SPL PATIENT PACKAGE INSERT
Patient Information
FLONASE®[flow′ naz] Nasal Spray, 50 mcg
(fluticasone propionate)
Read the Patient Information that comes with FLONASE Nasal Spray before you start using it and each time you get a refill. There may be new information. This Patient Information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is FLONASE Nasal Spray?
FLONASE Nasal Spray is an over-the-counter and prescription medicine used to treat non-allergy nasal symptoms such as runny nose, stuffy nose, sneezing, and nasal itching in adults and children aged 4 years and older.
It is not known if FLONASE Nasal Spray is safe and effective in children younger than 4 years of age.
Who should not use FLONASENasal Spray?
Do not use FLONASE Nasal Spray if you are allergic to fluticasone propionate or any of the ingredients in FLONASE Nasal Spray. See “What are the ingredients in FLONASE Nasal Spray?” below for a complete list of ingredients.
What should I tell my healthcare provider before using FLONASENasal Spray?
Tell your healthcare provider about all of your health conditions, including if you:
•have or have had nasal sores, nasal surgery, or nasal injury.•have eye problems, such as cataracts or glaucoma.•have an immune system problem.•are allergic to any of the ingredients in FLONASE Nasal Spray, any other medicines, or food products. See
“What are the ingredients in FLONASE Nasal Spray?”
below for a complete list of ingredients.•have any type of viral, bacterial, or fungal infection.•are exposed to chickenpox or measles.•have any other medical conditions.•are pregnant or planning to become pregnant. It is not known if FLONASE Nasal Spray may harm your unborn baby.•are breastfeeding or plan to breastfeed. It is not known if FLONASE Nasal Spray passes into your breast milk and if it can harm your baby.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. FLONASE Nasal Spray and certain other medicines may interact with each other. This may cause serious side effects. Especially, tell your healthcare provider if you take antifungal or anti-HIV medicines.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use FLONASE Nasal Spray?
Read the step-by-step instructions for using FLONASE Nasal Spray at the end of this Patient Information.
•FLONASE Nasal Spray is for use in your nose only. Do not spray it in your eyes or mouth.•Children should use FLONASE Nasal Spray with an adult’s help, as instructed by the child’s healthcare provider.•Use FLONASE Nasal Spray exactly as your healthcare provider tells you. Do not use FLONASE Nasal Spray more often than prescribed.•FLONASE Nasal Spray may take several days of regular use for your rhinitis symptoms to get better. If your symptoms do not improve or get worse, call your healthcare provider.•You will get the best results if you keep using FLONASE Nasal Spray regularly each day without missing a dose. After you begin to feel better, your healthcare provider may decrease your dose. Do not stop using FLONASE Nasal Spray unless your healthcare provider tells you to do so.
What are the possible side effects of FLONASE Nasal Spray?
FLONASE Nasal Spray may cause serious side effects, including:
•
nose problems.
Nose problems may include:•
nose bleeds.
•
sores (ulcers) in your nose.
•
a certain fungal infection in your nose, mouth, and/or throat (thrush).
•
hole in the cartilage of your nose (nasal septal perforation).
Symptoms of nasal septal perforation may include:•crusting in the nose•nose bleeds•runny nose•whistling sound when you breathe•
slow wound healing.
You should not use FLONASE Nasal Spray until your nose has healed if you have a sore in your nose, have had surgery on your nose, or if your nose has been injured.•
eye problems including glaucoma and cataracts.
You should have regular eye exams while you use FLONASE Nasal Spray.•
serious allergic reactions.
Call your healthcare provider or get emergency medical care if you get any of the following signs of a serious allergic reaction:•rash•hives•swelling of your face, mouth, and tongue•breathing problems•
weakened immune system and increased chance of getting infections (immunosuppression
). Taking medicines that weaken your immune system makes you more likely to get infections and can make certain infections worse. These infections may include tuberculosis (TB), ocular herpes simplex infections, and infections caused by fungi, bacteria, viruses, and parasites. Avoid contact with people who have a contagious disease such as chickenpox or measles while using FLONASE Nasal Spray. If you come in contact with someone who has chickenpox or measles call your healthcare provider right away. Symptoms of an infection may include
:
•fever•pain•aches•chills•feeling tired•nausea•vomiting•
lowered steroid hormone levels (adrenal insufficiency).
Adrenal insufficiency happens when your adrenal glands do not make enough steroid hormones. This can happen when you stop taking oral corticosteroid medicines (such as prednisone) and start taking medicine containing an inhaled steroid (such as FLONASE Nasal Spray). Symptoms of adrenal insufficiency may include:•feeling tired•lack of energy•weakness•nausea and vomiting•low blood pressure•
slowed growth in children.
A child’s growth should be checked often.
The most common side effects of FLONASENasal Spray include:
•headache•sore throat•nose bleeds•nose burning or itching•nausea and vomiting•trouble breathing•cough
Tell your healthcare provider about any side effect that bothers you or does not go away.
These are not all the side effects with FLONASE Nasal Spray. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store FLONASE Nasal Spray?
•Store FLONASE between 39°F and 86°F (4°C and 30°C).
Keep FLONASE Nasal Spray and all medicines out of the reach of children.
General information about the safe and effective use of FLONASE Nasal Spray.
Medicines are sometimes prescribed for purposes not mentioned in a Patient Information leaflet. Do not use FLONASE Nasal Spray for a condition for which it was not prescribed. Do not give your FLONASE Nasal Spray to other people, even if they have the same condition that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about FLONASE Nasal Spray. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about FLONASE Nasal Spray that was written for healthcare professionals.
For more information about FLONASE Nasal Spray, call 1-888-825-5249.
What are the ingredients in FLONASE Nasal Spray?
Active ingredient: fluticasone propionate.
Inactive ingredients: microcrystalline cellulose,carboxymethylcellulose sodium, dextrose, 0.02% w/w benzalkonium chloride, polysorbate 80, and 0.25% w/w phenylethyl alcohol.
Instructions for Use
FLONASE®[flow′ naz]
(fluticasone propionate)
Nasal Spray, 50 mcg
FLONASE Nasal Spray is for use in your nose only.
Read this information before you start using your FLONASE Nasal Spray.
Parts of your FLONASE Nasal Spray (See Figure A)

Figure A
Figure A
Your FLONASE Nasal Spray must be primed before you use it for the first time and when you have not used it for a week or more.
How to prime your FLONASE Nasal Spray
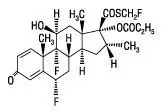
•Shake the bottle gently and then remove the dust cover (Figure B).
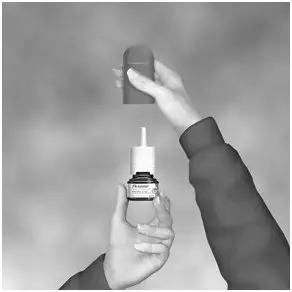
Figure B
Figure B
• Hold the bottle as shown (See Figure C) with the nasal applicator pointing away from you and with your forefinger and middle finger on either side of the nasal applicator and your thumb underneath the bottle. •Press down and release
6
times until a fine spray appears (See Figure C). The pump is now ready for use.
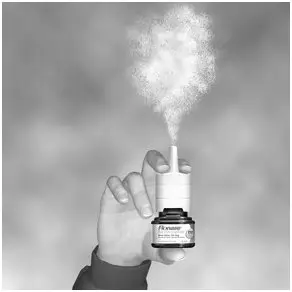
Figure C
Figure C
Using your FLONASE Nasal Spray:
Step 1. Blow your nose to clear your nostrils.
Step 2. Close 1 nostril. Tilt your head forward slightly and, keeping the bottle upright, carefully insert the nasal applicator into the other nostril (See Figure D).

Figure D
Figure D
Step 3. Start to breathe in through your nose, and while breathing in press firmly and quickly down 1 time on the applicator to release the spray. To get a full dose, use your forefinger and middle finger to spray while supporting the base of the bottle with your thumb. Avoid spraying in eyes. Breathe gently inwards through the nostril (See Figure E).
Figure E
Figure E
Step 4. Breathe out through your mouth.
Step 5. If a second spray is required in that nostril, repeat steps 2 through 4 .
Step 6. Repeat steps 2 through 5 in the other nostril.
Step 7. Wipe the nasal applicator with a clean tissue and replace the dust cover (See Figure F).

Figure F
Figure F
Do not use this bottle for more than the labeled number of sprays even though the bottle is not completely empty. Before you throw the bottle away, you should talk to your healthcare provider to see if a refill is needed. Do not take extra doses or stop taking FLONASE Nasal Spray without talking to your healthcare provider.
Cleaning your FLONASE Nasal Spray:
Your nasal spray should be cleaned at least 1 time each week.
1. Remove the dust cover and then gently pull upwards to free the nasal applicator.
2. Wash the applicator and dust cover under warm tap water. Allow to dry at room temperature.
3. Place the applicator and dust cover back on the bottle.
4. If the nasal applicator becomes blocked, it can be removed and left to soak in warm water. Rinse the nasal applicator with cold tap water. Dry the nasal applicator and place it back on the bottle. Do not try to unblock the nasal applicator by inserting a pin or other sharp object.
Storing your FLONASE Nasal Spray:
•Store FLONASE Nasal Spray between 39°F and 86°F (4°C and 30°C).•Do not use your FLONASE Nasal Spray after the date shown as “EXP” on the label or box.
FLONASE is a registered trademark of the GSK group of companies.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2015, the GSK group of companies. All rights reserved.
January 2015
FLN:1PIL