Drug Detail:Fluticasone and vilanterol (Fluticasone and vilanterol [ floo-tik-a-sone-and-vye-lan-ter-ol ])
Drug Class: Bronchodilator combinations
Highlights of Prescribing Information
Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder, for oral inhalation use
Initial U.S. Approval: 2013
Indications and Usage for Fluticasone and Vilanterol Inhalation Powder
Fluticasone Furoate/Vilanterol ELLIPTA is a combination of fluticasone furoate, an inhaled corticosteroid (ICS), and vilanterol, a long‑acting beta2-adrenergic agonist (LABA), indicated for:
- •
- Long-term, once-daily, maintenance treatment of airflow obstruction and reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD). (1.1)
- •
- Once-daily treatment of asthma in patients aged 18 years and older. (1.2)
Important limitation of use: Not indicated for relief of acute bronchospasm. (1.1, 1.2, 5.2)
Fluticasone and Vilanterol Inhalation Powder Dosage and Administration
- •
- For oral inhalation only. (2)
- •
- Maintenance treatment of COPD: 1 inhalation of Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder 100/25 mcg once daily. (2.1)
- •
- Asthma: 1 inhalation of Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder 100/25 mcg or Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder 200/25 mcg once daily. (2.2)
Dosage Forms and Strengths
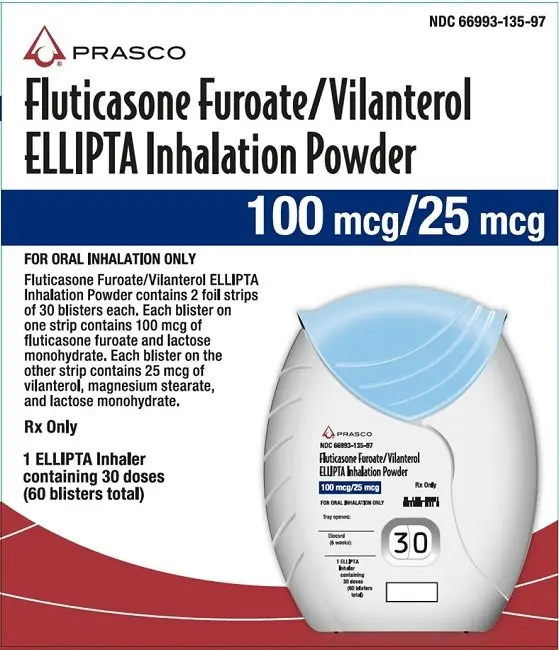
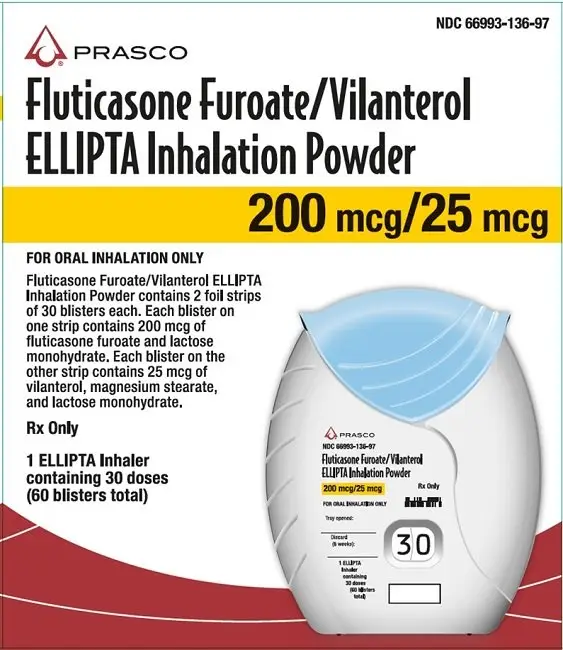
Inhalation powder: Inhaler containing 2 foil blister strips of powder formulation for oral inhalation. One strip contains fluticasone furoate 100 or 200 mcg per blister and the other contains vilanterol 25 mcg per blister. (3)
Contraindications
- •
- Primary treatment of status asthmaticus or acute episodes of COPD or asthma requiring intensive measures. (4)
- •
- Severe hypersensitivity to milk proteins or any ingredients. (4)
Warnings and Precautions
- •
- LABA monotherapy increases the risk of serious asthma-related events. (5.1)
- •
- Do not initiate in acutely deteriorating COPD or asthma. Do not use to treat acute symptoms. (5.2)
- •
- Do not use in combination with an additional medicine containing a LABA because of risk of overdose. (5.3)
- •
- Candida albicans infection of the mouth and pharynx may occur. Monitor patients periodically. Advise the patient to rinse his/her mouth with water without swallowing after inhalation to help reduce the risk. (5.4)
- •
- Increased risk of pneumonia in patients with COPD. Monitor patients for signs and symptoms of pneumonia. (5.5)
- •
- Potential worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infections; ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. (5.6)
- •
- Risk of impaired adrenal function when transferring from systemic corticosteroids. Taper patients slowly from systemic corticosteroids if transferring to Fluticasone Furoate/Vilanterol ELLIPTA. (5.7)
- •
- Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue Fluticasone Furoate/Vilanterol ELLIPTA slowly. (5.8)
- •
- If paradoxical bronchospasm occurs, discontinue Fluticasone Furoate/Vilanterol ELLIPTA and institute alternative therapy. (5.10)
- •
- Use with caution in patients with cardiovascular disorders because of beta-adrenergic stimulation. (5.12)
- •
- Assess for decrease in bone mineral density initially and periodically thereafter. (5.13)
- •
- Glaucoma and cataracts may occur with long-term use of ICS. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Furoate/Vilanterol ELLIPTA long term. (5.14)
- •
- Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis. (5.15)
- •
- Increased blood glucose levels have been reported. Also, be alert to hypokalemia. (5.16)
Adverse Reactions/Side Effects
- •
- COPD: Most common adverse reactions (incidence ≥3%) are nasopharyngitis, upper respiratory tract infection, headache, oral candidiasis, back pain, pneumonia, bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, hypertension, influenza, pharyngitis, and pyrexia. (6.1)
- •
- Asthma: Most common adverse reactions (incidence ≥2%) are nasopharyngitis, oral candidiasis, headache, influenza, upper respiratory tract infection, bronchitis, sinusitis, oropharyngeal pain, dysphonia, and cough. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Prasco Laboratories at 1-866-525-0688 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Strong cytochrome P450 3A4 inhibitors (e.g., ketoconazole): Use with caution. May cause systemic corticosteroid and cardiovascular effects. (7.1)
- •
- Monoamine oxidase inhibitors and tricyclic antidepressants: Use with extreme caution. May potentiate effect of vilanterol on vascular system. (7.2)
- •
- Beta-blockers: Use with caution. May block bronchodilatory effects of beta-agonists and produce severe bronchospasm. (7.3)
- •
- Diuretics: Use with caution. Electrocardiographic changes and/or hypokalemia associated with non–potassium-sparing diuretics may worsen with concomitant beta-agonists. (7.4)
Use In Specific Populations
Hepatic impairment: Fluticasone furoate systemic exposure may increase in patients with moderate or severe impairment. Monitor for systemic corticosteroid effects. (8.6, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2022
Full Prescribing Information
1. Indications and Usage for Fluticasone and Vilanterol Inhalation Powder
1.1 Maintenance Treatment of Chronic Obstructive Pulmonary Disease
Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg is indicated for the long-term, once-daily, maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema. Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg is also indicated to reduce exacerbations of COPD in patients with a history of exacerbations. Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg once daily is the only strength indicated for the treatment of COPD.
Important Limitation of Use
Fluticasone Furoate/Vilanterol ELLIPTA is NOT indicated for the relief of acute bronchospasm
1.2 Treatment of Asthma
Fluticasone Furoate/Vilanterol ELLIPTA is indicated for the once-daily treatment of asthma in patients aged 18 years and older. Fluticasone Furoate/Vilanterol ELLIPTA should be used for patients not adequately controlled on a long-term asthma control medication such as an inhaled corticosteroid (ICS) or whose disease warrants initiation of treatment with both an ICS and long-acting beta2-adrenergic agonist (LABA).
Important Limitation of Use
Fluticasone Furoate/Vilanterol ELLIPTA is NOT indicated for the relief of acute bronchospasm.
2. Fluticasone and Vilanterol Inhalation Powder Dosage and Administration
Fluticasone Furoate/Vilanterol ELLIPTA should be administered as 1 inhalation once daily by the orally inhaled route only.
Fluticasone Furoate/Vilanterol ELLIPTA should be used at the same time every day. Do not use Fluticasone Furoate/Vilanterol ELLIPTA more than 1 time every 24 hours.
After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis.
More frequent administration or a greater number of inhalations (more than 1 inhalation daily) of the prescribed strength of Fluticasone Furoate/Vilanterol ELLIPTA is not recommended as some patients are more likely to experience adverse effects with higher doses. Patients using Fluticasone Furoate/Vilanterol ELLIPTA should not use additional LABA for any reason. [See Warnings and Precautions (5.3, 5.5, 5.8, 5.12).]
2.1 Chronic Obstructive Pulmonary Disease
Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg should be administered as 1 inhalation once daily. The maximum recommended dosage is 1 inhalation of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg once daily, the only strength indicated for the treatment of COPD.
If shortness of breath occurs in the period between doses, an inhaled, short-acting beta2-agonist (rescue medicine, e.g., albuterol) should be taken for immediate relief.
2.2 Asthma
The recommended starting dosage is Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg or Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg administered as 1 inhalation once daily.
When choosing the starting dosage strength of Fluticasone Furoate/Vilanterol ELLIPTA, consider the patients’ disease severity, based on their previous asthma therapy, including the ICS dosage, as well as the patients’ current control of asthma symptoms and risk of future exacerbation.
The maximum recommended dosage is 1 inhalation of Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg once daily.
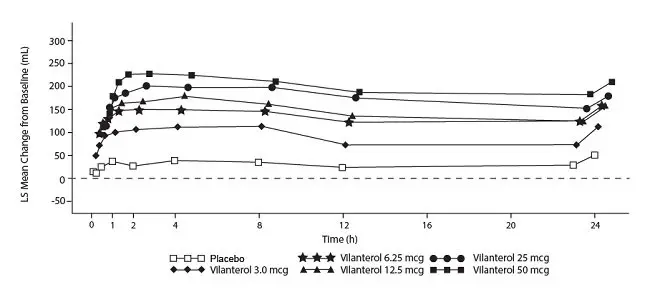
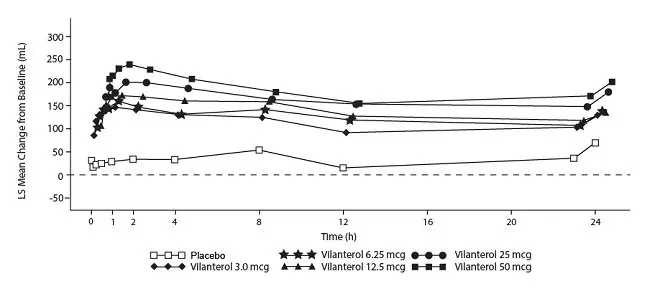
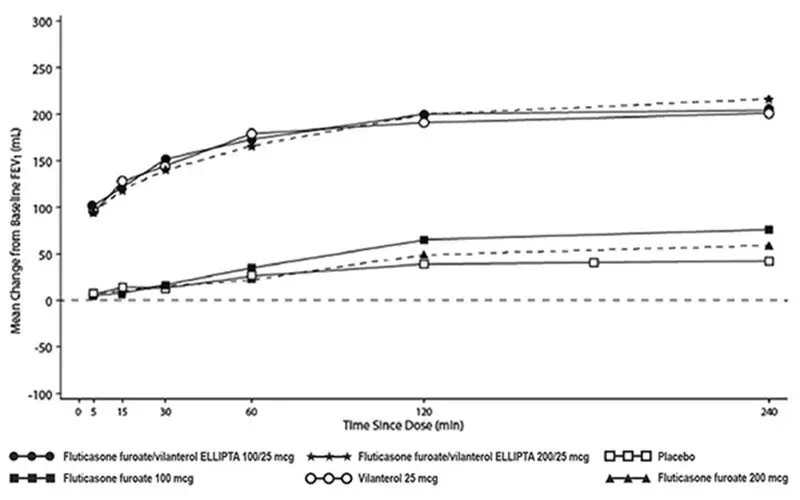
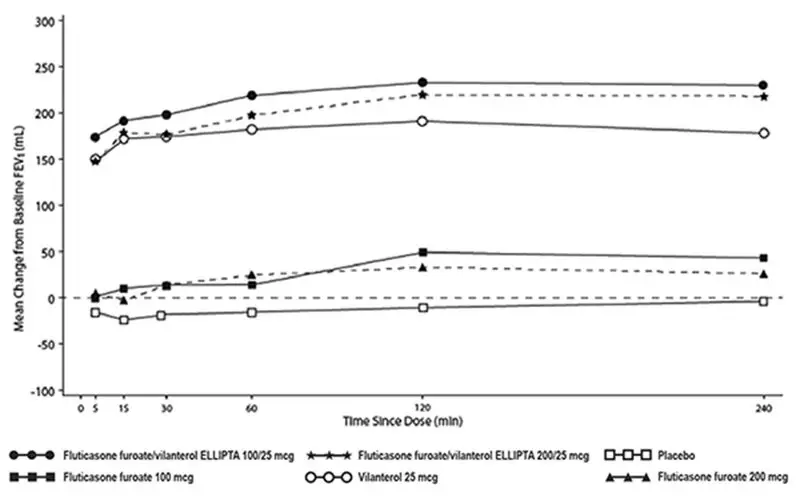
The median time to onset, defined as a 100-mL increase from baseline in mean forced expiratory volume in 1 second (FEV1), was approximately 15 minutes after beginning treatment. Individual patients will experience a variable time to onset and degree of symptom relief.
For patients who do not respond adequately to Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg, increasing the dose to Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg may provide additional improvement in asthma control.
If asthma symptoms arise in the period between doses, an inhaled, short-acting beta2-agonist (rescue medicine, e.g., albuterol) should be taken for immediate relief.
If a previously effective dosage regimen of Fluticasone Furoate/Vilanterol ELLIPTA fails to provide adequate improvement in asthma control, the therapeutic regimen should be reevaluated and additional therapeutic options (e.g., replacing the current strength of Fluticasone Furoate/Vilanterol ELLIPTA with a higher strength, adding additional ICS, initiating oral corticosteroids) should be considered.
3. Dosage Forms and Strengths
Inhalation powder: Disposable light grey and pale blue plastic inhaler containing 2 foil blister strips of powder intended for oral inhalation only. One strip contains fluticasone furoate (100 or 200 mcg per blister), and the other strip contains vilanterol (25 mcg per blister).
4. Contraindications
The use of Fluticasone Furoate/Vilanterol ELLIPTA is contraindicated in the following conditions:
- •
- Primary treatment of status asthmaticus or other acute episodes of COPD or asthma where intensive measures are required [see Warnings and Precautions (5.2)].
- •
- Severe hypersensitivity to milk proteins or demonstrated hypersensitivity to fluticasone furoate, vilanterol, or any of the excipients [see Warnings and Precautions (5.11), Description (11)].
5. Warnings and Precautions
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
Use of LABA as monotherapy (without ICS) for asthma is associated with an increased risk of asthma-related death [see Salmeterol Multicenter Asthma Research Trial (SMART)]. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma‑related events (hospitalizations, intubations, death) compared with ICS alone (see Serious Asthma-Related Events with Inhaled Corticosteroid/Long-acting Beta2-adrenergic Agonists).
Serious Asthma-Related Events with Inhaled Corticosteroid/Long-acting Beta2-adrenergic Agonists
Four (4) large, 26-week, randomized, double-blind, active-controlled clinical safety trials were conducted to evaluate the risk of serious asthma-related events when LABA were used in fixed‑dose combination with ICS compared with ICS alone in subjects with asthma. Three (3) trials included adult and adolescent subjects aged 12 years and older: 1 trial compared budesonide/formoterol with budesonide, 1 trial compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder, and 1 trial compared mometasone furoate/formoterol with mometasone furoate. The fourth trial included pediatric subjects aged 4 to 11 years and compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder. The primary safety endpoint for all 4 trials was serious asthma-related events (hospitalizations, intubations, death). A blinded adjudication committee determined whether events were asthma related.
The 3 adult and adolescent trials were designed to rule out a risk margin of 2.0, and the pediatric trial was designed to rule out a risk margin of 2.7. Each individual trial met its pre-specified objective and demonstrated non-inferiority of ICS/LABA to ICS alone. A meta-analysis of the 3 adult and adolescent trials did not show a significant increase in risk of a serious asthma-related event with ICS/LABA fixed-dose combination compared with ICS alone (Table 1). These trials were not designed to rule out all risk for serious asthma-related events with ICS/LABA compared with ICS.
| ICS = Inhaled Corticosteroid, LABA = Long-acting Beta2-adrenergic Agonist. | |||
| a Randomized subjects who had taken at least 1 dose of study drug. Planned treatment used for analysis. | |||
| b Estimated using a Cox proportional hazards model for time to first event with baseline hazards stratified by each of the 3 trials. | |||
| c Number of subjects with event that occurred within 6 months after the first use of study drug or 7 days after the last date of study drug, whichever date was later. Subjects can have one or more events, but only the first event was counted for analysis. A single, blinded, independent adjudication committee determined whether events were asthma related. | |||
|
ICS/LABA (n = 17,537)a |
ICS (n = 17,552)a |
ICS/LABA vs. ICS Hazard Ratio (95% CI)b |
|
|
Serious asthma-related eventc |
116 |
105 |
1.10 (0.85, 1.44) |
|
Asthma-related death |
2 |
0 | |
|
Asthma-related intubation (endotracheal) |
1 |
2 | |
|
Asthma-related hospitalization (≥24-hour stay) |
115 |
105 | |
The pediatric safety trial included 6,208 pediatric subjects aged 4 to 11 years who received ICS/LABA (fluticasone propionate/salmeterol inhalation powder) or ICS (fluticasone propionate inhalation powder). In this trial, 27/3,107 (0.9%) subjects randomized to ICS/LABA and 21/3,101 (0.7%) subjects randomized to ICS experienced a serious asthma-related event. There were no asthma-related deaths or intubations. ICS/LABA did not show a significantly increased risk of a serious asthma-related event compared with ICS based on the pre-specified risk margin (2.7), with an estimated hazard ratio of time to first event of 1.29 (95% CI: 0.73, 2.27).
Salmeterol Multicenter Asthma Research Trial (SMART)
A 28-week, placebo-controlled, U.S. trial that compared the safety of salmeterol with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in subjects receiving salmeterol (13/13,176 in subjects treated with salmeterol versus 3/13,179 in subjects treated with placebo; relative risk: 4.37 [95% CI: 1.25, 15.34]). Use of background ICS was not required in SMART. The increased risk of asthma-related death is considered a class effect of LABA monotherapy.
5.2 Deterioration of Disease and Acute Episodes
Fluticasone Furoate/Vilanterol ELLIPTA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD or asthma. Fluticasone Furoate/Vilanterol ELLIPTA has not been studied in subjects with acutely deteriorating COPD or asthma. The initiation of Fluticasone Furoate/Vilanterol ELLIPTA in this setting is not appropriate.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg no longer controls symptoms of bronchoconstriction; the patient’s inhaled, short-acting, beta2-agonist becomes less effective; or the patient needs more short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting a reevaluation of the patient and the COPD treatment regimen should be undertaken at once. For COPD, increasing the daily dose of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg is not appropriate in this situation.
Increasing use of inhaled, short-acting beta2-agonists is a marker of deteriorating asthma. In this situation, the patient requires immediate reevaluation with reassessment of the treatment regimen, giving special consideration to the possible need for replacing the current strength of Fluticasone Furoate/Vilanterol ELLIPTA with a higher strength, adding additional ICS, or initiating systemic corticosteroids. Patients should not use more than 1 inhalation once daily of Fluticasone Furoate/Vilanterol ELLIPTA.
Fluticasone Furoate/Vilanterol ELLIPTA should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Fluticasone Furoate/Vilanterol ELLIPTA has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
When beginning treatment with Fluticasone Furoate/Vilanterol ELLIPTA, patients who have been taking oral or inhaled, short-acting beta2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs and to use them only for symptomatic relief of acute respiratory symptoms. When prescribing Fluticasone Furoate/Vilanterol ELLIPTA, the healthcare provider should also prescribe an inhaled, short‑acting beta2-agonist and instruct the patient on how it should be used.
5.3 Excessive Use of Fluticasone Furoate/Vilanterol ELLIPTA and Use with Other Long-acting Beta2-agonists
Fluticasone Furoate/Vilanterol ELLIPTA should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medicines containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using Fluticasone Furoate/Vilanterol ELLIPTA should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
5.4 Local Effects of Inhaled Corticosteroids
In clinical trials, the development of localized infections of the mouth and pharynx with Candida albicans has occurred in subjects treated with fluticasone furoate/vilanterol ELLIPTA. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with Fluticasone Furoate/Vilanterol ELLIPTA continues, but at times therapy with Fluticasone Furoate/Vilanterol ELLIPTA may need to be interrupted. Advise the patient to rinse his/her mouth with water without swallowing following inhalation to help reduce the risk of oropharyngeal candidiasis.
5.5 Pneumonia
An increase in the incidence of pneumonia has been observed in subjects with COPD receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg in clinical trials. There was also an increased incidence of pneumonias resulting in hospitalization. In some incidences these pneumonia events were fatal. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of such infections overlap with the symptoms of COPD exacerbations.
In replicate 12-month trials in 3,255 subjects with moderate to severe COPD who had experienced a COPD exacerbation in the previous year, there was a higher incidence of pneumonia reported in subjects receiving fluticasone furoate/vilanterol 50/25 mcg: 6% (48 of 820 subjects); fluticasone furoate/vilanterol ELLIPTA 100/25 mcg: 6% (51 of 806 subjects); or fluticasone furoate/vilanterol ELLIPTA 200/25 mcg: 7% (55 of 811 subjects) than in subjects receiving vilanterol 25 mcg: 3% (27 of 818 subjects). There was no fatal pneumonia in subjects receiving vilanterol or fluticasone furoate/vilanterol 50/25 mcg. There was fatal pneumonia in 1 subject receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg and in 7 subjects receiving fluticasone furoate/vilanterol ELLIPTA 200/25 mcg (<1% for each treatment group).
In a mortality trial with a median treatment duration of 1.5 years in 16,568 subjects with moderate COPD and cardiovascular disease, the annualized incidence rate of pneumonia was 3.4 per 100 patient-years for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 3.2 for placebo, 3.3 for fluticasone furoate 100 mcg, and 2.3 for vilanterol 25 mcg. Adjudicated, on-treatment deaths due to pneumonia occurred in 13 subjects receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 9 subjects receiving placebo, 10 subjects receiving fluticasone furoate 100 mcg, and 6 subjects receiving vilanterol 25 mcg (<0.2 per 100 patient-years for each treatment group).
5.6 Immunosuppression
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
ICS should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.7 Transferring Patients from Systemic Corticosteroid Therapy
Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available ICS. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although Fluticasone Furoate/Vilanterol ELLIPTA may control COPD or asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress, a severe COPD exacerbation, or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress, a severe COPD exacerbation, or a severe asthma attack.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to Fluticasone Furoate/Vilanterol ELLIPTA. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg on a weekly basis during therapy with Fluticasone Furoate/Vilanterol ELLIPTA. Lung function (FEV1 or peak expiratory flow), beta-agonist use, and COPD or asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition, patients should be observed for signs and symptoms of adrenal insufficiency, such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Transfer of patients from systemic corticosteroid therapy to Fluticasone Furoate/Vilanterol ELLIPTA may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions).
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
5.8 Hypercorticism and Adrenal Suppression
Inhaled fluticasone furoate is absorbed into the circulation and can be systemically active. Effects of fluticasone furoate on the HPA axis are not observed with the therapeutic doses of Fluticasone Furoate/Vilanterol ELLIPTA. However, exceeding the recommended dosage or coadministration with a strong cytochrome P450 3A4 (CYP3A4) inhibitor may result in HPA dysfunction [see Warnings and Precautions (5.9), Drug Interactions (7.1)].
Because of the possibility of significant systemic absorption of ICS in sensitive patients, patients treated with Fluticasone Furoate/Vilanterol ELLIPTA should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, Fluticasone Furoate/Vilanterol ELLIPTA should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids, and other treatments for management of COPD or asthma symptoms should be considered.
5.9 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
Caution should be exercised when considering the coadministration of Fluticasone Furoate/Vilanterol ELLIPTA with ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, clarithromycin, conivaptan, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, saquinavir, telithromycin, troleandomycin, voriconazole) because increased systemic corticosteroid and increased cardiovascular adverse effects may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
5.10 Paradoxical Bronchospasm
As with other inhaled medicines, Fluticasone Furoate/Vilanterol ELLIPTA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with Fluticasone Furoate/Vilanterol ELLIPTA, it should be treated immediately with an inhaled, short-acting bronchodilator; Fluticasone Furoate/Vilanterol ELLIPTA should be discontinued immediately; and alternative therapy should be instituted.
5.11 Hypersensitivity Reactions, including Anaphylaxis
Hypersensitivity reactions such as anaphylaxis, angioedema, rash, and urticaria may occur after administration of Fluticasone Furoate/Vilanterol ELLIPTA. Discontinue Fluticasone Furoate/Vilanterol ELLIPTA if such reactions occur. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder medications containing lactose; therefore, patients with severe milk protein allergy should not use Fluticasone Furoate/Vilanterol ELLIPTA [see Contraindications (4)].
5.12 Cardiovascular Effects
Vilanterol, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles. If such effects occur, Fluticasone Furoate/Vilanterol ELLIPTA may need to be discontinued. In addition, beta‑agonists have been reported to produce electrocardiographic changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
In healthy subjects, large doses of inhaled fluticasone furoate/vilanterol (4 times the recommended dose of vilanterol, representing a 12- or 10-fold higher systemic exposure than seen in subjects with COPD or asthma, respectively) have been associated with clinically significant prolongation of the QTc interval, which has the potential for producing ventricular arrhythmias. Therefore, Fluticasone Furoate/Vilanterol ELLIPTA, like other sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
In a mortality trial with a median treatment duration of 1.5 years in 16,568 subjects with moderate COPD and cardiovascular disease, the annualized incidence rate of adjudicated cardiovascular events (composite of myocardial infarction, stroke, unstable angina, transient ischemic attack, or on-treatment death due to cardiovascular events) was 2.5 per 100 patient‑years for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 2.7 for placebo, 2.4 for fluticasone furoate 100 mcg, and 2.6 for vilanterol 25 mcg. Adjudicated, on-treatment deaths due to cardiovascular events occurred in 82 subjects receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 86 subjects receiving placebo, 80 subjects receiving fluticasone furoate 100 mcg, and 90 subjects receiving vilanterol 25 mcg (annualized incidence rate ranged from 1.2 to 1.3 per 100 patient-years for the treatment groups).
5.13 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing ICS. The clinical significance of small changes in BMD with regard to long‑term consequences such as fracture is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids) should be monitored and treated with established standards of care. Since patients with COPD often have multiple risk factors for reduced BMD, assessment of BMD is recommended prior to initiating Fluticasone Furoate/Vilanterol ELLIPTA and periodically thereafter. If significant reductions in BMD are seen and Fluticasone Furoate/Vilanterol ELLIPTA is still considered medically important for that patient’s COPD therapy, use of medicine to treat or prevent osteoporosis should be strongly considered.
In replicate 12-month trials in 3,255 subjects with moderate to severe COPD, bone fractures were reported by 2% of subjects receiving the fluticasone furoate/vilanterol combination (50/25 mcg: 2% [14 of 820 subjects]; 100/25 mcg: 2% [19 of 806 subjects]; or 200/25 mcg: 2% [14 of 811 subjects]) compared with <1% of subjects receiving vilanterol 25 mcg alone (8 of 818 subjects).
Similar findings were seen in a mortality trial with a median treatment duration of 1.5 years in 16,568 subjects with moderate COPD and cardiovascular disease.
5.14 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients with COPD or asthma following the long-term administration of ICS. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Furoate/Vilanterol ELLIPTA long term.
5.15 Coexisting Conditions
Fluticasone Furoate/Vilanterol ELLIPTA, like all medicines containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2‑adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.16 Hyperglycemia and Hypokalemia
There have been reports of increases in blood glucose levels with fluticasone furoate/vilanterol ELLIPTA. This should be considered in patients with a history of, or with risk factors for, diabetes mellitus [see Adverse Reactions (6.3)].
Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. In clinical trials evaluating fluticasone furoate/vilanterol ELLIPTA in subjects with COPD or asthma, there was no evidence of a treatment effect on serum potassium.
6. Adverse Reactions/Side Effects
Use of LABA may result in the following:
- •
- Serious asthma-related events – hospitalizations, intubations, death [see Warnings and Precautions (5.1)]
- •
- Cardiovascular effects [see Warnings and Precautions (5.12)]
Systemic and local corticosteroid use may result in the following:
- •
- Candida albicans infection [see Warnings and Precautions (5.4)]
- •
- Increased risk of pneumonia in COPD [see Warnings and Precautions (5.5)]
- •
- Immunosuppression [see Warnings and Precautions (5.6)]
- •
- Hypercorticism and adrenal suppression [see Warnings and Precautions (5.8)]
- •
- Reduction in bone mineral density [see Warnings and Precautions (5.13)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience in Chronic Obstructive Pulmonary Disease
The clinical program for fluticasone furoate/vilanterol ELLIPTA included more than 24,000 subjects with COPD in two 6-month lung function trials, two 12-month exacerbation trials, 1 mortality trial, and 6 other trials of shorter duration. A total of 6,174 subjects with COPD received at least 1 dose of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, and 1,087 subjects received a higher strength of fluticasone furoate/vilanterol. The safety data described below are based on the confirmatory 6- and 12-month trials. Adverse reactions observed in the other trials were similar to those observed in the confirmatory trials.
6-Month Trials
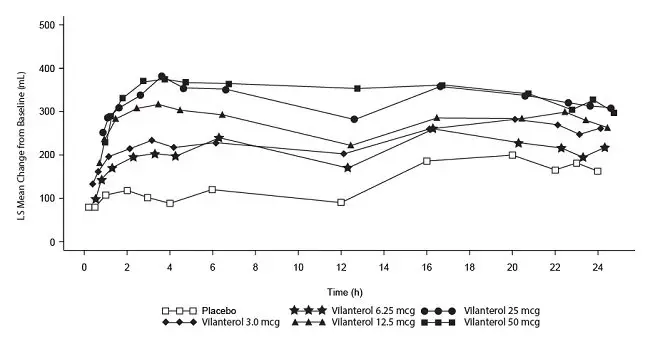
The incidence of adverse reactions associated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg in Table 2 is based on 2 placebo-controlled, 6-month clinical trials (Trials 1 and 2; n = 1,224 and n = 1,030, respectively). Of the 2,254 subjects, 70% were male and 84% were white. They had a mean age of 62 years and an average smoking history of 44 pack years, with 54% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 48% (range: 14% to 87%), the mean postbronchodilator FEV1/forced vital capacity (FVC) ratio was 47% (range: 17% to 88%), and the mean percent reversibility was 14% (range: -41% to 152%).
Subjects received 1 inhalation once daily of the following: fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate/vilanterol 50 /25 mcg, fluticasone furoate 100 mcg, fluticasone furoate 200 mcg, vilanterol 25 mcg, or placebo.
| a Includes oral candidiasis, oropharyngeal candidiasis, candidiasis, and fungal oropharyngitis. | ||||
|
Adverse Reaction |
Fluticasone Furoate/
100/25 mcg (n = 410) % |
Vilanterol 25 mcg (n = 408) % |
Fluticasone Furoate 100 mcg (n = 410) % |
Placebo (n = 412) % |
|
Infections and infestations | ||||
|
Nasopharyngitis |
9 |
10 |
8 |
8 |
|
Upper respiratory tract infection |
7 |
5 |
4 |
3 |
|
Oropharyngeal candidiasisa |
5 |
2 |
3 |
2 |
|
Nervous system disorders | ||||
|
Headache |
7 |
9 |
7 |
5 |
12-Month Trials
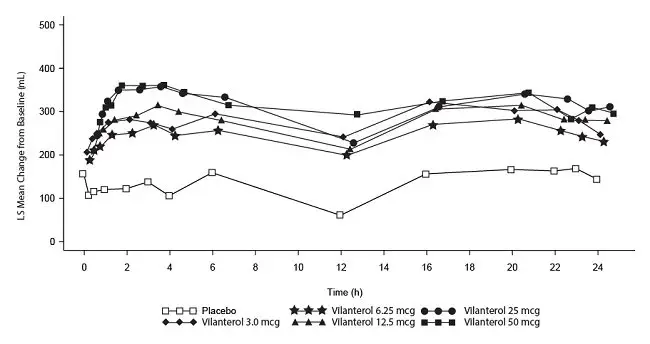
Long-term safety data are based on two 12-month trials (Trials 3 and 4; n = 1,633 and n = 1,622, respectively). Trials 3 and 4 included 3,255 subjects, of which 57% were male and 85% were white. They had a mean age of 64 years and an average smoking history of 46 pack years, with 44% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 45% (range: 12% to 91%), and the mean postbronchodilator FEV1/FVC ratio was 46% (range: 17% to 81%), indicating that the subject population had moderate to very severely impaired airflow obstruction. Subjects received 1 inhalation once daily of the following: fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate/vilanterol 50/25 mcg, or vilanterol 25 mcg. In addition to the reactions shown in Table 2, adverse reactions occurring in ≥3% of the subjects treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 806) for 12 months included back pain, pneumonia [see Warnings and Precautions (5.5)], bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, influenza, pharyngitis, and pyrexia.
Mortality Trial
Safety data are available from a mortality trial in subjects with moderate COPD (moderate airflow limitation [≥50% and ≤70% predicted FEV1]) who either had a history of, or were at risk of, cardiovascular disease and were treated for up to 4 years (median treatment duration of 1.5 years). The trial included 16,568 subjects, 4,140 of whom received fluticasone furoate/vilanterol ELLIPTA 100/25 mcg. In addition to the events in COPD trials shown in Table 2, adverse reactions occurring in ≥3% of the subjects treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg and more common than placebo included pneumonia, back pain, hypertension, and influenza.
6.2 Clinical Trials Experience in Asthma
Fluticasone furoate/vilanterol ELLIPTA for the treatment of asthma was studied in 18 double-blind, parallel-group, controlled trials (11 with placebo) of 4 to 76 weeks’ duration, which enrolled 9,969 subjects with asthma. Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was studied in 2,369 subjects and fluticasone furoate/vilanterol ELLIPTA 200/25 mcg was studied in 956 subjects. While subjects aged 12 to 17 years were included in these trials, fluticasone furoate/vilanterol ELLIPTA is not approved for use in this age group [see Use in Specific Populations (8.4)]. The safety data described below are based on two 12-week efficacy trials, one 24-week efficacy trial, and 2 long-term trials.
12-Week Trials
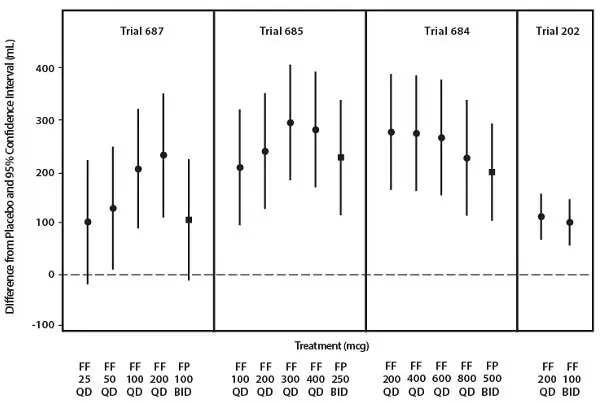
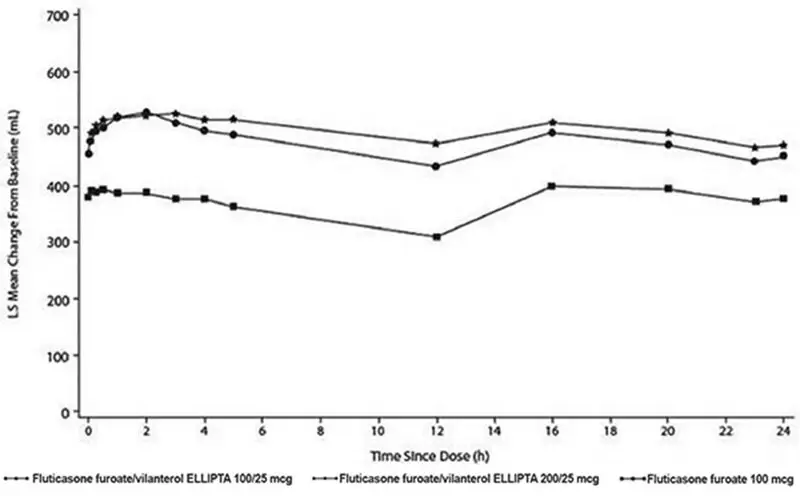
Trial 1 was a 12-week trial that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg in adult and adolescent subjects with asthma compared with fluticasone furoate 100 mcg and placebo. Of the 609 subjects, 58% were female and 84% were white; the mean age was 40 years. The incidence of adverse reactions associated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg is shown in Table 3.
| a Includes oral candidiasis and oropharyngeal candidiasis. | |||
|
Adverse Reaction |
Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg (n = 201) % |
Fluticasone Furoate 100 mcg (n = 205) % |
Placebo (n = 203) % |
|
Infections and infestations | |||
|
Nasopharyngitis |
10 |
7 |
7 |
|
Oral candidiasisa |
2 |
2 |
0 |
|
Nervous system disorders | |||
|
Headache |
5 |
4 |
4 |
|
Respiratory, thoracic, and mediastinal disorders | |||
|
Oropharyngeal pain |
2 |
2 |
1 |
|
Dysphonia |
2 |
1 |
0 |
Trial 2 was a 12-week trial that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, and fluticasone furoate 100 mcg in adult and adolescent subjects with asthma. This trial did not have a placebo arm. Of the 1,039 subjects, 60% were female and 88% were white; the mean age was 46 years. The incidence of adverse reactions associated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg and fluticasone furoate/vilanterol ELLIPTA 200/25 mcg is shown in Table 4.
|
Adverse Reaction |
Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg (n = 346) % |
Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg (n = 346) % |
Fluticasone Furoate 100 mcg (n = 347) % |
|
Nervous system disorders | |||
|
Headache |
8 |
8 |
9 |
|
Infections and infestations | |||
|
Nasopharyngitis |
7 |
6 |
7 |
|
Influenza |
3 |
3 |
1 |
|
Upper respiratory tract infection |
2 |
2 |
3 |
|
Sinusitis |
2 |
1 |
<1 |
|
Bronchitis |
2 |
<1 |
2 |
|
Respiratory, thoracic and mediastinal disorders | |||
|
Oropharyngeal pain |
2 |
2 |
1 |
|
Cough |
1 |
2 |
1 |
24-Week Trial
Trial 3 was a 24-week trial that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 200/25 mcg once daily, fluticasone furoate 200 mcg once daily, and fluticasone propionate 500 mcg twice daily in adult and adolescent subjects with asthma. Of the 586 subjects, 59% were female and 84% were white; the mean age was 46 years. This trial did not have a placebo arm. In addition to the reactions shown in Tables 3 and 4, adverse reactions occurring in ≥2% of subjects treated with fluticasone furoate/vilanterol ELLIPTA 200/25 mcg included viral respiratory tract infection, pharyngitis, pyrexia, and arthralgia.
12-Month Trial
Long-term safety data are based on a 12-month trial that evaluated the safety of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg once daily (n = 201), fluticasone furoate/vilanterol ELLIPTA 200/25 mcg once daily (n = 202), and fluticasone propionate 500 mcg twice daily (n = 100) in adult and adolescent subjects with asthma (Trial 4). Overall, 63% were female and 67% were white. The mean age was 39 years; adolescents (aged 12 to 17 years) made up 16% of the population. In addition to the reactions shown in Tables 3 and 4, adverse reactions occurring in ≥2% of the subjects treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg or fluticasone furoate/vilanterol ELLIPTA 200/25 mcg for 12 months included pyrexia, back pain, extrasystoles, upper abdominal pain, respiratory tract infection, allergic rhinitis, pharyngitis, rhinitis, arthralgia, supraventricular extrasystoles, ventricular extrasystoles, acute sinusitis, and pneumonia.
Exacerbation Trial
In a 24- to 76-week trial, subjects received fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 1,009) or fluticasone furoate 100 mcg (n = 1,010) (Trial 5). Subjects participating in this trial had a history of 1 or more asthma exacerbations that required treatment with oral/systemic corticosteroids or emergency department visit or in-patient hospitalization for the treatment of asthma in the year prior to trial entry. Overall, 67% were female and 73% were white; the mean age was 42 years (adolescents aged 12 to 17 years made up 14% of the population). While subjects aged 12 to 17 years were included in this trial, fluticasone furoate/vilanterol ELLIPTA is not approved for use in this age group [see Use in Specific Populations (8.4)]. Asthma-related hospitalizations occurred in 10 subjects (1%) treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with 7 subjects (0.7%) treated with fluticasone furoate 100 mcg. Among subjects aged 12 to 17 years, asthma-related hospitalizations occurred in 4 subjects (2.6%) treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 151) compared with 0 subjects treated with fluticasone furoate 100 mcg (n = 130). There were no asthma-related deaths or asthma-related intubations observed in this trial.
6.3 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postapproval use of fluticasone furoate/vilanterol ELLIPTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone furoate/vilanterol ELLIPTA or a combination of these factors.
Cardiac Disorders
Palpitations, tachycardia.
Immune System Disorders
Hypersensitivity reactions, including anaphylaxis, angioedema, rash, and urticaria.
Metabolism and Nutrition Disorders
Hyperglycemia.
Musculoskeletal and Connective Tissue Disorders
Muscle spasms.
Nervous System Disorders
Tremor.
Psychiatric Disorders
Nervousness.
Respiratory, Thoracic, and Mediastinal Disorders
Paradoxical bronchospasm.
7. Drug Interactions
7.1 Inhibitors of Cytochrome P450 3A4
Fluticasone furoate and vilanterol, the individual components of Fluticasone Furoate/Vilanterol ELLIPTA, are both substrates of CYP3A4. Concomitant administration of the strong CYP3A4 inhibitor ketoconazole increases the systemic exposure to fluticasone furoate and vilanterol. Caution should be exercised when considering the coadministration of Fluticasone Furoate/Vilanterol ELLIPTA with ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, clarithromycin, conivaptan, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, saquinavir, telithromycin, troleandomycin, voriconazole) [see Warnings and Precautions (5.9), Clinical Pharmacology (12.3)].
7.2 Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
Vilanterol, like other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval or within 2 weeks of discontinuation of such agents, because the effect of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval have an increased risk of ventricular arrhythmias.
7.3 Beta-adrenergic Receptor Blocking Agents
Beta-blockers not only block the pulmonary effect of beta-agonists, such as vilanterol, a component of Fluticasone Furoate/Vilanterol ELLIPTA, but may also produce severe bronchospasm in patients with COPD or asthma. Therefore, patients with COPD or asthma should not normally be treated with beta-blockers. However, under certain circumstances, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents for these patients; cardioselective beta-blockers could be considered, although they should be administered with caution.
7.4 Non–Potassium-Sparing Diuretics
The electrocardiographic changes and/or hypokalemia that may result from the administration of non–potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with non–potassium-sparing diuretics.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are insufficient data on the use of fluticasone furoate/vilanterol ELLIPTA, fluticasone furoate, or vilanterol in pregnant women. There are clinical considerations with use of fluticasone furoate/vilanterol ELLIPTA in pregnant women. (See Clinical Considerations.) In an animal reproduction study, fluticasone furoate and vilanterol administered by inhalation alone or in combination to pregnant rats during the period of organogenesis produced no fetal structural abnormalities. The highest fluticasone furoate and vilanterol doses in this study were approximately 5 and 40 times the maximum recommended human daily inhalation doses (MRHDID) of 200 and 25 mcg in adults, respectively. (See Data.)
The estimated risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryofetal Risk: In women with poorly or moderately controlled asthma, there is an increased risk of several perinatal outcomes such as pre-eclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. Pregnant women should be closely monitored and medication adjusted as necessary to maintain optimal control of asthma.
Labor and Delivery: There are no human studies evaluating the effects of fluticasone furoate/vilanterol ELLIPTA during labor and delivery. Because of the potential for beta-agonist interference with uterine contractility, use of Fluticasone Furoate/Vilanterol ELLIPTA during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Data
Animal Data: Fluticasone Furoate and Vilanterol: In an embryofetal developmental study, pregnant rats received fluticasone furoate and vilanterol during the period of organogenesis at doses up to approximately 5 and 40 times the MRHDID, respectively, alone or in combination (on a mcg/m2 basis at inhalation doses up to approximately 95 mcg/kg/day). No evidence of structural abnormalities was observed.
Fluticasone Furoate: In 2 separate embryofetal developmental studies, pregnant rats and rabbits received fluticasone furoate during the period of organogenesis at doses up to approximately 4 and 1 times the MRHDID, respectively (on a mcg/m2 basis at maternal inhalation doses up to 91 and 8 mcg/kg/day). No evidence of structural abnormalities in fetuses was observed in either species. In a perinatal and postnatal developmental study in rats, dams received fluticasone furoate during late gestation and lactation periods at doses up to approximately 1 time the MRHDID (on a mcg/m2 basis at maternal inhalation doses up to 27 mcg/kg/day). No evidence of effects on offspring development was observed.
Vilanterol: In 2 separate embryofetal developmental studies, pregnant rats and rabbits received vilanterol during the period of organogenesis at doses up to approximately 13,000 and 1,000 times, respectively, the MRHDID (on a mcg/m2 basis at maternal inhalation doses up to 33,700 mcg/kg/day in rats and on an AUC basis at maternal inhaled doses up to 5,740 mcg/kg/day in rabbits). No evidence of structural abnormalities was observed at any dose in rats or in rabbits up to approximately 160 times the MRHDID (on an AUC basis at maternal doses up to 591 mcg/kg/day). However, fetal skeletal variations were observed in rabbits at approximately 1,000 times the MRHDID (on an AUC basis at maternal inhaled or subcutaneous doses of 5,740 or 300 mcg/kg/day, respectively). The skeletal variations included decreased or absent ossification in cervical vertebral centrum and metacarpals. In a perinatal and postnatal developmental study in rats, dams received vilanterol during late gestation and the lactation periods at doses up to approximately 3,900 times the MRHDID (on a mcg/m2 basis at maternal oral doses up to 10,000 mcg/kg/day). No evidence of effects in offspring development was observed.
8.2 Lactation
Risk Summary
There is no information available on the presence of fluticasone furoate or vilanterol in human milk, the effects on the breastfed child, or the effects on milk production. Low concentrations of other ICS have been detected in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Fluticasone Furoate/Vilanterol ELLIPTA and any potential adverse effects on the breastfed child from fluticasone furoate or vilanterol or from the underlying maternal condition.
8.4 Pediatric Use
Fluticasone Furoate/Vilanterol ELLIPTA is not indicated for use in children and adolescents. The safety and efficacy in pediatric patients (aged 17 years and younger) have not been established.
In a 24- to 76-week exacerbation trial, subjects received fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 1,009) or fluticasone furoate 100 mcg (n = 1,010). Subjects had a mean age of 42 years and a history of 1 or more asthma exacerbations that required treatment with oral/systemic corticosteroids or emergency department visit or in-patient hospitalization for the treatment of asthma in the year prior to study entry. [See Clinical Studies (14.2).] Adolescents aged 12 to 17 years made up 14% of the study population (n = 281), with a mean exposure of 352 days for subjects in this age group treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 151) and 355 days for subjects in this age group treated with fluticasone furoate 100 mcg (n = 130). In this age group, 10% of subjects treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reported an asthma exacerbation compared with 7% for subjects treated with fluticasone furoate 100 mcg. Among the adolescents, asthma-related hospitalizations occurred in 4 subjects (2.6%) treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with 0 subjects treated with fluticasone furoate 100 mcg. There were no asthma‑related deaths or asthma-related intubations observed in the adolescent age group.
Effects on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to children and adolescents. A reduction of growth velocity in children and adolescents may occur as a result of poorly controlled asthma or from use of corticosteroids, including ICS. The effects of long-term treatment of children and adolescents with ICS, including fluticasone furoate, on final adult height are not known.
Controlled clinical trials have shown that ICS may cause a reduction in growth in children. In these trials, the mean reduction in growth velocity was approximately 1 cm/year (range: 0.3 to 1.8 cm/year) and appears to be related to dose and duration of exposure. This effect has been observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in children than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied. The growth of children and adolescents receiving orally inhaled corticosteroids, including Fluticasone Furoate/Vilanterol ELLIPTA, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including Fluticasone Furoate/Vilanterol ELLIPTA, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
A randomized, double-blind, parallel-group, multicenter, 1-year, placebo-controlled trial evaluated the effect of once-daily treatment with 110 mcg of fluticasone furoate in the nasal spray formulation on growth velocity assessed by stadiometry. The subjects were 474 prepubescent children (girls aged 5 to 7.5 years and boys aged 5 to 8.5 years). Mean growth velocity over the 52-week treatment period was lower in the subjects receiving fluticasone furoate nasal spray (5.19 cm/year) compared with placebo (5.46 cm/year). The mean reduction in growth velocity was 0.27 cm/year (95% CI: 0.06, 0.48) [see Warnings and Precautions (5.17)].
8.5 Geriatric Use
Based on available data, no adjustment of the dosage of Fluticasone Furoate/Vilanterol ELLIPTA in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
Clinical trials of fluticasone furoate/vilanterol ELLIPTA for COPD included 4,820 subjects aged 65 and older and 1,118 subjects aged 75 and older. Clinical trials of fluticasone furoate/vilanterol ELLIPTA for asthma included 854 subjects aged 65 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects.
8.6 Hepatic Impairment
Fluticasone furoate systemic exposure increased by up to 3‑fold in subjects with hepatic impairment compared with healthy subjects. Hepatic impairment had no effect on vilanterol systemic exposure. Use Fluticasone Furoate/Vilanterol ELLIPTA with caution in patients with moderate or severe hepatic impairment. Monitor patients for corticosteroid-related side effects [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
There were no significant increases in either fluticasone furoate or vilanterol exposure in subjects with severe renal impairment (CrCl <30 mL/min) compared with healthy subjects. No dosage adjustment is required in patients with renal impairment [see Clinical Pharmacology (12.3)].
10. Overdosage
No human overdosage data has been reported for fluticasone furoate/vilanterol ELLIPTA.
Fluticasone Furoate/Vilanterol ELLIPTA contains both fluticasone furoate and vilanterol; therefore, the risks associated with overdosage for the individual components described below apply to Fluticasone Furoate/Vilanterol ELLIPTA. Treatment of overdosage consists of discontinuation of Fluticasone Furoate/Vilanterol ELLIPTA together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta‑receptor blocker may be considered, bearing in mind that such medicine can produce bronchospasm. Cardiac monitoring is recommended in cases of overdosage.
10.1 Fluticasone Furoate
Because of low systemic bioavailability (15.2%) and an absence of acute drug-related systemic findings in clinical trials, overdosage of fluticasone furoate is unlikely to require any treatment other than observation. If used at excessive doses for prolonged periods, systemic effects such as hypercorticism may occur [see Warnings and Precautions (5.8)].
Single- and repeat-dose trials of fluticasone furoate at doses of 50 to 4,000 mcg have been studied in human subjects. Decreases in mean serum cortisol were observed at dosages of 500 mcg or higher given once daily for 14 days.
10.2 Vilanterol
The expected signs and symptoms with overdosage of vilanterol are those of excessive beta‑adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms of beta-adrenergic stimulation (e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, hypokalemia, metabolic acidosis). As with all inhaled sympathomimetic medicines, cardiac arrest and even death may be associated with an overdose of vilanterol.
11. Fluticasone and Vilanterol Inhalation Powder Description
Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg and Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg are inhalation powders for oral inhalation that contain a combination of fluticasone furoate (an ICS) and vilanterol (a LABA).
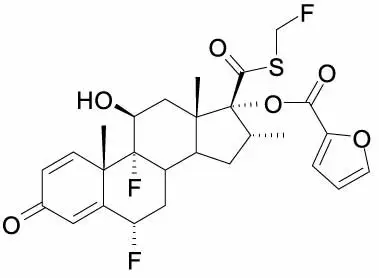
One active component of Fluticasone Furoate/Vilanterol ELLIPTA is fluticasone furoate, a synthetic trifluorinated corticosteroid having the chemical name (6α,11β,16α,17α)-6,9-difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl 2-furancarboxylate and the following chemical structure:
Fluticasone furoate is a white powder with a molecular weight of 538.6, and the empirical formula is C27H29F3O6S. It is practically insoluble in water.
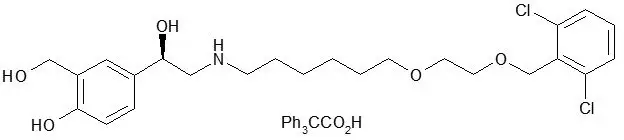
The other active component of Fluticasone Furoate/Vilanterol ELLIPTA is vilanterol trifenatate, a LABA with the chemical name triphenylacetic acid-4-{(1R)-2-[(6-{2-[2,6-dicholorobenzyl)oxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol (1:1) and the following chemical structure:
Vilanterol trifenatate is a white powder with a molecular weight of 774.8, and the empirical formula is C24H33Cl2NO5•C20H16O2. It is practically insoluble in water.
Fluticasone Furoate/Vilanterol ELLIPTA is a light grey and pale blue plastic inhaler containing 2 foil blister strips. Each blister on one strip contains a white powder mix of micronized fluticasone furoate (100 or 200 mcg) and lactose monohydrate (12.4 or 12.3 mg), and each blister on the other strip contains a white powder mix of micronized vilanterol trifenatate (40 mcg equivalent to 25 mcg of vilanterol), magnesium stearate (125 mcg), and lactose monohydrate (12.34 mg). The lactose monohydrate contains milk proteins. After the inhaler is activated, the powder within both blisters is exposed and ready for dispersion into the airstream created by the patient inhaling through the mouthpiece.
Under standardized in vitro test conditions, fluticasone furoate/vilanterol ELLIPTA delivers 92 and 184 mcg of fluticasone furoate and 22 mcg of vilanterol per blister when tested at a flow rate of 60 L/min for 4 seconds.
In adult subjects with obstructive lung disease and severely compromised lung function (COPD with FEV1/FVC <70% and FEV1 <30% predicted or FEV1 <50% predicted plus chronic respiratory failure), mean peak inspiratory flow through the ELLIPTA inhaler was 66.5 L/min (range: 43.5 to 81.0 L/min).
In adult subjects with severe asthma, mean peak inspiratory flow through the ELLIPTA inhaler was 96.6 L/min (range: 72.4 to 124.6 L/min).
The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
12. Fluticasone and Vilanterol Inhalation Powder - Clinical Pharmacology
12.1 Mechanism of Action
Fluticasone Furoate/Vilanterol ELLIPTA
Since Fluticasone Furoate/Vilanterol ELLIPTA contains both fluticasone furoate and vilanterol, the mechanisms of action described below for the individual components apply to Fluticasone Furoate/Vilanterol ELLIPTA. These drugs represent 2 different classes of medications (a synthetic corticosteroid and a LABA) that have different effects on clinical and physiological indices.
Fluticasone Furoate
Fluticasone furoate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone furoate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is approximately 29.9 times that of dexamethasone and 1.7 times that of fluticasone propionate. The clinical relevance of these findings is unknown.
The precise mechanism through which fluticasone furoate affects COPD and asthma symptoms is not known. Inflammation is an important component in the pathogenesis of COPD and asthma. Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in inflammation. Specific effects of fluticasone furoate demonstrated in in vitro and in vivo models included activation of the glucocorticoid response element, inhibition of pro-inflammatory transcription factors such as NFkB, and inhibition of antigen-induced lung eosinophilia in sensitized rats. These anti-inflammatory actions of corticosteroids may contribute to their efficacy.
Vilanterol
Vilanterol is a LABA. In vitro tests have shown the functional selectivity of vilanterol was similar to salmeterol. The clinical relevance of this in vitro finding is unknown.
Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenoceptor agonist drugs, including vilanterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′,5′-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Healthy Subjects: QTc interval prolongation was studied in a double-blind, multiple-dose, placebo- and positive-controlled crossover study in 85 healthy volunteers. The maximum mean (95% upper confidence bound) difference in QTcF from placebo after baseline-correction was 4.9 (7.5) milliseconds and 9.6 (12.2) milliseconds seen 30 minutes after dosing for fluticasone furoate/vilanterol 200 mcg/25 mcg and fluticasone furoate/vilanterol 800 mcg/100 mcg, respectively.
A dose-dependent increase in heart rate was also observed. The maximum mean (95% upper confidence bound) difference in heart rate from placebo after baseline-correction was 7.8 (9.4) beats/min and 17.1 (18.7) beats/min seen 10 minutes after dosing for fluticasone furoate/vilanterol 200 mcg/25 mcg and fluticasone furoate/vilanterol 800 mcg/100 mcg, respectively.
Hypothalamic-Pituitary-Adrenal Axis Effects
Healthy Subjects: Inhaled fluticasone furoate at repeat doses up to 400 mcg was not associated with statistically significant decreases in serum or urinary cortisol in healthy subjects. Decreases in serum and urine cortisol levels were observed at fluticasone furoate exposures several-fold higher than exposures observed at the therapeutic dose.
Subjects with Chronic Obstructive Pulmonary Disease: In a trial with subjects with COPD, treatment with fluticasone furoate (50, 100, or 200 mcg)/vilanterol 25 mcg, vilanterol 25 mcg, and fluticasone furoate (100 or 200 mcg) for 6 months did not affect 24-hour urinary cortisol excretion. A separate trial with subjects with COPD demonstrated no effects on serum cortisol after 28 days of treatment with fluticasone furoate (50, 100, or 200 mcg)/vilanterol 25 mcg.
Subjects with Asthma: A randomized, double-blind, parallel-group trial in 185 subjects with asthma showed no difference between once-daily treatment with fluticasone furoate/vilanterol 100 mcg/25 mcg or fluticasone furoate/vilanterol 200 mcg/25 mcg compared with placebo on serum cortisol weighted mean (0 to 24 hours), serum cortisol AUC(0-24), and 24‑hour urinary cortisol after 6 weeks of treatment, whereas prednisolone 10 mg given once daily for 7 days resulted in significant cortisol suppression.
12.3 Pharmacokinetics
Linear pharmacokinetics was observed for fluticasone furoate (200 to 800 mcg) and vilanterol (25 to 100 mcg). On repeated once-daily inhalation administration, steady state of fluticasone furoate and vilanterol plasma concentrations was achieved after 6 days, and the accumulation was up to 2.6-fold for fluticasone furoate and 2.4-fold for vilanterol as compared with single dose.
Absorption
Fluticasone Furoate: Fluticasone furoate plasma levels may not predict therapeutic effect. Peak plasma concentrations are reached within 0.5 to 1 hour. Absolute bioavailability of fluticasone furoate when administrated by inhalation was 15.2%, primarily due to absorption of the inhaled portion of the dose delivered to the lung. Oral bioavailability from the swallowed portion of the dose is low (approximately 1.3%) due to extensive first-pass metabolism. Systemic exposure (AUC) in subjects with COPD or asthma was 46% or 7% lower, respectively, than observed in healthy subjects.
Vilanterol: Vilanterol plasma levels may not predict therapeutic effect. Peak plasma concentrations are reached within 10 minutes following inhalation. Absolute bioavailability of vilanterol when administrated by inhalation was 27.3%, primarily due to absorption of the inhaled portion of the dose delivered to the lung. Oral bioavailability from the swallowed portion of the dose of vilanterol is low (<2%) due to extensive first-pass metabolism. Systemic exposure (AUC) in subjects with COPD was 24% higher than observed in healthy subjects. Systemic exposure (AUC) in subjects with asthma was 21% lower than observed in healthy subjects.
Distribution
Fluticasone Furoate: Following intravenous administration to healthy subjects, the mean volume of distribution at steady state was 661 L. Binding of fluticasone furoate to human plasma proteins was high (99.6%).
Vilanterol: Following intravenous administration to healthy subjects, the mean volume of distribution at steady state was 165 L. Binding of vilanterol to human plasma proteins was 93.9%.
Metabolism
Fluticasone Furoate: Fluticasone furoate is cleared from systemic circulation principally by hepatic metabolism via CYP3A4 to metabolites with significantly reduced corticosteroid activity. There was no in vivo evidence for cleavage of the furoate moiety resulting in the formation of fluticasone.
Vilanterol: Vilanterol is mainly metabolized, principally via CYP3A4, to a range of metabolites with significantly reduced β1- and β2-agonist activity.
Elimination
Fluticasone Furoate: Fluticasone furoate and its metabolites are eliminated primarily in the feces, accounting for approximately 101% and 90% of the orally and intravenously administered doses, respectively. Urinary excretion accounted for approximately 1% and 2% of the orally and intravenously administered doses, respectively. Following repeat-dose inhaled administration, the plasma elimination phase half-life averaged 24 hours.
Vilanterol: Following oral administration, vilanterol was eliminated mainly by metabolism followed by excretion of metabolites in urine and feces (approximately 70% and 30% of the recovered radioactive dose, respectively). The plasma elimination half-life of vilanterol, as determined from inhalation administration of multiple doses of vilanterol 25 mcg, is 21.3 hours in subjects with COPD and 16.0 hours in subjects with asthma.
Specific Populations
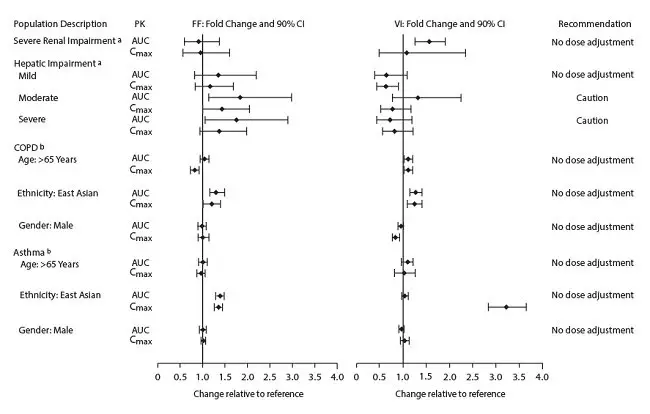
The effect of renal and hepatic impairment and other intrinsic factors on the pharmacokinetics of fluticasone furoate and vilanterol is shown in Figure 1.
Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination
Racial or Ethnic Groups: Systemic exposure [AUC(0-24)] to inhaled fluticasone furoate 200 mcg was 27% to 49% higher in healthy subjects of Japanese, Korean, and Chinese heritage compared with white subjects. Similar differences were observed for subjects with COPD or asthma (Figure 1). However, there is no evidence that this higher exposure to fluticasone furoate results in clinically relevant effects on urinary cortisol excretion or on efficacy in these racial groups.
There was no effect of race on the pharmacokinetics of vilanterol in subjects with COPD. In subjects with asthma, vilanterol Cmax is estimated to be higher (3-fold) and AUC(0-24) comparable for those subjects from an Asian heritage compared with subjects from a non-Asian heritage. However, the higher Cmax values are similar to those seen in healthy subjects.
Patients with Hepatic Impairment: Fluticasone Furoate: Following repeat dosing of fluticasone furoate/vilanterol 200 mcg/25 mcg (100 mcg/12.5 mcg in the severe impairment group) for 7 days, there was an increase of 34%, 83%, and 75% in fluticasone furoate systemic exposure (AUC) in subjects with mild, moderate, and severe hepatic impairment, respectively, compared with healthy subjects (Figure 1).
In subjects with moderate hepatic impairment receiving fluticasone furoate/vilanterol 200 mcg/25 mcg, mean serum cortisol (0 to 24 hours) was reduced by 34% (90% CI: 11%, 51%) compared with healthy subjects. In subjects with severe hepatic impairment receiving fluticasone furoate/vilanterol 100 mcg/12.5 mcg, mean serum cortisol (0 to 24 hours) was increased by 14% (90% CI: -16%, 55%) compared with healthy subjects. Patients with moderate to severe hepatic disease should be closely monitored.
Vilanterol: Hepatic impairment had no effect on vilanterol systemic exposure [Cmax and AUC(0-24) on Day 7] following repeat-dose administration of fluticasone furoate/vilanterol 200 mcg/25 mcg (100 mcg/12.5 mcg in the severe impairment group) for 7 days (Figure 1).
There were no additional clinically relevant effects of the fluticasone furoate/vilanterol combinations on heart rate or serum potassium in subjects with mild or moderate hepatic impairment (vilanterol 25 mcg combination) or with severe hepatic impairment (vilanterol 12.5 mcg combination) compared with healthy subjects.
Patients with Renal Impairment: Fluticasone furoate systemic exposure was not increased and vilanterol systemic exposure [AUC(0-24)] was 56% higher in subjects with severe renal impairment compared with healthy subjects (Figure 1). There was no evidence of greater corticosteroid or beta-agonist class-related systemic effects (assessed by serum cortisol, heart rate, and serum potassium) in subjects with severe renal impairment compared with healthy subjects.
Drug Interaction Studies
There were no clinically relevant differences in the pharmacokinetics or pharmacodynamics of either fluticasone furoate or vilanterol when administered in combination compared with administration alone. The potential for fluticasone furoate and vilanterol to inhibit or induce metabolic enzymes and transporter systems is negligible at low inhalation doses.
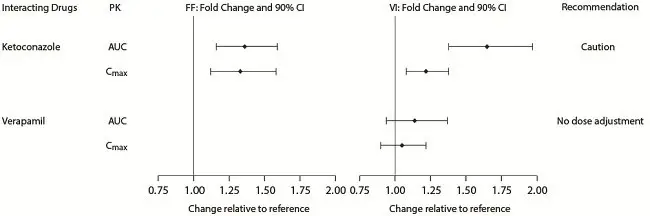
Inhibitors of Cytochrome P450 3A4: The exposure (AUC) of fluticasone furoate and vilanterol were 36% and 65% higher, respectively, when coadministered with ketoconazole 400 mg compared with placebo (Figure 2). The increase in fluticasone furoate exposure was associated with a 27% reduction in weighted mean serum cortisol (0 to 24 hours). The increase in vilanterol exposure was not associated with an increase in beta-agonist–related systemic effects on heart rate or blood potassium.
Figure 2. Impact of Coadministered Drugsa on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination or Vilanterol Coadministered with a Long-acting Muscarinic Antagonist
Inhibitors of P-glycoprotein: Fluticasone furoate and vilanterol are both substrates of P‑glycoprotein (P-gp). Coadministration of repeat-dose (240 mg once daily) verapamil (a potent P-gp inhibitor and moderate CYP3A4 inhibitor) did not affect the vilanterol Cmax or AUC in healthy subjects (Figure 2). Drug interaction trials with a specific P-gp inhibitor and fluticasone furoate have not been conducted.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Fluticasone Furoate/Vilanterol ELLIPTA
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with fluticasone furoate/vilanterol ELLIPTA; however, studies are available for the individual components, fluticasone furoate and vilanterol, as described below.
Fluticasone Furoate
Fluticasone furoate produced no treatment-related increases in the incidence of tumors in 2-year inhalation studies in rats and mice at inhaled doses up to 9 and 19 mcg/kg/day, respectively (approximately 0.5 times the MRHDID in adults on a mcg/m2 basis).
Fluticasone furoate did not induce gene mutation in bacteria or chromosomal damage in a mammalian cell mutation test in mouse lymphoma L5178Y cells in vitro. There was also no evidence of genotoxicity in the in vivo micronucleus test in rats.
No evidence of impairment of fertility was observed in male and female rats at inhaled fluticasone furoate doses up to 29 and 91 mcg/kg/day, respectively (approximately 3 and 8 times, respectively, the MRHDID based on AUC) [see Use in Specific Populations (8.1)].
Vilanterol
In a 2-year carcinogenicity study in mice, vilanterol caused a statistically significant increase in ovarian tubulostromal adenomas in females at an inhalation dose of 29,500 mcg/kg/day (approximately 8,750 times the MRHDID in adults on an AUC basis). No increase in tumors was seen at an inhalation dose of 615 mcg/kg/day (approximately 530 times the MRHDID in adults on an AUC basis).
In a 2-year carcinogenicity study in rats, vilanterol caused statistically significant increases in mesovarian leiomyomas in females and shortening of the latency of pituitary tumors at inhalation doses greater than or equal to 84.4 mcg/kg/day (greater than or equal to approximately 45 times the MRHDID in adults on an AUC basis). No tumors were seen at an inhalation dose of 10.5 mcg/kg/day (approximately 2 times the MRHDID in adults on an AUC basis).
These tumor findings in rodents are similar to those reported previously for other beta-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Vilanterol tested negative in the following genotoxicity assays: the in vitro Ames assay, in vivo rat bone marrow micronucleus assay, in vivo rat unscheduled DNA synthesis (UDS) assay, and in vitro Syrian hamster embryo (SHE) cell assay. Vilanterol tested equivocal in the in vitro mouse lymphoma assay.
No evidence of impairment of fertility was observed in male and female rats at inhaled vilanterol doses up to 31,500 and 37,100 mcg/kg/day, respectively (approximately 5,490 times the MRHDID based on AUC).
14. Clinical Studies
14.1 Chronic Obstructive Pulmonary Disease
The safety and efficacy of fluticasone furoate/vilanterol ELLIPTA were evaluated in more than 24,000 subjects with COPD. The development program included 4 confirmatory trials of 6 and 12 months’ duration, three 12-week active comparator trials with fluticasone propionate/salmeterol 250/50 mcg, 1 long-term trial, and dose-ranging trials of shorter duration. The efficacy of fluticasone furoate/vilanterol ELLIPTA is based primarily on the dose-ranging trials and the 4 confirmatory trials described below.
Dose Selection for Vilanterol
Dose selection for vilanterol in COPD was supported by a 28-day, randomized, double-blind, placebo-controlled, parallel-group trial evaluating 5 doses of vilanterol (3 to 50 mcg) or placebo dosed in the morning in 602 subjects with COPD. Results demonstrated dose-related increases from baseline in FEV1 at Day 1 and Day 28 (Figure 3).
Figure 3. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28
Day 1
Day 28
The differences in trough FEV1 on Day 28 from placebo for the 3-, 6.25-, 12.5-, 25-, and 50-mcg doses were 92 mL (95% CI: 39, 144), 98 mL (95% CI: 46, 150), 110 mL (95% CI: 57, 162), 137 mL (95% CI: 85, 190), and 165 mL (95% CI: 112, 217), respectively. These results supported the evaluation of vilanterol 25 mcg once daily in the confirmatory trials for COPD.
Dose Selection for Fluticasone Furoate
Dose selection of fluticasone furoate for Phase 3 trials in subjects with COPD was based on dose-ranging trials conducted in subjects with asthma; these trials are described in detail below [see Clinical Studies (14.2)].
Confirmatory Trials
The 4 confirmatory trials evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA on lung function (Trials 1 and 2) and exacerbations (Trials 3 and 4).
Lung Function: Trials 1 and 2 were 24-week, randomized, double-blind, placebo-controlled trials designed to evaluate the efficacy of fluticasone furoate/vilanterol ELLIPTA on lung function in subjects with COPD. In Trial 1, subjects were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate 100 mcg, fluticasone furoate 200 mcg, vilanterol 25 mcg, and placebo. In Trial 2, subjects were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol 50/25 mcg, fluticasone furoate 100 mcg, vilanterol 25 mcg, and placebo. All treatments were administered as 1 inhalation once daily.
Of the 2,254 patients, 70% were male and 84% were white. They had a mean age of 62 years and an average smoking history of 44 pack years, with 54% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 48% (range: 14% to 87%), mean postbronchodilator FEV1/FVC ratio was 47% (range: 17% to 88%), and the mean percent reversibility was 14% (range: -41% to 152%).
The co-primary efficacy variables in both trials were weighted mean FEV1 (0 to 4 hours) postdose on Day 168 and change from baseline in trough FEV1 on Day 169 (the mean of the FEV1 values obtained 23 and 24 hours after the final dose on Day 168). The weighted mean comparison of the fluticasone furoate/vilanterol combination with fluticasone furoate was assessed to evaluate the contribution of vilanterol to fluticasone furoate/vilanterol ELLIPTA. The trough FEV1 comparison of the fluticasone furoate/vilanterol combination with vilanterol was assessed to evaluate the contribution of fluticasone furoate to fluticasone furoate/vilanterol ELLIPTA.
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg demonstrated a larger increase in the weighted mean FEV1 (0 to 4 hours) relative to placebo and fluticasone furoate 100 mcg at Day 168 (Table 5).
| a At Day 168. | ||||||
| b At Day 169. | ||||||
|
Treatment |
n |
Weighted Mean FEV1 (0-4 h)a (mL) |
Trough FEV1b (mL) |
|||
|
Difference from |
Difference from |
|||||
|
Placebo (95% CI) |
Fluticasone Furoate 100 mcg (95% CI) |
Fluticasone Furoate 200 mcg (95% CI) |
Placebo (95% CI) |
Vilanterol 25 mcg (95% CI) |
||
|
Trial 1 |
||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
204 |
214 (161, 266) |
168 (116, 220) |
–– |
144 (91, 197) |
45 (-8, 97) |
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
205 |
209 (157, 261) |
–– |
168 (117, 219) |
131 (80, 183) |
32 (-19, 83) |
|
Trial 2 |
||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
206 |
173 (123, 224) |
120 (70, 170) |
–– |
115 (60, 169) |
48 (-6, 102) |
Serial spirometric evaluations were performed predose and up to 4 hours after dosing. Results from Trial 1 at Day 1 and Day 168 are shown in Figure 4. Similar results were seen in Trial 2 (not shown).
Figure 4. Raw Mean Change from Baseline in Postdose Serial FEV1 (0-4 h) (mL) on Days 1 and 168
Day 1
Day 168
The second co-primary variable was change from baseline in trough FEV1 following the final treatment day. At Day 169, both Trials 1 and 2 demonstrated significant increases in trough FEV1 for all strengths of the fluticasone furoate/vilanterol combination compared with placebo (Table 5). The comparison of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg with vilanterol did not achieve statistical significance (Table 5).
Trials 1 and 2 evaluated FEV1 as a secondary endpoint. Peak FEV1 was defined as the maximum postdose FEV1 recorded within 4 hours after the first dose of trial medicine on Day 1 (measurements recorded at 5, 15, and 30 minutes and 1, 2, and 4 hours). In both trials, differences in mean change from baseline in peak FEV1 were observed for the groups receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo (152 and 139 mL, respectively). The median time to onset, defined as a 100-mL increase from baseline in FEV1, was 16 minutes in subjects receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg.
Exacerbations: Trials 3 and 4 were randomized, double-blind, 52-week trials designed to evaluate the effect of fluticasone furoate/vilanterol ELLIPTA on the rate of moderate and severe COPD exacerbations. All subjects were treated with fluticasone propionate/salmeterol 250/50 mcg twice daily during a 4-week run-in period prior to being randomly assigned to 1 of the following treatment groups: fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate/vilanterol 50/25 mcg, or vilanterol 25 mcg.
The primary efficacy variable in both trials was the annual rate of moderate/severe exacerbations. The comparison of the fluticasone furoate/vilanterol combination with vilanterol was assessed to evaluate the contribution of fluticasone furoate to fluticasone furoate/vilanterol ELLIPTA. In these 2 trials, exacerbations were defined as worsening of 2 or more major symptoms (dyspnea, sputum volume, and sputum purulence) or worsening of any 1 major symptom together with any 1 of the following minor symptoms: sore throat, colds (nasal discharge and/or nasal congestion), fever without other cause, and increased cough or wheeze for at least 2 consecutive days. COPD exacerbations were considered to be of moderate severity if treatment with systemic corticosteroids and/or antibiotics was required and were considered to be severe if hospitalization was required.
Trials 3 and 4 included 3,255 subjects, of which 57% were male and 85% were white. They had a mean age of 64 years and an average smoking history of 46 pack years, with 44% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 45% (range: 12% to 91%), and mean postbronchodilator FEV1/FVC ratio was 46% (range: 17% to 81%), indicating that the subject population had moderate to very severely impaired airflow obstruction. The mean percent reversibility was 15% (range: -65% to 313%).
Subjects treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg had a lower annual rate of moderate/severe COPD exacerbations compared with vilanterol in both trials (Table 6).
|
Treatment |
n |
Mean Annual Rate (exacerbations/year) |
Ratio vs. Vilanterol |
95% CI |
|
Trial 3 |
||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
403 |
0.90 |
0.79 |
0.64, 0.97 |
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
409 |
0.79 |
0.69 |
0.56, 0.85 |
|
Fluticasone furoate/vilanterol 50/25 mcg |
412 |
0.92 |
0.81 |
0.66, 0.99 |
|
Vilanterol 25 mcg |
409 |
1.14 |
–– |
–– |
|
Trial 4 |
||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
403 |
0.70 |
0.66 |
0.54, 0.81 |
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
402 |
0.90 |
0.85 |
0.70, 1.04 |
|
Fluticasone furoate/vilanterol 50/25 mcg |
408 |
0.92 |
0.87 |
0.72, 1.06 |
|
Vilanterol 25 mcg |
409 |
1.05 |
–– |
–– |
Comparator Trials
Three 12-week, randomized, double-blind, double-dummy trials were conducted with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg once daily versus fluticasone propionate/salmeterol 250/50 mcg twice daily to evaluate the efficacy of serial lung function of fluticasone furoate/vilanterol ELLIPTA in subjects with COPD. The primary endpoint of each study was change from baseline in weighted mean FEV1 (0 to 24 hours) on Day 84. Of the 519 patients in Trial 5, 64% were male and 97% were white; mean age was 61 years; average smoking history was 40 pack years, with 55% identified as current smokers. At screening in the treatment group using fluticasone furoate/vilanterol ELLIPTA 100/25mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 19% to 70%), the mean (SD) FEV1/FVC ratio was 0.51 (0.11), and the mean percent reversibility was 11% (range: -12% to 83%). At screening in the treatment group using fluticasone propionate/salmeterol 250/50 mcg, the mean postbronchodilator percent predicted FEV1 was 47% (range: 14% to 71%), the mean (SD) FEV1/FVC ratio was 0.49 (0.10), and the mean percent reversibility was 11% (range: -13% to 50%).
Of the 511 patients in Trial 6, 68% were male and 94% were white; mean age was 62 years; average smoking history was 35 pack years, with 52% identified as current smokers. At screening in the treatment group using fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 18% to 70%), the mean (SD) FEV1/FVC ratio was 0.51 (0.10), and the mean percent reversibility was 12% (range: -56% to 77%). At screening in the treatment group using fluticasone propionate/salmeterol 250/50 mcg, the mean postbronchodilator percent predicted FEV1 was 49% (range: 15% to 70%), the mean (SD) FEV1/FVC ratio was 0.50 (0.10), and the mean percent reversibility was 12% (range: -66% to 72%).
Of the 828 patients in Trial 7, 72% were male and 98% were white; mean age was 61 years; average smoking history was 38 pack years, with 60% identified as current smokers. At screening in the treatment group using fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 18% to 70%), the mean (SD) FEV1/FVC ratio was 0.52 (0.10), and the mean percent reversibility was 12% (range: -26% to 84%). At screening in the treatment group using fluticasone propionate/salmeterol 250/50 mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 16% to 70%), the mean (SD) FEV1/FVC ratio was 0.51 (0.10), and the mean percent reversibility was 12% (range: -15% to 67%).
In Trial 5, the mean (SE) change from baseline in weighted mean FEV1 (0 to 24 hours) with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was 174 (15) mL compared with 94 (16) mL with fluticasone propionate/salmeterol 250/50 mcg (treatment difference 80 mL; 95% CI: 37, 124; P<0.001). In Trials 6 and 7, the mean (SE) change from baseline in weighted mean FEV1 (0 to 24 hours) with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was 142 (18) mL and 168 (12) mL, respectively, compared with 114 (18) mL and 142 (12) mL, respectively, for fluticasone propionate/salmeterol 250/50 mcg (Trial 6 treatment difference 29 mL; 95% CI: -22, 80; P = 0.267; Trial 7 treatment difference 25 mL; 95% CI: -8, 59; P = 0.137).
Mortality Trial
A randomized, double-blind, multicenter, multinational trial prospectively evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo on survival. The trial was event-driven and patients were followed until a sufficient number of deaths occurred. In this trial, 16,568 subjects aged 40 to 80 years received fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 4,140), fluticasone furoate 100 mcg (n = 4,157), vilanterol 25 mcg (n = 4,140), or placebo (n = 4,131). Subjects were treated for up to 4 years, with a median treatment duration of 1.5 years. Median duration of follow-up for the endpoint of survival was 1.8 years for all treatment groups. All subjects had COPD with moderate airflow limitation (≥50% and ≤70% predicted FEV1) and either had a history of, or were at risk of, cardiovascular disease. The primary endpoint was all-cause mortality. Secondary efficacy endpoints included the rate of decline in FEV1, annual rate of moderate/severe COPD exacerbations, and health-related quality of life as measured by the St. George’s Respiratory Questionnaire for COPD patients (SGRQ-C).
Survival: Survival with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was not significantly improved compared with placebo (hazard ratio 0.88; 95% CI: 0.74, 1.04). Mortality per 100 patient-years was 3.1 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 3.5 for placebo, 3.2 for fluticasone furoate, and 3.4 for vilanterol.
Lung Function: A reduction of 8 mL/year was estimated on-treatment for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo in the rate of lung function decline as measured by FEV1 (95% CI: 1, 15).
Exacerbations: Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the on-treatment annual rate of moderate/severe exacerbations by 29% compared with placebo (95% CI: 22, 35). Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the annual rate of moderate/severe exacerbations by 19% compared with fluticasone furoate (95% CI: 12, 26) and by 21% compared with vilanterol (95% CI: 14, 28). The on-treatment annual rate of moderate/severe exacerbations was 0.25 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 0.35 for placebo, 0.31 for fluticasone furoate, and 0.31 for vilanterol.
Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the on-treatment annual rate of severe exacerbations (i.e., requiring hospitalization) by 27% compared with placebo (95% CI: 13, 39). Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the on-treatment annual rate of exacerbations requiring hospitalization by 11% compared with fluticasone furoate (95% CI: -6, 25) and by 9% compared with vilanterol (95% CI: -8, 24).
Health-Related Quality of Life: The St. George’s Respiratory Questionnaire (SGRQ) is a disease-specific patient-reported instrument that measures symptoms, activities, and impact on daily life. The SGRQ-C, a shorter version derived from the original SGRQ, was used in this trial. Results were transformed to the SGRQ for reporting purposes. In a subset of 4,443 subjects, the on-treatment SGRQ responder rates at 1 year (defined as a change in score of 4 or more as threshold) were 49% for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 47% for placebo, 48% for fluticasone furoate, and 48% for vilanterol (odds ratio 1.18; 95% CI: 0.97, 1.44 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo).
14.2 Asthma
The safety and efficacy of fluticasone furoate/vilanterol ELLIPTA were evaluated in 9,969 subjects with asthma. The development program included 4 confirmatory trials (2 of 12 weeks’ duration, 1 of 24 weeks’ duration, 1 exacerbation trial of 24 to 76 weeks’ duration), one 24-week active comparator trial with fluticasone propionate/salmeterol 250/50 mcg, and dose‑ranging trials of shorter duration. The efficacy of fluticasone furoate/vilanterol ELLIPTA is based primarily on the dose-ranging trials and the 4 confirmatory trials described below.
Dose Selection for Vilanterol
Dose selection for vilanterol in asthma was supported by a 28-day, randomized, double-blind, placebo-controlled, parallel-group trial evaluating 5 doses of vilanterol (3 to 50 mcg) or placebo dosed in the evening in 607 subjects with asthma. Results demonstrated dose-related increases from baseline in FEV1 at Day 1 and Day 28 (Figure 5).
Figure 5. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28
Day 1
Day 28
The differences in trough FEV1 on Day 28 from placebo for the 3-, 6.25-, 12.5-, 25-, and 50-mcg doses were 64 mL (95% CI: -36, 164), 69 mL (95% CI: -29, 168), 130 mL (95% CI: 30, 230), 121 mL (95% CI: 23, 220), and 162 mL (95% CI: 62, 261), respectively. These results and results of the secondary endpoints supported the evaluation of vilanterol 25 mcg once daily in the confirmatory trials for asthma.
Dose Selection for Fluticasone Furoate
Eight doses of fluticasone furoate ranging from 25 to 800 mcg once daily were evaluated in 3 randomized, double-blind, placebo-controlled, 8-week trials in subjects with asthma. A dose‑related increase in trough FEV1 at Week 8 was seen for doses from 25 to 200 mcg with no consistent additional benefit for doses above 200 mcg. To evaluate dosing frequency, a separate trial compared fluticasone furoate 200 mcg once daily and fluticasone furoate 100 mcg twice daily. The results supported the selection of the once-daily dosing frequency (Figure 6).
Confirmatory Trials
The efficacy of fluticasone furoate/vilanterol ELLIPTA was evaluated in 4 randomized, double‑blind, parallel-group clinical trials in adult and adolescent subjects with asthma. Three (3) trials were designed to evaluate the safety and efficacy of fluticasone furoate/vilanterol ELLIPTA given once daily in subjects who were not controlled on their current treatments of ICS or combination therapy consisting of an ICS plus a LABA (Trials 1, 2, and 3). A 24- to 76‑week exacerbation trial was designed to demonstrate that treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg significantly decreased the risk of asthma exacerbations as measured by time to first asthma exacerbation when compared with fluticasone furoate 100 mcg (Trial 5). This trial enrolled subjects who had 1 or more asthma exacerbations in the year prior to trial entry. The demographics of these 4 trials and the comparator trial (Trial 6) are provided in Table 7. While subjects aged 12 to 17 years were included in these trials, fluticasone furoate/vilanterol ELLIPTA is not approved for use in this age group [see Indications (1.2), Adverse Reactions (6.2), Use in Specific Populations (8.4)].
| N/A = Data not collected. | |||||
| a Trials did not include current smokers; past smokers had fewer than 10 packs per year history. | |||||
|
Parameter |
Trial 1 n = 609 |
Trial 2 n = 1,039 |
Trial 3 n = 586 |
Trial 5 n = 2,019 |
Trial 6 n = 806 |
|
Mean age (years) (range) |
40 (12, 84) |
46 (12, 82) |
46 (12, 76) |
42 (12, 82) |
43 (12, 80) |
|
Female (%) |
58 |
60 |
59 |
67 |
61 |
|
White (%) |
84 |
88 |
84 |
73 |
59 |
|
Duration of asthma (years) |
12 |
18 |
16 |
16 |
21 |
|
Never smokeda (%) |
N/A |
84 |
N/A |
86 |
81 |
|
Predose FEV1 (L) at baseline |
2.32 |
1.97 |
2.15 |
2.20 |
2.03 |
|
Mean percent predicted FEV1 at baseline (%) |
70 |
62 |
67 |
72 |
68 |
|
% Reversibility |
29 |
30 |
29 |
24 |
28 |
|
Absolute reversibility (mL) |
614 |
563 |
571 |
500 |
512 |
Trials 1, 2, and 3 were 12- or 24-week trials that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA on lung function in subjects with asthma. In Trial 1, subjects were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate 100 mcg, or placebo. In Trial 2, subjects were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, or fluticasone furoate 100 mcg. In Trial 3, subjects were randomized to fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate 200 mcg, or fluticasone propionate 500 mcg. All inhalations were administered once daily, with the exception of fluticasone propionate, which was administered twice daily. Subjects receiving an ICS or an ICS plus a LABA (doses of ICS varied by trial and asthma severity) entered a 4-week run-in period during which LABA treatment was stopped. Subjects reporting symptoms and/or rescue beta2‑agonist medication use during the run‑in period were continued in the trial.
In Trials 1 and 3, change from baseline in weighted mean FEV1 (0 to 24 hours) and change from baseline in trough FEV1 at approximately 24 hours after the last dose at study endpoint (12 and 24 weeks, respectively) were co-primary efficacy endpoints. In Trial 2, change from baseline in weighted mean FEV1 (0 to 24 hours) at Week 12 was the primary efficacy endpoint; change from baseline in trough FEV1 at approximately 24 hours after the last dose at Week 12 was a secondary endpoint. (See Table 8.) Weighted mean FEV1 (0 to 24 hours) was derived from serial measurements taken within 30 minutes prior to dosing and postdose assessments at 5, 15, and 30 minutes and 1, 2, 3, 4, 5, 12, 16, 20, 23, and 24 hours after the final dose. Other secondary endpoints included change from baseline in percentage of rescue-free 24-hour periods and percentage of symptom-free 24-hour periods over the treatment period.
| ICS = inhaled corticosteroid, LABA = long-acting beta2-adrenergic agonist. | ||||||||||
|
Study (Duration) Background Treatment |
n |
Weighted Mean FEV1 (0-24 h) (mL) |
||||||||
|
Difference from |
||||||||||
|
Treatment |
Placebo (95% CI) |
Fluticasone Furoate 100 mcg (95% CI) |
Fluticasone Furoate 200 mcg (95% CI) |
|||||||
|
Trial 1 (12 Weeks) Low- to mid-dose ICS or low-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
108 |
302 (178, 426) |
116 (-5, 236) |
–– |
||||||
|
Trial 2 (12 Weeks) Mid- to high-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
312 |
–– |
108 (45, 171) |
–– |
||||||
|
Trial 3 (24 Weeks) High-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
89 |
–– |
–– |
136 (1, 270) |
||||||
|
Study (Duration) Background Treatment |
n |
Trough FEV1 (mL) |
||||||||
|
Difference from |
||||||||||
|
Treatment |
Placebo (95% CI) |
Fluticasone Furoate 100 mcg (95% CI) |
Fluticasone Furoate 200 mcg (95% CI) |
|||||||
|
Trial 1 (12 Weeks) Low- to mid-dose ICS or low-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
200 |
172 (87, 258) |
36 (-48, 120) |
–– |
||||||
|
Trial 2 (12 Weeks) Mid- to high-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
334 |
–– |
77 (16, 138) |
–– |
||||||
|
Trial 3 (24 Weeks) High-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
187 |
–– |
–– |
193 (108, 277) |
||||||
In Trial 1, weighted mean FEV1 (0 to 24 hours) was assessed in a subset of subjects (n = 309). At Week 12, change from baseline in weighted mean FEV1 (0 to 24 hours) was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo (302 mL; 95% CI: 178, 426; P<0.001) (Table 8); change from baseline in weighted mean FEV1 (0 to 24 hours) for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was numerically greater than fluticasone furoate 100 mcg, but not statistically significant (116 mL; 95% CI: -5, 236). At Week 12, change from baseline in trough FEV1 was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo (172 mL; 95% CI: 87, 258; P<0.001) (Table 8); change from baseline in trough FEV1 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was numerically greater than fluticasone furoate 100 mcg, but not statistically significant (36 mL; 95% CI: -48, 120).
In Trial 2, the change from baseline in weighted mean FEV1 (0 to 24 hours) was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg (108 mL; 95% CI: 45, 171; P<0.001) at Week 12 (Table 8). In a descriptive analysis, the change from baseline in weighted mean FEV1 (0 to 24 hours) for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg was numerically greater than fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (24 mL; 95% CI: -37, 86) at Week 12. The change from baseline in trough FEV1 was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg (77 mL, 95% CI: 16, 138; P = 0.014) at Week 12 (Table 8). In a descriptive analysis, the change from baseline in trough FEV1 for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg was numerically greater than fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (16 mL; 95% CI: -46, 77) at Week 12.
In Trial 3, the change from baseline in weighted mean FEV1 (0 to 24 hours) was significantly greater for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg compared with fluticasone furoate 200 mcg (136 mL; 95% CI: 1, 270; P = 0.048) at Week 24 (Table 8). The change from baseline in trough FEV1 was significantly greater for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg compared with fluticasone furoate 200 mcg (193 mL, 95% CI: 108, 277; P<0.001) at Week 24.
Lung function improvements were demonstrated through weighted mean FEV1 (0 to 24 hours) over the 24-hour period following the final dose of fluticasone furoate/vilanterol ELLIPTA in Trials 2 and 3. Serial FEV1 measurements were taken within 30 minutes prior to dosing and postdose assessments at 5, 15, and 30 minutes and 1, 2, 3, 4, 5, 12, 16, 20, 23, and 24 hours in Trials 1, 2, and 3. A representative figure is shown from Trial 2 in Figure 7.
Subjects receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (Trial 2) or fluticasone furoate/vilanterol ELLIPTA 200/25 mcg (Trial 3) had significantly greater improvements from baseline in percentage of 24-hour periods without need of beta2-agonist rescue medication use and percentage of 24-hour periods without asthma symptoms compared with subjects receiving fluticasone furoate 100 mcg or fluticasone furoate 200 mcg, respectively. In a descriptive analysis (Trial 2), subjects receiving fluticasone furoate/vilanterol ELLIPTA 200/25 mcg had numerical improvements from baseline in percentage of 24-hour periods without need of beta2‑agonist rescue medication use and percentage of 24-hour periods without asthma symptoms compared with subjects receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg.
Trial 5 was a 24- to 76-week event-driven exacerbation trial that evaluated whether fluticasone furoate/vilanterol ELLIPTA 100/25 mcg significantly decreased the risk of asthma exacerbations as measured by time to first asthma exacerbation when compared with fluticasone furoate 100 mcg in subjects with asthma. Subjects receiving low- to high-dose ICS (fluticasone propionate 100 mcg to 500 mcg twice daily or equivalent) or low- to mid-dose ICS plus a LABA (fluticasone propionate/salmeterol 100/50 mcg to 250/50 mcg twice daily or equivalent) and a history of 1 or more asthma exacerbations that required treatment with oral/systemic corticosteroid or emergency department visit or in-patient hospitalization for the treatment of asthma in the year prior to trial entry, entered a 2-week run-in period during which LABA treatment was stopped. Subjects reporting symptoms and/or rescue beta2-agonist medication use during the run-in period were continued in the trial.
The primary endpoint was time to first asthma exacerbation. Asthma exacerbation was defined as deterioration of asthma requiring the use of systemic corticosteroid for at least 3 days or an in‑patient hospitalization or emergency department visit due to asthma that required systemic corticosteroid. Rate of asthma exacerbation was a secondary endpoint. The hazard ratio from the Cox Model for the analysis of time to first asthma exacerbation for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg was 0.795 (95% CI: 0.642, 0.985). This represents a 20% reduction in the risk of experiencing an asthma exacerbation for subjects treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg (P = 0.036). Mean yearly rates of asthma exacerbations of 0.14 and 0.19 in subjects treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg, respectively, were observed (25% reduction in rate; 95% CI: 5%, 40%).
Comparator Trial
Trial 6 was a 24-week trial that compared the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg once daily with fluticasone propionate/salmeterol 250/50 mcg twice daily (N = 806). Subjects receiving mid-dose ICS (fluticasone propionate 250 mcg twice daily or equivalent) entered a 4‑week run-in period during which all subjects received fluticasone propionate 250 mcg twice daily. The primary endpoint was change from baseline in weighted mean FEV1 (0 to 24 hours) at Week 24.
The mean change (SE) from baseline in weighted mean FEV1 (0 to 24 hours) for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was 341 (18.4) mL compared with 377 (18.5) mL for fluticasone propionate/salmeterol 250/50 mcg (treatment difference -37 mL; 95% CI: -88, 15; P = 0.162).
16. How is Fluticasone and Vilanterol Inhalation Powder supplied
Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder is supplied as a disposable light grey and pale blue plastic inhaler containing 2 foil strips, each with 30 blisters. One strip contains fluticasone furoate (100 or 200 mcg per blister), and the other strip contains vilanterol (25 mcg per blister). A blister from each strip is used to create 1 dose. The inhaler is packaged within a moisture‑protective foil tray with a desiccant and a peelable lid in the following packs:
- NDC 66993-135-97 100/25 mcg 30 inhalations (60 blisters)
- NDC 66993-136-97 200/25 mcg 30 inhalations (60 blisters)
Store at room temperature between 68°F and 77°F (20°C and 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Store in a dry place away from direct heat or sunlight. Keep out of reach of children.
Fluticasone Furoate/Vilanterol ELLIPTA should be stored inside the unopened moisture‑protective foil tray and only removed from the tray immediately before initial use. Discard Fluticasone Furoate/Vilanterol ELLIPTA 6 weeks after opening the foil tray or when the counter reads “0” (after all blisters have been used), whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Asthma-Related Events
Inform patients with asthma that LABA when used alone increases the risk of asthma-related hospitalization or asthma-related death. Available data show that when ICS and LABA are used together, such as with Fluticasone Furoate/Vilanterol ELLIPTA, there is not a significant increase in the risk of these events.
Not for Acute Symptoms
Inform patients that Fluticasone Furoate/Vilanterol ELLIPTA is not meant to relieve acute symptoms of COPD or asthma and extra doses should not be used for that purpose. Advise patients to treat acute symptoms with an inhaled, short-acting beta2-agonist such as albuterol. Provide patients with such medication and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
- •
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- •
- Need for more inhalations than usual of inhaled, short-acting beta2-agonists
- •
- Significant decrease in lung function as outlined by the physician
Tell patients they should not stop therapy with Fluticasone Furoate/Vilanterol ELLIPTA without physician/provider guidance since symptoms may recur after discontinuation.
Do Not Use Additional Long-acting Beta2-agonists
Instruct patients not to use other LABA for COPD and asthma.
Local Effects
Inform patients that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, treat it with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing therapy with Fluticasone Furoate/Vilanterol ELLIPTA, but at times therapy with Fluticasone Furoate/Vilanterol ELLIPTA may need to be temporarily interrupted under close medical supervision. Advise patients to rinse the mouth with water without swallowing after inhalation to help reduce the risk of thrush.
Pneumonia
Patients with COPD have a higher risk of pneumonia; instruct them to contact their healthcare providers if they develop symptoms of pneumonia.
Immunosuppression
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Hypercorticism and Adrenal Suppression
Advise patients that Fluticasone Furoate/Vilanterol ELLIPTA may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, inform patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to Fluticasone Furoate/Vilanterol ELLIPTA.
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk.
Glaucoma and Cataracts
Advise patients that long-term use of ICS may increase the risk of some eye problems (cataracts or glaucoma); consider regular eye examinations.
Risks Associated with Beta-agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness.
Hypersensitivity Reactions, Including Anaphylaxis
Advise patients that hypersensitivity reactions (e.g., anaphylaxis, angioedema, rash, urticaria) may occur after administration of Fluticasone Furoate/Vilanterol ELLIPTA. Instruct patients to discontinue Fluticasone Furoate/Vilanterol ELLIPTA if such reactions occur. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder medications containing lactose; therefore, patients with severe milk protein allergy should not use Fluticasone Furoate/Vilanterol ELLIPTA.
ELLIPTA is a registered trademark of the GSK group of companies.
Manufactured for:
Prasco Laboratories
Mason, OH 45040 USA
Manufactured by:
GlaxoSmithKline
Research Triangle Park, NC 27709
BRE-PS:1PI
|
PATIENT INFORMATION Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder for oral inhalation use |
|
|
What is Fluticasone Furoate/Vilanterol ELLIPTA?
|
|
|
Do not use Fluticasone Furoate/Vilanterol ELLIPTA:
|
|
|
Before using Fluticasone Furoate/Vilanterol ELLIPTA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Fluticasone Furoate/Vilanterol ELLIPTA and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take antifungal or anti-HIV medicines. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
|
How should I use Fluticasone Furoate/Vilanterol ELLIPTA? Read the step-by-step instructions for using Fluticasone Furoate/Vilanterol ELLIPTA at the end of this Patient Information.
|
|
|
What are the possible side effects of Fluticasone Furoate/Vilanterol ELLIPTA? Fluticasone Furoate/Vilanterol ELLIPTA can cause serious side effects, including:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Common side effects of Fluticasone Furoate/Vilanterol ELLIPTA include: COPD: |
|
|
|
|
|
|
|
|
|
|
Asthma: |
|
|
|
|
|
|
These are not all the possible side effects of Fluticasone Furoate/Vilanterol ELLIPTA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store Fluticasone Furoate/Vilanterol ELLIPTA?
Keep Fluticasone Furoate/Vilanterol ELLIPTA and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of Fluticasone Furoate/Vilanterol ELLIPTA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Fluticasone Furoate/Vilanterol ELLIPTA for a condition for which it was not prescribed. Do not give Fluticasone Furoate/Vilanterol ELLIPTA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Fluticasone Furoate/Vilanterol ELLIPTA that is written for health professionals. |
|
|
What are the ingredients in Fluticasone Furoate/Vilanterol ELLIPTA? Active ingredients: fluticasone furoate, vilanterol trifenatate Inactive ingredients: lactose monohydrate (contains milk proteins), magnesium stearate For more information about Fluticasone Furoate/Vilanterol ELLIPTA, call 1-866-525-0688 or visit our website at www.Prasco.com. ELLIPTA is a registered trademark of the GSK group of companies. Manufactured for: Prasco Laboratories Mason, OH 45040 USA Manufactured by: GlaxoSmithKline Research Triangle Park, NC 27709 BRE-PS:1PIL |
|
- This Patient Information has been approved by the U.S. Food and Drug Administration Revised: February 2022
|
INSTRUCTIONS FOR USE Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder for oral inhalation use |
|
|
Read this before you start:
Your Fluticasone Furoate/Vilanterol ELLIPTA inhaler 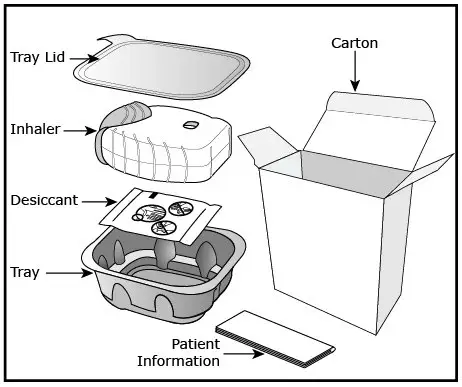 How to use your inhaler
|
|
|  |
|
Important Notes:
Check the counter. See Figure C. |
|
 Figure C |
|
|
Prepare your dose: Wait to open the cover until you are ready to take your dose. |
|
 Figure D |
Step 1. Open the cover of the inhaler. See Figure D.
|
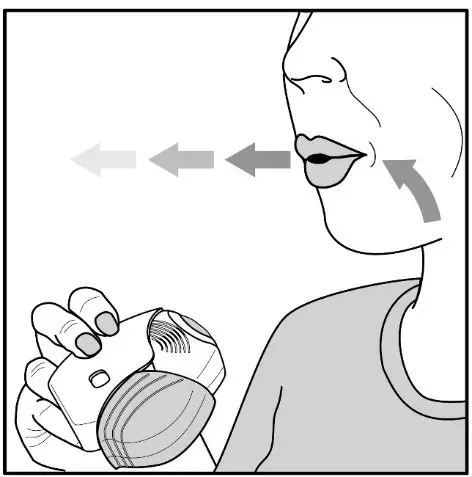 Figure E |
Step 2. Breathe out. See Figure E.
|
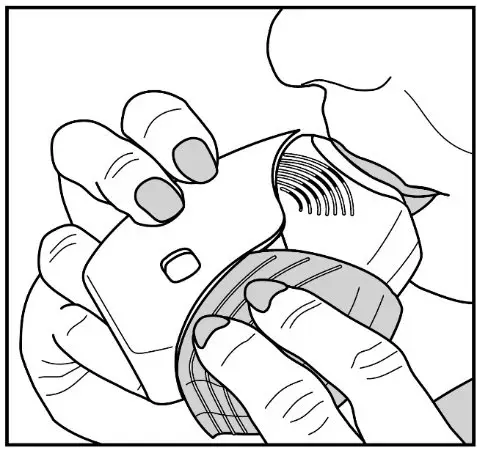 Figure F |
Step 3. Inhale your medicine. See Figure F.
|
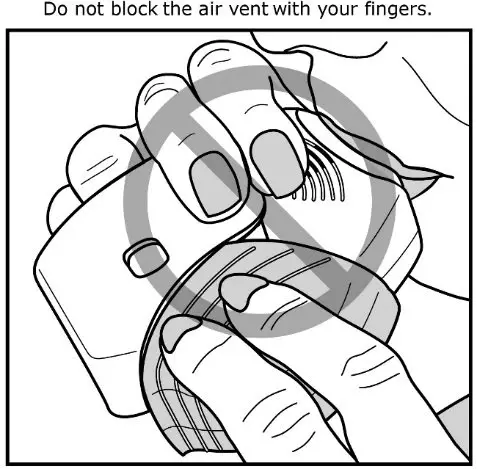 Figure G |
|
 Figure H |
|
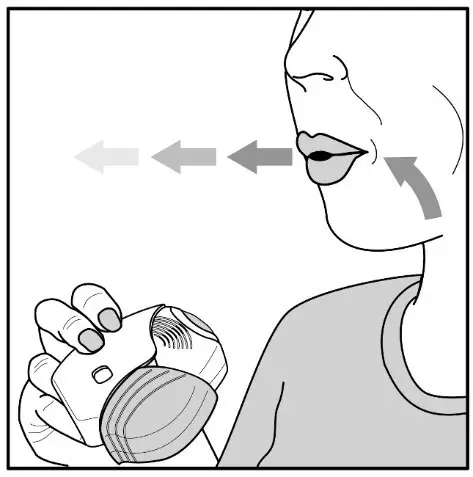 Figure I |
|
 Figure J |
|
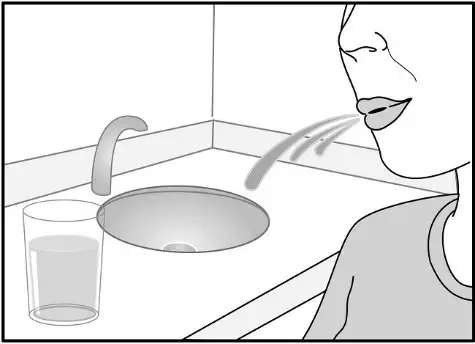 Figure K |
|
|
|
 Figure L |
|
|
For more information about Fluticasone Furoate/Vilanterol ELLIPTA or how to use your inhaler, call 1-866-525-0688 or visit our website at www.Prasco.com. ELLIPTA is a registered trademark of the GSK group of companies. Manufactured for: Prasco Laboratories Mason, OH 45040 USA Manufactured by: GlaxoSmithKline Research Triangle Park, NC 27709 BRE-PS:1IFU |
|
- This Instructions for Use has been approved by the U.S. Food and Drug Administration Revised: February 2022
Principal Display Panel
NDC 66993-135-97
Fluticasone Furoate/Vilanterol
ELLIPTA Inhalation Powder
100 mcg/25 mcg
PRASCO
FOR ORAL INHALATION ONLY
Fluticasone Furoate/Vilanterol ELLIPTA Inhalation Powder contains 2 foil strips of 30 blisters each. Each blister on one strip contains 100 mcg of fluticasone furoate and lactose monohydrate. Each blister on the other strip contains 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate.
Rx Only
1 ELLIPTA Inhaler containing 30 doses
(60 blisters total)
62000000074871
Principal Display Panel
NDC 66993-136-97
Fluticasone Furoate/Vilanterol
ELLIPTA Inhalation Powder
200 mcg/25 mcg
PRASCO
FOR ORAL INHALATION ONLY
Fluticasone Furoate/Vilanterol ELLIPTA Inhalation Powder contains 2 foil strips of 30 blisters each. Each blister on one strip contains 200 mcg of fluticasone furoate and lactose monohydrate. Each blister on the other strip contains 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate.
Rx Only
1 ELLIPTA Inhaler containing 30 doses
(60 blisters total)
62000000074875
| FLUTICASONE FUROATE AND VILANTEROL
fluticasone furoate and vilanterol powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| FLUTICASONE FUROATE AND VILANTEROL
fluticasone furoate and vilanterol powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Prasco Laboratories (065969375) |
| Registrant - GlaxoSmithKline LLC (167380711) |