Drug Detail:Filspari (Sparsentan)
Drug Class: Miscellaneous cardiovascular agents
Highlights of Prescribing Information
FILSPARITM (sparsentan) tablets, for oral use
Initial U.S. Approval: 2023
WARNING: HEPATOTOXICITY and EMBRYO- FETAL TOXICITY
See full prescribing information for complete boxed warning.
- FILSPARI is only available through a restricted distribution program called the FILSPARI Risk Evaluation and Mitigation Strategies (REMS) because of these risks (5.3):
- Some endothelin receptor antagonists have caused elevations of aminotransferases, hepatotoxicity, and liver failure (5.1).
- Measure liver aminotransferases and total bilirubin prior to initiation of treatment and ALT and AST monthly for 12 months, then every 3 months during treatment (2.2, 2.5, 5.1).
- Interrupt treatment and closely monitor patients developing aminotransferase elevations more than 3x Upper Limit of Normal (ULN) (2.2, 2.5).
- Based on animal data, FILSPARI can cause major birth defects if used during pregnancy (4, 5.2, 8.1).
- Pregnancy testing is required before, during, and after treatment (2.2, 4, 5.2, 8.1).
- Patients who can become pregnant must use effective contraception prior to initiation of treatment, during treatment, and for one month after (4, 5.3, 8.1, 8.3).
Indications and Usage for Filspari
FILSPARI is an endothelin and angiotensin II receptor antagonist indicated to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression, generally a urine protein-to-creatinine ratio (UPCR) ≥1.5 g/g. (1, 12.1).
This indication is approved under accelerated approval based on reduction of proteinuria. It has not been established whether FILSPARI slows kidney function decline in patients with IgAN. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory clinical trial.
Filspari Dosage and Administration
- Prior to initiating treatment with FILSPARI, discontinue use of renin- angiotensin-aldosterone system (RAAS) inhibitors, endothelin receptor antagonists (ERAs) or aliskiren (2.1, 4, 7.1).
- Initiate treatment with FILSPARI at 200 mg orally once daily. After 14 days, increase to the recommended dose of 400 mg once daily, as tolerated. When resuming treatment with FILSPARI after an interruption, consider titration of FILSPARI, starting at 200 mg once daily. After 14 days, increase to the recommended dose of 400 mg once daily (2.3).
- Instruct patients to swallow tablets whole with water prior to the morning or evening meal (2.4).
Dosage Forms and Strengths
Tablets: 200 mg and 400 mg (3)
Contraindications
- Pregnancy (4).
- Do not coadminister FILSPARI with angiotensin receptor blockers, endothelin receptor antagonists, or aliskiren (4).
Warnings and Precautions
- Hepatotoxicity (5.1)
- Embryo-Fetal Toxicity (5.2)
- Hypotension (5.4)
- Acute Kidney Injury (5.5)
- Hyperkalemia (5.6)
- Fluid Retention (5.7)
Adverse Reactions/Side Effects
Most common adverse reactions (≥5%) are peripheral edema , hypotension (including orthostatic hypotension), dizziness, hyperkalemia, and anemia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Travere Therapeutics at 1-877-659-5518 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Renin-Angiotensin System (RAS) inhibitors and ERAs: Contraindicated. Increased risk of hypotension, hyperkalemia (2.1, 4, 7.1).
- Strong CYP3A inhibitors: Avoid concomitant use. Increased sparsentan exposure (2.6, 7.2, 12.3).
- Moderate CYP3A inhibitors: Monitor adverse reactions. Increased sparsentan exposure (7.2, 12.3).
- Strong CYP3A inducers: Avoid concomitant use. Decreased sparsentan exposure (7.3, 12.3).
- Antacids: Avoid use within 2 hours before or after use of sparsentan. May decrease exposure to sparsentan (7.4, 11).
- Acid reducing agents: Avoid concomitant use. May decrease exposure to sparsentan (7.4).
- Nonsteroidal anti-inflammatory drugs (NSAIDs) including selective cyclooxygenase (COX-2) inhibitors: Monitor for signs of worsening renal function. Increased risk of kidney injury (7.5).
- CYP2B6, 2C9, and 2C19 substrates: Monitor for efficacy of the concurrently administered substrates. Decreased exposure of these substrates (7.6, 12.3).
- Sensitive P-gp and BCRP substrates: Avoid concomitant use. Increased exposure to substrates (7.7, 12.3).
- Agents Increasing Serum Potassium: Increased risk of hyperkalemia, monitor serum potassium frequently (5.6, 7.8).
Use In Specific Populations
- Lactation: Advise not to breastfeed (8.2).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2023
Related/similar drugs
budesonide, cyclophosphamide, Cytoxan, Tarpeyo, sparsentanFull Prescribing Information
WARNING: HEPATOTOXICITY and EMBRYO-FETAL TOXICITY
Hepatotoxicity
Some Endothelin Receptor Antagonists (ERAs) have caused elevations of aminotransferases, hepatotoxicity, and liver failure. In clinical studies, elevations in aminotransferases (ALT or AST) of at least 3-times the Upper Limit of Normal (ULN) have been observed in up to 2.5% of FILSPARI-treated patients, including cases confirmed with rechallenge.
Measure transaminases and bilirubin before initiating treatment and monthly for the first 12 months, and then every 3 months during treatment. Interrupt treatment and closely monitor patients who develop aminotransferase elevations more than 3x ULN [see Dosage and Administration (2.3, 2.5), Warning and Precautions (5.1)].
Embryo-Fetal Toxicity
FILSPARI can cause major birth defects if used by pregnant patients based on animal data [see Warnings and Precautions (5.2), Use in Specific Populations (8.1)]. Therefore, pregnancy testing is required before the initiation of treatment, during treatment, and one month after discontinuation of treatment with FILSPARI. Patients who can become pregnant must use effective contraception before the initiation of treatment, during treatment, and for one month after discontinuation of treatment with FILSPARI [see Dosage and Administration (2.2), Contraindications (4), Warnings and Precautions (5.2), Use in Specific Populations (8.1, 8.3)].
Filspari Dosage and Administration
2.2 Monitoring
Initiate treatment with FILSPARI in patients who can become pregnant only after confirmation of a negative pregnancy test. Pregnancy tests are required monthly during treatment and one month after discontinuation of treatment with FILSPARI [see Warnings and Precautions (5.2), Use in Specific Populations (8.1, 8.3)].
2.5 Dosage Adjustment for Aminotransferase Elevations
If aminotransferase levels increase, adjust monitoring and treatment plan according to Table 1.
Do not resume treatment in patients who have experienced clinical symptoms of hepatotoxicity or in patients whose hepatic enzyme levels and bilirubin have not returned to pretreatment levels.
| ALT = alanine aminotransferase; AST = aspartate aminotransferase; INR = international normalized ratio; ULN = upper limit of normal. | ||
| ALT/AST levels | Treatment and monitoring recommendations | |
| >3x and ≤8x ULN |
Confirm elevation with a repeat measure. If confirmed, interrupt treatment, and monitor aminotransferase levels and bilirubin at least weekly, and INR as needed, until the levels return to pretreatment values and the patient is asymptomatic. Do not resume treatment if any of the following occurs without other cause found:
If treatment is resumed, initiate FILSPARI at 200 mg once daily, with reassessment of hepatic enzyme levels and bilirubin within 3 days. Close monitoring is required in these patients [see Dosage and Administration (2.2, 2.3)]. |
|
| >8x ULN | Stop treatment permanently if no other cause found. | |
Dosage Forms and Strengths
FILSPARI is supplied as film-coated, modified oval, white to off-white tablets debossed on one side and plain on the other in the following strengths:
200 mg debossed with “105”
400 mg debossed with “021”
Warnings and Precautions
5.1 Hepatotoxicity
Advise patients with symptoms suggesting hepatotoxicity (nausea, vomiting, right upper quadrant pain, fatigue, anorexia, jaundice, dark urine, fever, or itching) to immediately stop treatment with FILSPARI and seek medical attention . If aminotransferase levels are abnormal at any time during treatment, interrupt FILSPARI and monitor as recommended [see Dosage and Administration (2.5)].
Consider re-initiation of FILSPARI only when hepatic enzyme levels and bilirubin return to pretreatment values and only in patients who have not experienced clinical symptoms of hepatotoxicity. [see Dosage and Administration (2.2, 2.5)].
Avoid initiation of FILSPARI in patients with elevated aminotransferases (>3x ULN) prior to drug initiation because monitoring hepatotoxicity in these patients may be more difficult and these patients may be at increased risk for serious hepatotoxicity [see Dosage and Administration (2.2, 2.5) and Warnings and Precautions (5.3)].
5.3 FILSPARI REMS
Important requirements of the FILSPARI REMS include the following:
- Prescribers must be certified with the FILSPARI REMS by enrolling and completing training.
- All patients must enroll in the FILSPARI REMS prior to initiating treatment and comply with monitoring requirements [see Dosage and Administration (2.2, 2.5, 2.6), Warnings and Precautions (5.1, 5.2), Use in Specific Populations (8.3)].
- Pharmacies that dispense FILSPARI must be certified with the FILSPARI REMS and must dispense only to patients who are authorized to receive FILSPARI.
Further information is available at www.filsparirems.com or 1-833-513-1325.
Adverse Reactions/Side Effects
Clinically significant adverse reactions that appear in other sections of the label include:
- Hepatotoxicity [see Warnings and Precautions (5.1)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.2)]
- Hypotension [see Warnings and Precautions (5.4)]
- Acute Kidney Injury [see Warnings and Precautions (5.5)]
- Hyperkalemia [see Warnings and Precautions (5.6)]
- Fluid Retention [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
NCT03762850), an ongoing, randomized, double-blind, active-controlled clinical study in adults with IgAN.
| 1 Data presented include all Treatment-Emergent Adverse Events reported 2 Elevations in ALT or AST >3-fold ULN reported as Adverse Events of Interest |
||
| FILSPARI (N = 202) n (%) | Irbesartan (N = 202) n (%) |
|
| Peripheral edema | 29 (14) | 19 (9) |
| Hypotension (including orthostatic hypotension) | 28 (14) | 12 (6) |
| Dizziness | 27 (13) | 11 (5) |
| Hyperkalemia | 27 (13) | 21 (10) |
| Anemia | 10 (5) | 5 (2) |
| Acute kidney injury | 9 (4) | 2 (1) |
| Transaminase elevations2 | 5 (2.5) | 4 (2) |
Initiation of FILSPARI may cause an initial small decrease in estimated glomerular filtration rate (eGFR) that occurs within the first 4 weeks of starting therapy and then stabilizes.
The incidence of a hemoglobin decrease >2 g/dL compared to baseline and below the lower limit of normal was greater for the FILSPARI arm (11%) compared to the irbesartan arm (5%). This decrease is thought to be in part due to hemodilution. There were no treatment discontinuations due to anemia or hemoglobin decrease in the PROTECT study.
Drug Interactions
7.1 Renin-Angiotensin System (RAS) Inhibitors and ERAs
Combined use of these agents is associated with increased risks of hypotension, syncope, hyperkalemia, and changes in renal function (including acute renal failure).
7.2 Strong and Moderate CYP3A Inhibitors
Avoid concomitant use of FILSPARI with strong CYP3A inhibitors. If a strong CYP3A inhibitor cannot be avoided, interrupt treatment with FILSPARI. When resuming treatment with FILSPARI, consider dose titration [see Dosage and Administration (2.3, 2.6), Clinical Pharmacology (12.3)].
Monitor blood pressure, serum potassium, edema, and kidney function regularly when used concomitantly with moderate CYP3A inhibitors [see Warnings and Precautions (5.4, 5.5, 5.6, 5.7)]. No FILSPARI dose adjustment is needed.
Sparsentan is a CYP3A substrate. Concomitant use with a strong CYP3A inhibitor increases sparsentan Cmax and AUC [see Clinical Pharmacology (12.3)], which may increase the risk of FILSPARI adverse reactions.
7.3 Strong CYP3A Inducers
Avoid concomitant use with a strong CYP3A inducer. Sparsentan is a CYP3A substrate. Concomitant use with a strong CYP3A inducer decreases sparsentan Cmax and AUC [see Clinical Pharmacology (12.3)], which may reduce FILSPARI efficacy.
7.4 Antacids and Acid Reducing Agents
Administer FILSPARI 2 hours before or after administration of antacids. Avoid concomitant use of acid reducing agents (histamine H2 receptor antagonist and PPI proton pump inhibitor) with FILSPARI. Sparsentan exhibits pH-dependent solubility [see Description (11)]. Antacids or acid reducing agents may decrease sparsentan exposure which may reduce FILSPARI efficacy.
7.5 Non-Steroidal Anti-Inflammatory Agents (NSAIDs), Including Selective Cyclooxygenase-2 (COX-2) Inhibitors
Monitor for signs of worsening renal function with concomitant use with NSAIDs (including selective COX-2 inhibitors). In patients with volume depletion (including those on diuretic therapy) or with impaired kidney function, concomitant use of NSAIDs (including selective COX-2 inhibitors) with drugs that antagonize the angiotensin II receptor may result in deterioration of kidney function, including possible kidney failure [see Warnings and Precautions (5.5)]. These effects are usually reversible.
7.6 CYP2B6, 2C9, and 2C19 Substrates
Monitor for efficacy of the concurrently administered CYP2B6, 2C9, and 2C19 substrates and consider dosage adjustment in accordance with the Prescribing Information. Sparsentan is an inducer of CYP2B6, 2C9, and 2C19. Sparsentan decreases exposure of these substrates [see Clinical Pharmacology (12.3)], which may reduce efficacy related to these substrates.
7.7 P-gp and BCRP Substrates
Avoid concomitant use of sensitive substrates of P-gp and BCRP with FILSPARI. Sparsentan is an inhibitor of P-gp and BCRP. Sparsentan may increase exposure of these transporter substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions related to these substrates.
7.8 Agents Increasing Serum Potassium
Monitor serum potassium frequently in patients treated with FILSPARI and other agents that increase serum potassium. Concomitant use of FILSPARI with potassium-sparing diuretics, potassium supplements, potassium-containing salt substitutes, or other drugs that raise serum potassium levels may result in hyperkalemia [see Warnings and Precautions (5.6)].
Use In Specific Populations
8.4 Pediatric Use
The safety and efficacy of FILSPARI in pediatric patients have not been established.
Filspari - Clinical Pharmacology
12.3 Pharmacokinetics
The apparent volume of distribution at steady state is 61.4 L at the approved recommended dosage.
Sparsentan is >99% bound to human plasma proteins.
The half-life of sparsentan is estimated to be 9.6 hours at steady state.
Cytochrome P450 3A is the major isozyme responsible for the metabolism of sparsentan.
Clinical Studies and Model-Informed Approaches
Effect of Other Drugs on Sparsentan
Clinical Studies
NCT03762850) in adults with biopsy-proven IgAN, eGFR ≥30 mL/min/1.73 m2, and total urine protein ≥1.0 g/day on a maximized stable dose of RAS inhibitor treatment that was at least 50% of maximum labeled dose. Patients with other glomerulopathies or those who had been recently treated with systemic immunosuppressants were excluded.
|
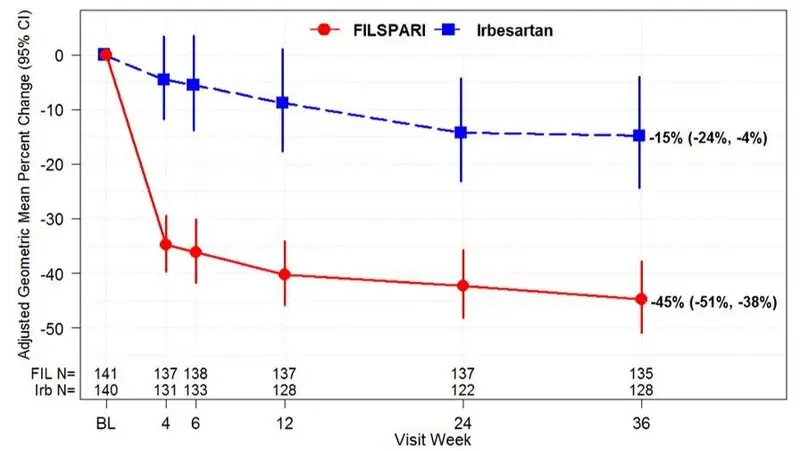
*Adjusted GM of UPCR was based on MMRM stratified by screening eGFR and total urine protein excretion. MMRM analysis includes UPCR data during the double-blind period up to week 36 from the first 281 randomized and treated subjects at the interim analysis. Baseline was defined as the last non-missing observation on or prior to the start of dosing. Missing data were imputed using multiple imputation under the missing at random assumption. Data observed while on randomized treatment and after treatment discontinuation were included in the analysis regardless of treatment discontinuation and initiation of rescue therapy (treatment policy strategy). Rescue immunosuppressive treatment was initiated in 1.4% and 5.7% of FILSPARI and irbesartan patients, respectively. |
||
| FILSPARI N=141 | Irbesartan N=140 |
|
| Adjusted GM of UPCR*, g/g | ||
| Baseline | 1.2 (n=141) | 1.2 (n=140) |
| Week 36 | 0.7 (n=135) | 1.0 (n=128) |
| Adjusted GMPC from Baseline in UPCR at Week 36 (95% CI) | ‑45% (-51%, -38%) | ‑15% (-24%, -4%) |
| FILSPARI versus Irbesartan: Ratio of adjusted GM relative to baseline at week 36 (95% CI) | 0.65 (0.55, 0.77) | |
| p-value | <0.0001 | |
| Adjusted GMPC of UPCR relative to baseline were based on the same MMRM analysis as used in Table 3. Counts in axis table represent number of subjects with UPCR data by visit and treatment group. BL=baseline; CI=confidence interval; FIL=FILSPARI; GMPC=geometric mean percent change; Irb=irbesartan; IAS=interim analysis data set; MMRM=mixed model repeated measures; N=number of subjects in each group; UPCR=urine protein-to-creatinine ratio. |
|
|
How is Filspari supplied
FILSPARI is supplied in bottles of 30 film-coated tablets.
- 200 mg tablets are film-coated, modified oval, white to off-white, debossed with “105” on one side and plain on the other side, available in bottles of 30 tablets with child-resistant caps (NDC 68974-200-30).
- 400 mg tablets are film-coated, modified oval, white to off-white, debossed with “021” on one side and plain on the other side, available in bottles of 30 tablets with child-resistant caps (NDC 68974-400-30).
Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Medication Guide).
Advise the patient that FILSPARI is only available through a restricted access program called the FILSPARI REMS.
Instruct patients that the risks associated with FILSPARI include:
Hepatotoxicity
Advise patients with symptoms suggesting hepatotoxicity (nausea, vomiting, right upper quadrant pain, fatigue, anorexia, jaundice, dark urine, fever, or itching) to immediately stop taking FILSPARI and seek medical attention [see Dosage and Administration (2.2, 2.3, 2.5), Warnings and Precautions (5.1)].
Educate and counsel patients who can become pregnant about the need to use reliable methods of contraception prior to treatment with FILSPARI, during treatment and for one month after treatment discontinuation. Patients who can become pregnant must have pregnancy tests prior to treatment with FILSPARI, monthly during treatment, and one month after treatment discontinuation [see Dosage and Administration (2.2, 2.3, 2.5)), Contraindications (4), Warnings and Precautions (5.2), Use in Specific Populations (8.1, 8.3)].
Advise patients to contact their gynecologist or healthcare provider if they want to change the form of birth control which is used to ensure that another acceptable form of birth control is selected.
Advise the patient that FILSPARI is available only from certified pharmacies that are enrolled in the FILSPARI REMS.
Patients must sign the FILSPARI REMS Patient Enrollment Form to confirm that they understand the risks of FILSPARI.
Other Risks Associated with FILSPARI
Inform patients of other risks associated with FILSPARI, including:
- Hypotension: Advise patients to remain hydrated [see Warnings and Precautions (5.4)].
- Hyperkalemia: Advise patients not to use potassium supplements or salt substitutes that contain potassium without consulting their healthcare provider [see Warnings and Precautions (5.6)].
Distributed by Travere Therapeutics, Inc., San Diego, CA 92130
FILSPARI is a registered trademark of Travere Therapeutics, Inc.
| Medication Guide
FILSPARITM (fil spah’ ree) (sparsentan) tablets |
||
|
What is the most important information I should know about FILSPARI? FILSPARI is only available through the FILSPARI REMS Program. Before you begin taking FILSPARI, you must read and agree to all of the instructions in the FILSPARI REMS Program. FILSPARI can cause serious side effects, including: Liver problems.
|
||
|
|
|
|
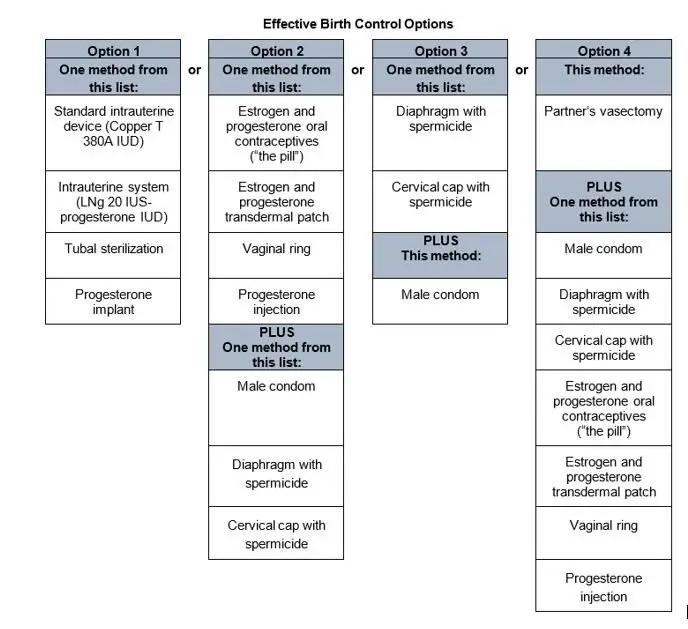
Serious birth defects. FILSPARI can cause serious birth defects if taken during pregnancy.
Patients who can become pregnant must use effective birth control during treatment with FILSPARI and for 1 month after stopping FILSPARI because the medicine may still be in your body.
See the chart below for acceptable birth control options during treatment with FILSPARI.
|
||
|
What is FILSPARI? FILSPARI is a prescription medicine used to reduce levels of protein in the urine (proteinuria) in adults with a kidney disease called primary immunoglobulin A nephropathy (IgAN), who are at risk of their disease progressing quickly. It is not known if FILSPARI is safe and effective in children. |
||
|
Do not take FILSPARI if you:
|
||
|
Before taking FILSPARI, tell your healthcare provider about all of your medical conditions, including if you have:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements, and grapefruit. FILSPARI and other medicines may affect each other and cause side effects. Do not start any new medicine until you check with your healthcare provider. Especially tell your healthcare provider if you take:
|
||
|
How should I take FILSPARI?
|
||
|
What should I avoid while taking FILSPARI?
|
||
|
What are the possible side effects of FILSPARI? FILSPARI can cause serious side effects, including:
|
||
|
|
|
|
These are not all the possible side effects of FILSPARI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store FILSPARI?
|
||
|
General information about the safe and effective use of FILSPARI Medicines are sometimes prescribed for purposes other than those listed in the Medication Guide. Do not use FILSPARI for a condition for which it was not prescribed. Do not give FILSPARI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about FILSPARI that is written for health professionals. |
||
|
What are the ingredients in FILSPARI? Active ingredient: sparsentan Inactive ingredients: colloidal silicon dioxide, lactose anhydrous, magnesium stearate, silicified microcrystalline cellulose, and sodium starch glycolate. Tablets are film coated with material containing macrogol/polyethylene glycol, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide. |
||
|
Distributed by Travere Therapeutics, Inc., San Diego, CA 92130 |
||
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Issued: February 2023 | |
| FILSPARI
sparsentan tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| FILSPARI
sparsentan tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Travere Therapeutics, Inc. (965454502) |
| Registrant - Travere Therapeutics, Inc. (965454502) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Travere Therapeutics, Inc. | 965454502 | LABEL(68974-200, 68974-400) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent CTS, LLC | 962674474 | manufacture(68974-200, 68974-400) | |