 2 Oral Antidiabetic Therapies |
|||
|
Intent-to-Treat Population |
N=194† |
N=211† |
N=193† |
|
HbA1c (%) |
|||
|
Baseline (mean) |
8.6 |
8.6 |
8.5 |
|
Change from baseline (adjusted mean‡) |
-0.9 |
-0.8 |
-0.3 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-0.6§ (-0.7, -0.5) |
-0.5§ (-0.7, -0.4) | |
|
FPG (mg/dL) |
|||
|
Baseline (mean) |
173.7 |
NTà |
170.0 |
|
Change from baseline (adjusted mean‡) |
-21.7 |
NTà |
3.3 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-25.0§ (-34.3, -15.8) |
NTà | |
|
Body Weight (kg) |
|||
|
Baseline (mean) |
94.6 |
93.2 |
94.2 |
|
Change from baseline (adjusted mean‡) |
-1.7 |
-1.0 |
0.0 |
|
Difference from placebo (adjusted mean‡) (95% CI) |
-1.7§ (-2.2, -1.2) |
-1.0§ (-1.5, -0.5) | |
Combination Therapy with Exenatide-Extended Release as Add-On to Metformin
A total of 694 adult patients with type 2 diabetes mellitus and inadequate glycemic control (HbA1c ≥8.0 and ≤12.0%) on metformin, were evaluated in a 28-week double-blind, active-controlled study to compare FARXIGA in combination with exenatide extended-release (a GLP-1 receptor agonist) to FARXIGA alone and exenatide extended-release alone, as add-on to metformin (NCT02229396). Patients on metformin at a dose of at least 1,500 mg per day were randomized following a 1-week placebo lead-in period to receive either FARXIGA 10 mg once daily (QD) in combination with exenatide extended-release 2 mg once weekly (QW), FARXIGA 10 mg QD, or exenatide extended–release 2 mg QW.
At Week 28, FARXIGA in combination with exenatide extended-release provided statistically significantly greater reductions in HbA1c (-1.77%) compared to FARXIGA alone (-1.32%, p=0.001) and exenatide extended-release alone (-1.42%, p=0.012). FARXIGA in combination with exenatide extended-release provided statistically significantly greater reductions in FPG (-57.35 mg/dL) compared to FARXIGA alone (-44.72 mg/dL, p=0.006) and exenatide extended-release alone (-40.53, p <0.001).
Use in Patients with Type 2 Diabetes Mellitus and Moderate Renal Impairment
FARXIGA was assessed in two placebo-controlled studies of patients with type 2 diabetes mellitus and moderate renal impairment.
Patients with type 2 diabetes mellitus and an eGFR between 45 to less than 60 mL/min/1.73 m2 inadequately controlled on current diabetes therapy participated in a 24-week, double-blind, placebo-controlled clinical study (NCT02413398). Patients were randomized to either FARXIGA 10 mg or placebo, administered orally once daily. At Week 24, FARXIGA provided statistically significant reductions in HbA1c compared with placebo (Table 14).
| FARXIGA 10 mg | Placebo | |
|---|---|---|
| Number of patients: | N=160 | N=161 |
|
||
|
HbA1c (%) |
||
|
Baseline (mean) |
8.3 |
8.0 |
|
Change from baseline (adjusted mean*) |
-0.4 |
-0.1 |
|
Difference from placebo (adjusted mean*) (95% CI) |
-0.3† (-0.5, - 0.1) | |
14.2 Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus
Dapagliflozin Effect on Cardiovascular Events (DECLARE, NCT01730534) was an international, multicenter, randomized, double-blind, placebo-controlled, clinical study conducted to determine the effect of FARXIGA relative to placebo on cardiovascular (CV) outcomes when added to current background therapy. All patients had type 2 diabetes mellitus and either established CV disease or two or more additional CV risk factors (age ≥55 years in men or ≥60 years in women and one or more of dyslipidemia, hypertension, or current tobacco use). Concomitant antidiabetic and atherosclerotic therapies could be adjusted, at the discretion of investigators, to ensure participants were treated according to the standard care for these diseases.
Of 17160 randomized patients, 6974 (40.6%) had established CV disease and 10186 (59.4%) did not have established CV disease. A total of 8582 patients were randomized to FARXIGA 10 mg, 8578 to placebo, and patients were followed for a median of 4.2 years.
Approximately 80% of the trial population was White, 4% Black or African-American, and 13% Asian. The mean age was 64 years, and approximately 63% were male.
Mean duration of diabetes was 11.9 years and 22.4% of patients had diabetes for less than 5 years. Mean eGFR was 85.2 mL/min/1.73 m2. At baseline, 23.5% of patients had microalbuminuria (UACR ≥30 to ≤300 mg/g) and 6.8% had macroalbuminuria (UACR >300 mg/g). Mean HbA1c was 8.3% and mean BMI was 32.1 kg/m2. At baseline, 10% of patients had a history of heart failure.
Most patients (98.1%) used one or more antihyperglycemic medications at baseline. 82.0% of the patients were being treated with metformin, 40.9% with insulin, 42.7% with a sulfonylurea, 16.8% with a DPP4 inhibitor, and 4.4% with a GLP-1 receptor agonist.
Approximately 81.3% of patients were treated with angiotensin converting enzyme inhibitors or angiotensin receptor blockers, 75.0% with statins, 61.1% with antiplatelet therapy, 55.5% with acetylsalicylic acid, 52.6% with beta-blockers, 34.9% with calcium channel blockers, 22.0% with thiazide diuretics, and 10.5% with loop diuretics.
A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio (HR) of the composite of CV death, myocardial infarction (MI), or ischemic stroke (MACE) and if non-inferiority was demonstrated, to test for superiority on the two primary endpoints: 1) the composite of hospitalization for heart failure or CV death, and 2) MACE.
The incidence rate of MACE was similar in both treatment arms: 2.30 MACE events per 100 patient-years on dapagliflozin vs 2.46 MACE events per 100 patient-years on placebo. The estimated hazard ratio of MACE associated with dapagliflozin relative to placebo was 0.93 with a 95% CI of (0.84, 1.03). The upper bound of this confidence interval, 1.03, excluded the pre-specified non-inferiority margin of 1.3.
FARXIGA was superior to placebo in reducing the incidence of the primary composite endpoint of hospitalization for heart failure or CV death (HR 0.83 [95% CI 0.73, 0.95]).
The treatment effect was due to a significant reduction in the risk of hospitalization for heart failure in subjects randomized to FARXIGA (HR 0.73 [95% CI 0.61, 0.88]), with no change in the risk of CV death (Table 15 and Figures 4 and 5).
|
|||
|
Patients with events n (%) | |||
|
Efficacy Variable (time to first occurrence) |
FARXIGA 10 mg N=8582 |
Placebo N=8578 |
Hazard ratio (95% CI) |
|
Primary Endpoints |
|||
|
Composite of Hospitalization for Heart Failure, CV Death† |
417 (4.9) |
496 (5.8) |
0.83 (0.73, 0.95) |
|
Composite Endpoint of CV Death, MI, Ischemic Stroke |
756 (8.8) |
803 (9.4) |
0.93 (0.84, 1.03) |
|
Components of the composite endpoints‡ |
|||
|
Hospitalization for Heart Failure |
212 (2.5) |
286 (3.3) |
0.73 (0.61, 0.88) |
|
CV Death |
245 (2.9) |
249 (2.9) |
0.98 (0.82, 1.17) |
|
Myocardial Infarction |
393 (4.6) |
441 (5.1) |
0.89 (0.77, 1.01) |
|
Ischemic Stroke |
235 (2.7) |
231 (2.7) |
1.01 (0.84, 1.21) |
|
N=Number of patients, CI=Confidence interval, CV=Cardiovascular, MI=Myocardial infarction. |
|||
Figure 4: Time to First Occurrence of Hospitalization for Heart Failure or CV Death in the DECLARE Study
Figure 5: Time to First Occurrence of Hospitalization for Heart Failure in the DECLARE Study
14.3 Heart Failure with Reduced Ejection Fraction
Dapagliflozin And Prevention of Adverse outcomes in Heart Failure (DAPA-HF, NCT03036124) was an international, multicenter, randomized, double-blind, placebo-controlled study in patients with heart failure (New York Heart Association [NYHA] functional class II-IV) with reduced ejection fraction (left ventricular ejection fraction [LVEF] 40% or less) to determine whether FARXIGA reduces the risk of cardiovascular death and hospitalization for heart failure.
Of 4744 patients, 2373 were randomized to FARXIGA 10 mg and 2371 to placebo and were followed for a median of 18 months. The mean age of the study population was 66 years, 77% were male and 70% were White, 5% Black or African-American, and 24% Asian.
At baseline, 68% patients were classified as NYHA class II, 32% class III, and 1% class IV; median LVEF was 32%. History of type 2 diabetes mellitus was present in 42%, and an additional 3% had type 2 diabetes mellitus based on a HbA1c ≥6.5% at both enrollment and randomization.
At baseline, 94% of patients were treated with ACEi, ARB or angiotensin receptor-neprilysin inhibitor (ARNI, including sacubitril/valsartan 11%), 96% with beta-blocker, 71% with mineralocorticoid receptor antagonist (MRA), 93% with diuretic, and 26% had an implantable device.
FARXIGA reduced the incidence of the primary composite endpoint of CV death, hospitalization for heart failure or urgent heart failure visit (HR 0.74 [95% CI 0.65, 0.85]; p<0.0001). All three components of the primary composite endpoint individually contributed to the treatment effect. The FARXIGA and placebo event curves separated early and continued to diverge over the study period (Table 16, Figures 6A, 6B and 6C).
| Patients with events (event rate) | ||||
|---|---|---|---|---|
| Efficacy Variable
(time to first occurrence) | FARXIGA 10 mg
N=2373 | Placebo
N=2371 | Hazard ratio
(95% CI) | p-value† |
|
||||
|
Composite of Hospitalization for Heart Failure, CV Death or Urgent Heart Failure Visit |
386 (11.6) |
502 (15.6) |
0.74 (0.65, 0.85) |
<0.0001 |
|
Composite of CV Death or Hospitalization for Heart Failure |
382 (11.4) |
495 (15.3) |
0.75 (0.65, 0.85) |
<0.0001 |
|
Components of the composite endpoints |
||||
|
CV Death |
227 (6.5) |
273 (7.9) |
0.82 (0.69, 0.98) | |
|
Hospitalization for Heart Failure or Urgent Heart |
237 (7.1) |
326 (10.1) |
0.70 (0.59, 0.83) | |
|
Hospitalization for Heart Failure |
231 (6.9) |
318 (9.8) |
0.70 (0.59, 0.83) | |
|
Urgent Heart Failure Visit |
10 (0.3) |
23 (0.7) |
0.43 (0.20, 0.90) | |
|
All-Cause Mortality |
276 (7.9) |
329 (9.5) |
0.83 (0.71, 0.97) | |
|
N=Number of patients, CI=Confidence interval, CV=Cardiovascular. |
||||
NOTE: Time to first event was analyzed in a Cox proportional hazards model. The number of first events for the single components are the actual number of first events for each component and does not add up to the number of events in the composite endpoint. Event rates are presented as the number of subjects with event per 100 patient years of follow-up.
Figure 6: Kaplan-Meier Curves for the Primary Composite Endpoint (A), Cardiovascular Death (B), and Heart Failure Hospitalization (C) (DAPA-HF Study)
Figure 6A: Time to the First Occurrence of the Composite of Cardiovascular Death, Hospitalization for Heart Failure or Urgent Heart Failure Visit
NOTE: An urgent heart failure visit was defined as an urgent, unplanned, assessment by a physician, e.g., in an Emergency Department, and requiring treatment for worsening heart failure (other than just an increase in oral diuretics).
Patients at risk is the number of patients at risk at the beginning of the period.
HR=Hazard ratio, CI=Confidence interval.
Figure 6B: Time to the First Occurrence of Cardiovascular Death
Patients at risk is the number of patients at risk at the beginning of the period.
HR=Hazard ratio, CI=Confidence interval.
Figure 6C: Time to the First Occurrence of Heart Failure Hospitalization
Patients at risk is the number of patients at risk at the beginning of the period.
HR=Hazard ratio, CI=Confidence interval.
FARXIGA reduced the total number of hospitalizations for heart failure (first and recurrent) events and CV death, with 567 and 742 total events in the FARXIGA-treated vs placebo group (Rate Ratio 0.75 [95% CI 0.65, 0.88]; p=0.0002).
The results of the primary composite endpoint were consistent across the subgroups examined, including heart failure patients with and without type 2 diabetes mellitus (Figure 7).
Figure 7: Treatment Effects for Primary Composite Endpoint (Cardiovascular Death and Heart Failure Events) Subgroup Analysis (DAPA-HF Study)
aHazard ratio estimates are not presented for subgroups with less than 15 events in total, both arms combined.
n/N# Number of subjects with event/number of subjects in the subgroup.
NT-proBNP = N-terminal pro b-type natriuretic peptide, HF = Heart failure, MRA = mineralocorticoid receptor antagonist,
ECG = electrocardiogram, eGFR = estimated glomerular filtration rate.
Note: The figure above presents effects in various subgroups, all of which are baseline characteristics. The 95% confidence limits that are shown do not take into account the number of comparisons made and may not reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
14.4 Chronic Kidney Disease
The Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease (DAPA-CKD, NCT03036150) was an international, multicenter, randomized, double-blind, placebo-controlled study in patients with chronic kidney disease (CKD) (eGFR between 25 and 75 mL/min/1.73 m2) and albuminuria (urine albumin creatinine ratio [UACR] between 200 and 5000 mg/g) who were receiving standard of care background therapy, including a maximally tolerated, labeled daily dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). The trial excluded patients with autosomal dominant or autosomal recessive polycystic kidney disease, lupus nephritis, or ANCA-associated vasculitis and patients requiring cytotoxic, immunosuppressive, or immunomodulatory therapies in the preceding 6 months.
The primary objective was to determine whether FARXIGA reduces the incidence of the composite endpoint of ≥50% sustained decline in eGFR, progression to end-stage kidney disease (ESKD) (defined as sustained eGFR<15 mL/min/1.73 m2, initiation of chronic dialysis treatment or renal transplant), CV or renal death.
A total of 4304 patients were randomized equally to FARXIGA 10 mg or placebo and were followed for a median of 28.5 months.
The mean age of the study population was 62 years and 67% were male. The population was 53% White, 4% Black or African-American, and 34% Asian; 25% were of Hispanic or Latino ethnicity.
At baseline, mean eGFR was 43 mL/min/1.73 m2, 44% of patients had an eGFR 30 mL/min/1.73m2 to less than 45 mL/min/1.73m2, and 15% of patients had an eGFR less than 30 mL/min/1.73m2. Median UACR was 950 mg/g. A total of 68% of the patients had type 2 diabetes mellitus at randomization. The most common etiologies of CKD were diabetic nephropathy (58%), ischemic/hypertensive nephropathy (16%), and IgA nephropathy (6%).
At baseline, 97% of patients were treated with ACEi or ARB. Approximately 44% were taking antiplatelet agents, and 65% were on a statin.
FARXIGA reduced the incidence of the primary composite endpoint of ≥50% sustained decline in eGFR, progression to ESKD, CV or renal death (HR 0.61 [95% CI 0.51,0.72]; p<0.0001). The FARXIGA and placebo event curves separate by Month 4 and continue to diverge over the study period. The treatment effect reflected a reduction in ≥50% sustained decline in eGFR, progression to ESKD, and CV death. There were few renal deaths during the trial (Table 17, Figure 8).
FARXIGA also reduced the incidence of the composite endpoint of CV death or hospitalization for heart failure (HR 0.71 [95% CI 0.55, 0.92], p=0.0089) and all-cause mortality (HR 0.69 [95% CI 0.53, 0.88], p=0.0035).
|
||||
|
Patients with events (event rate) | ||||
|
Efficacy Variable (time to first occurrence) |
FARXIGA 10 mg
|
Placebo
|
Hazard ratio
|
p-value |
|
Composite of ≥50% sustained eGFR decline, ESKD, CV or renal death |
197 (4.6) |
312 (7.5) |
0.61 |
<0.0001 |
|
≥50% sustained eGFR decline |
112 (2.6) |
201 (4.8) |
0.53 | |
|
ESKD* |
109 (2.5) |
161 (3.8) |
0.64 | |
|
CV Death |
65 (1.4) |
80 (1.7) |
0.81 | |
|
Renal Death |
2 (0.0) |
6 (0.1) | ||
|
≥50% sustained eGFR decline, ESKD or renal death |
142 (3.3) |
243 (5.8) |
0.56 |
<0.0001 |
|
CV death or Hospitalization for Heart Failure |
100 (2.2) |
138 (3.0) |
0.71 |
0.0089 |
|
Hospitalization for Heart Failure |
37 (0.8) |
71 (1.6) |
0.51 | |
|
All-Cause Mortality |
101 (2.2) |
146 (3.1) |
0.69 |
0.0035 |
|
N=Number of patients, CI=Confidence interval, CV=Cardiovascular, ESKD=End stage kidney disease. |
||||
|
NOTE: Time to first event was analyzed in a Cox proportional hazards model. Event rates are presented as the number of subjects with event per 100 patient years of follow-up. There were too few events of renal death to compute a reliable hazard ratio. |
||||
Figure 8: Time to First Occurrence of the Primary Composite Endpoint, ≥50% Sustained Decline in eGFR, ESKD, CV or Renal Death (DAPA-CKD Study)
Patients at risk is the number of subjects at risk at the beginning of the period. 1 month corresponds to 30 days. 2-sided p-value is displayed. HR, CI and p-value are from the Cox proportional hazard model.
HR=hazard ratio; CI=confidence interval; eGFR=estimated glomerular filtration rate; ESKD=end stage kidney disease; CV=cardiovascular; vs=versus.
The results of the primary composite endpoint were consistent across the subgroups examined, including CKD patients with and without type 2 diabetes mellitus, causes of CKD, age, biological sex, race, UACR, and eGFR.
DAPA-CKD enrolled a population with relatively advanced CKD at high risk of progression. Exploratory analyses of a randomized, double-blind, placebo-controlled trial conducted to determine the effect of FARXIGA on CV outcomes (the DECLARE trial) support the conclusion that FARXIGA is also likely to be effective in patients with less advanced CKD.
16. How is Farxiga supplied
How Supplied
FARXIGA (dapagliflozin) tablets have markings on both sides and are available in the strengths and packages listed in Table 18.
| Tablet Strength | Film-Coated Tablet
Color/Shape | Tablet
Markings | Package Size | NDC Code |
|---|---|---|---|---|
|
5 mg |
yellow, |
“5” engraved on one |
Bottles of approximately 1440 Tablets |
55154-6932-8 |
|
10 mg |
yellow, |
“10” engraved on one side and “1428” engraved on the other side |
Bottles of approximately 840 tablets |
55154-6933-8 |
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ketoacidosis
Inform patients with diabetes mellitus that ketoacidosis is a serious life-threatening condition and that cases of ketoacidosis have been reported during use of FARXIGA with diabetes mellitus, sometimes associated with illness or surgery among other risk factors. Instruct patients to check ketones (when possible) if symptoms consistent with ketoacidosis occur even if blood glucose is not elevated. If symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness and labored breathing) occur, instruct patients to discontinue FARXIGA and seek medical attention immediately [see Warnings and Precautions (5.1)].
Volume Depletion
Inform patients that symptomatic hypotension may occur with FARXIGA and advise them to contact their healthcare provider if they experience such symptoms [see Warnings and Precautions (5.2)]. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake.
Serious Urinary Tract Infections
Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice promptly if such symptoms occur [see Warnings and Precautions (5.3)].
Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
Inform patients that necrotizing infections of the perineum (Fournier’s Gangrene) have occurred with FARXIGA in patients with diabetes mellitus. Counsel patients to promptly seek medical attention if they develop pain or tenderness, redness, or swelling of the genitals or the area from the genitals back to the rectum, along with a fever above 100.4°F or malaise [see Warnings and Precautions (5.5)].
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infections. Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.6)].
Genital Mycotic Infections in Males (e.g., Balanitis)
Inform male patients that yeast infections of the penis (e.g., balanitis or balanoposthitis) may occur, especially in patients with prior history. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice [see Warnings and Precautions (5.6)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions (e.g., urticaria, anaphylactic reactions, and angioedema) have been reported with FARXIGA. Advise patients to immediately report any signs or symptoms suggesting allergic reaction or angioedema, and to take no more of the drug until they have consulted prescribing physicians.
Pregnancy
Advise pregnant patients of the potential risk to a fetus with treatment with FARXIGA. Instruct patients to immediately inform their healthcare provider if pregnant or planning to become pregnant [see Use in Specific Populations (8.1)].
Lactation
Advise patients that use of FARXIGA is not recommended while breastfeeding [see Use in Specific Populations (8.2)].
Laboratory Tests
Due to its mechanism of action, patients taking FARXIGA will test positive for glucose in their urine.
Missed Dose
If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of FARXIGA at the same time.
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
FARXIGA® is a registered trademark of the AstraZeneca group of companies.
Distributed by:
Cardinal Health
Dublin, OH 43017
L7248A
L7249B
|
MEDICATION GUIDE
|
|||
|
What is the most important information I should know about FARXIGA? FARXIGA can cause serious side effects, including:
Talk to your healthcare provider about what to do if you get symptoms of a yeast infection of the vagina or penis. Your healthcare provider may suggest you use an over-the-counter antifungal medicine. Talk to your healthcare provider right away if you use an over-the-counter antifungal medication and your symptoms do not go away. |
|||
|
What is FARXIGA?
|
|||
|
Who should not take FARXIGA? Do not take FARXIGA if you:
|
|||
|
What should I tell my healthcare provider before taking FARXIGA? Before you take FARXIGA, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. FARXIGA may affect the way other medicines work, and other medicines may affect how FARXIGA works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|||
|
How should I take FARXIGA?
|
|||
|
What are the possible side effects of FARXIGA? FARXIGA may cause serious side effects, including: See “What is the most important information I should know about FARXIGA?”
|
|||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|||
|
|
|
|
|
|
The most common side effects of FARXIGA include:
These are not all the possible side effects of FARXIGA. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
How should I store FARXIGA? Store FARXIGA at room temperature between 68°F to 77°F (20°C to 25°C). |
|||
|
General information about the safe and effective use of FARXIGA Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use FARXIGA for a condition for which it is not prescribed. Do not give FARXIGA to other people, even if they have the same symptoms you have. It may harm them. This Medication Guide summarizes the most important information about FARXIGA. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about FARXIGA that is written for healthcare professionals. For more information about FARXIGA, go to www.farxiga.com or call 1-800-236-9933. |
|||
|
What are the ingredients in FARXIGA? Active ingredient: dapagliflozin. Inactive ingredients: microcrystalline cellulose, anhydrous lactose, crospovidone, silicon dioxide, and magnesium stearate. The film coating contains: polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and yellow iron oxide.
FARXIGA is a registered trademark of the AstraZeneca group of companies. Distributed by: Cardinal Health Dublin, OH 43017 L7248A Rev.A L7249A Rev.B |
|||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised 01/2023
| FARXIGA
dapagliflozin tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| FARXIGA
dapagliflozin tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Cardinal Health 107, LLC (118546603) |