Drug Detail:Fosaprepitant (Fosaprepitant [ fos-a-prep-i-tan-t ])
Drug Class: NK1 receptor antagonists
Highlights of Prescribing Information
FOCINVEZ (fosaprepitant injection) for intravenous use
Initial U.S. Approval: 2008
Indications and Usage for Focinvez Injection
FOCINVEZ is a substance P/neurokinin-1 (NK 1) receptor antagonist, indicated in adults and pediatric patients 6 months of age and older, in combination with other antiemetic agents, for the prevention of (1):
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
Limitations of Use (1)
- FOCINVEZ has not been studied for treatment of established nausea and vomiting.
Focinvez Injection Dosage and Administration
Recommended Adult Dosage ( 2.1)
- FOCINVEZ 150mg on Day 1 as an intravenous infusion over 20 to 30 minutes;
- Complete the infusion approximately 30 minutes prior to chemotherapy.
Recommended Dosage for Pediatric Patients (6 months to 17 years) Weighing at Least 6 kg ( 2.2)
- See full prescribing information for pediatric dosage regimens by age.
- Single dose chemotherapy regimens: single dose of FOCINVEZ on Day 1.
- Single or multi-day chemotherapy regimens:3-day regimen of FOCINVEZ on Day 1 and aprepitant capsules or aprepitant for oral suspension on Days 2 and 3.
- Administer FOCINVEZ through a central venous catheter on Day 1 as an intravenous infusion over 30 minutes (12 years to 17 years) or 60 minutes (6 months to less than 12 years).
- Complete the infusion approximately 30 minutes prior to chemotherapy.
Concomitant Antiemetics
- See full prescribing information for additional information. ( 2.1, 2.2)
Dosage Forms and Strengths
Injection: 150 mg/50 mL (3 mg/mL) of fosaprepitant, in a single-dose vial. (3)
Contraindications
- Known hypersensitivity to any component of this drug. ( 4, 5.2)
- Concurrent use with pimozide. ( 4)
Warnings and Precautions
- CYP3A4 Interactions:Fosaprepitant is a weak inhibitor of CYP3A4, and aprepitant, the active moiety, is a substrate, inhibitor, and inducer of CYP3A4; see Full Prescribing Information for recommendations regarding contraindications, risk of adverse reactions, and dosage adjustment of FOCINVEZ and concomitant drugs. ( 4, 5.1, 7.1, 7.2)
- Hypersensitivity Reactions (including anaphylaxis and anaphylactic shock): May occur during or soon after infusion. If symptoms occur, discontinue the drug. Do not reinitiate FOCINVEZ if symptoms occur with previous use. ( 4, 5.2)
- Infusion Site Reactions (including thrombophlebitis, necrosis, and vasculitis): Majority of reactions reported in patients receiving vesicant chemotherapy. Avoid infusion into small veins. Discontinue infusion and administer treatment if a severe reaction develops. ( 5.3)
- Warfarin (a CYP2C9 substrate):Risk of decreased INR of prothrombin time; monitor INR in 2–week period, particularly at 7 to 10 days, following initiation of FOCINVEZ. ( 5.4, 7.1)
- Hormonal Contraceptives: Efficacy of contraceptives may be reduced during treatment and for 1 month following administration of the last dose of either fosaprepitant or oral aprepitant. Use effective alternative or back-up methods of contraception. ( 5.5, 7.1, 8.3)
Adverse Reactions/Side Effects
- Most common adverse reactions in adults (≥2%) are: fatigue, diarrhea, neutropenia, asthenia, anemia, peripheral neuropathy, leukopenia, dyspepsia, urinary tract infection, pain in extremity. ( 6.1)
- Adverse reactions in pediatrics are similar to adults.
To report SUSPECTED ADVERSE REACTIONS, contact Spes Pharmaceuticals Inc., at 1-732-354-3630 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
See full prescribing information for a list of clinically significant drug interactions. ( 4, 5.1, 5.4, 5.5, 7.1, 7.2)
Additional pediatric use information is approved for Merck Sharp & Dohme LLC’s EMEND (fosaprepitant) for injection. However, due to Merck Sharp & Dohme LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for FDA-approved patient labeling.
Revised: 8/2023
Full Prescribing Information
1. Indications and Usage for Focinvez Injection
FOCINVEZ, in combination with other antiemetic agents, is indicated in adults and pediatric patients 6 months of age and older for the prevention of:
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
Limitations of Use
- FOCINVEZ has not been studied for treatment of established nausea and vomiting.
2. Focinvez Injection Dosage and Administration
2.1 Recommended Dosage for the Prevention of Nausea and Vomiting Associated with HEC and MEC in Adult Patients
The recommended dosage of FOCINVEZ, dexamethasone, and a 5-HT 3antagonist for the prevention of nausea and vomiting associated with administration of HEC or MEC in adults is shown in Table 1 or Table 2, respectively. Administer FOCINVEZ as an intravenous infusion on Day 1 over 20 to 30 minutes, completing the infusion approximately 30 minutes prior to chemotherapy.
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| aThe concentration of FOCINVEZ is 3 mg/mL. | ||||
| bAdminister dexamethasone 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. Also administer dexamethasone in the evenings on Days 3 and 4. A 50% dosage reduction of dexamethasone on Days 1 and 2 is recommended to account for a drug interaction with FOCINVEZ [see Clinical Pharmacology ( 12.3)] . | ||||
| FOCINVEZ a |
150 mg intravenously over 20 to 30 minutes | none | none | none |
| Dexamethasone b | 12 mg orally |
8 mg orally |
8 mg orally twice daily |
8 mg orally twice daily |
| 5-HT 3antagonist |
See selected 5-HT3 antagonist prescribing information for the recommended dosage | none | none | none |
| Day 1 | ||||
|---|---|---|---|---|
| aThe concentration of FOCINVEZ is 3 mg/mL | ||||
| bAdminister dexamethasone 30 minutes prior to chemotherapy treatment on Day 1. A 50% dosage reduction of dexamethasone is recommended to account for a drug interaction with FOCINVEZ [see Clinical Pharmacology ( 12.3)] . | ||||
| FOCINVEZ a | 150 mg intravenously over 20 to 30 minutes | |||
| Dexamethasone b | 12 mg orally | |||
| 5-HT 3antagonist | See selected 5-HT3 antagonist prescribing information for the recommended dosage | |||
2.2 Recommended Dosage for the Prevention of Nausea and Vomiting Associated with HEC and MEC in Pediatric Patients
The recommended pediatric dose regimens of FOCINVEZ, to be administered with a 5-HT 3antagonist, with or without a corticosteroid, for the prevention of nausea and vomiting associated with administration of single or multi-day chemotherapy regimens of HEC or MEC, are shown in Tables 3 and 4. Single-day chemotherapy regimens include those regimens in which HEC or MEC is administered for a single day only. Multi-day chemotherapy regimens include chemotherapy regimens in which HEC or MEC is administered for 2 or more days.
FOCINVEZ Dosage Regimens for Use with Single-Day Chemotherapy Regimens
For pediatric patients weighing at least 6 kg receiving single-day HEC or MEC, FOCINVEZ may be administered as:
- a single dose regimen of FOCINVEZ infused through a central venous catheter on Day 1, as shown in Table 3; or
- as a 3-day fosaprepitant/aprepitant regimen consisting of FOCINVEZ as an intravenous infusion through a central venous catheter on Day 1 and aprepitant capsules or aprepitant for oral suspension on Days 2 and 3, as shown in Table 4.
Administer FOCINVEZ on Day 1 over 30 minutes (12 years to 17 years) or 60 minutes (6 months to less than 12 years), completing the infusion approximately 30 minutes prior to chemotherapy.
| Drug | Age | Regimen |
|---|---|---|
| aDosing in pediatric patients less than 6 kg is not recommended | ||
| bThe concentration of FOCINVEZ is 3 mg/mL. | ||
| cAdminister dexamethasone 30 minutes prior to chemotherapy treatment on Day 1 | ||
| FOCINVEZ b | 12 Years to 17 Years | 150 mg intravenously over 30 minutes |
| 2 Years to less than 12 Years | 4 mg/kg (maximum dose 150 mg) intravenously over 60 minutes | |
| 6 Months to less than 2 Years | 5 mg/kg (maximum dose 150 mg) intravenously over 60 minutes | |
| Dexamethasone c | 6 Months to 17 Years | If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 and 2. |
| 5-HT 3antagonist | 6 Months to 17 Years | See selected 5-HT3 antagonist prescribing information for the recommended dosage |
FOCINVEZ Dosage Regimen for Use with Multi-Day Chemotherapy Regimens
For pediatric patients weighing at least 6 kg receiving multi-day regimens of HEC or MEC, administer FOCINVEZ as an intravenous infusion through a central venous catheter on Day 1 and aprepitant capsules or aprepitant for oral suspension on Days 2 and 3, as shown in Table 4.
Administer FOCINVEZ over 30 minutes (12 years to 17 years) or 60 minutes (6 months to less than 12 years), completing the infusion approximately 30 minutes prior to chemotherapy.
| Age Group | Drug | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|
| aDosing in pediatric patients less than 6 kg is not recommended | ||||
| bThe concentration of FOCINVEZ is 3 mg/mL. | ||||
| cFor patients 12 years to 17 years who cannot swallow oral capsules, aprepitant for oral suspension can be used instead. | ||||
| dFor patients less than 12 years of age who weigh at least 40 kg and who are able to swallow oral capsules, aprepitant capsules can be used on Days 2 and 3. | ||||
| eAdminister dexamethasone 30 minutes prior to chemotherapy treatment on Day 1. | ||||
|
12 Years to 17 Years | FOCINVEZ b | 115 mg intravenously over 30 minutes | -- | -- |
| Aprepitant capsules c | -- | 80 mg orally | 80 mg orally | |
| 6 Months to Less than 12 Years | FOCINVEZ | 3 mg/kg
(maximum dose 115 mg) intravenously over 60 minutes | -- | -- |
| Aprepitant for oral suspension d | -- | 2 mg/kg orally (maximum 80 mg) | 2 mg/kg orally (maximum 80 mg) | |
| 6 Months to 17 Years | Dexamethasone e | If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 | ||
| 6 Months to 17 Years | 5-HT 3antagonist | See selected 5-HT3 antagonist prescribing information for the recommended dosage | ||
Additional pediatric use information is approved for Merck Sharp & Dohme LLC’s EMEND (fosaprepitant) for injection. However, due to Merck Sharp & Dohme LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
2.3 Preparation of FOCINVEZ
FOCINVEZ is ready-to-usefor intravenous infusion. The concentration of FOCINVEZ is 3 mg/mL.
Determine the volume to be administered from the injection vial directly based on the recommended dose [see Dosage and Administration ( 2.1, 2.2)].
Adults
The entire volume of the vial (50 mL) should be administered.
Pediatrics
In patients 12 years and older, the volume to be administered is calculated as follows:
- Volume to administer (mL) = the recommended dose (mg) / 3 (mg/mL) *
In patients 6 months to less than 12 years, the volume to be administered is calculated as follows:
- Volume to administer (mL) = the recommended dose (mg/kg) x weight (kg) / 3 (mg/mL)*
Note: Do not exceed the maximum dose [see Dosage and Administration ( 2.2)]
- In pediatric patients, the entire volume in vial may NOTbe required. Discard the unused portion.
- * The recommended dose of FOCINVEZ is based on the patient’s age and weight. The concentration of FOCINVEZ is 3 mg/mL.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Do not use if solution is cloudy, contains precipitates, or if the red flip top cap is not intact.
- Vial may be inverted for use with a medical infusion set. The vial hanger can be seperated from the vial label and hanged from an IV pole.
- Allow FOCINVEZ to come to room temperature before use if the injection is NOTstored at room temperature.
- Caution:Do not mix FOCINVEZ with solutions for which physical and chemical compatibility have not been established. FOCINVEZ is incompatible with any solutions containing divalent cations (e.g., Ca 2+, Mg 2+), including Lactated Ringer’s Solution and Hartmann's Solution.
FOCINVEZ is compatible with 0.9% Sodium Chloride Injection.
3. Dosage Forms and Strengths
Injection: 150 mg/50 mL (3 mg/mL) of fosaprepitant, a clear and colorless solution, in a single-dose vial.
4. Contraindications
FOCINVEZ is contraindicated in patients:
- who are hypersensitive to any component of the product. Hypersensitivity reactions including anaphylactic reactions, flushing, erythema, and dyspnea have been reported [see Warnings and Precautions (5.2), and Adverse Reactions ( 6.2)] .
- taking pimozide. Inhibition of CYP3A4 by aprepitant, the active moiety, could result in elevated plasma concentrations of this drug, which is a CYP3A4 substrate, potentially causing serious or life-threatening reactions, such as QT prolongation, a known adverse reaction of pimozide [see Warnings and Precautions ( 5.1)] .
5. Warnings and Precautions
5.1 Clinically Significant CYP3A4 Drug Interactions
Fosaprepitant, a prodrug of aprepitant, is a weak inhibitor of CYP3A4, and aprepitant is a substrate, inhibitor, and inducer of CYP3A4.
- Use of FOCINVEZ with other drugs that are CYP3A4 substrates, may result in increased plasma concentration of the concomitant drug.
- Use of pimozide with FOCINVEZ is contraindicated due to the risk of significantly increased plasma concentrations of pimozide, potentially resulting in prolongation of the QT interval, a known adverse reaction of pimozide [see Contraindications (4)] .
- Use of FOCINVEZ with strong or moderate CYP3A4 inhibitors (e.g., ketoconazole, diltiazem) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to FOCINVEZ.
- Use of FOCINVEZ with strong CYP3A4 inducers (e.g., rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of FOCINVEZ.
See Table 7 and Table 8 for a listing of potentially significant drug interactions [see Drug Interactions ( 7.1, 7.2)] .
5.2 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis and anaphylactic shock, during or soon after infusion of fosaprepitant have occurred. Symptoms including flushing, erythema, dyspnea, hypotension and syncope have been reported [see Adverse Reactions ( 6.2)] .
Monitor patients during and after infusion. If hypersensitivity reactions occur, discontinue the infusion and administer appropriate medical therapy. Do not reinitiate FOCINVEZ in patients who experience these symptoms with previous use
[see Contraindications (
4)]
.
5.3 Infusion Site Reactions
Infusion site reactions (ISRs) have been reported with the use of intravenous fosaprepitant [see Adverse Reactions (6.1)]. The majority of severe ISRs, including thrombophlebitis and vasculitis, were reported with concomitant vesicant (anthracycline-based) chemotherapy administration, particularly when associated with extravasation. Necrosis was also reported in some patients with concomitant vesicant chemotherapy. Most ISRs occurred with the first, second or third exposure to single doses of intravenous fosaprepitant and in some cases, reactions persisted for two weeks or longer. Treatment of severe ISRs consisted of medical, and in some cases surgical, intervention.
Avoid infusion of FOCINVEZ into small veins or through a butterfly catheter. If a severe ISR develops during infusion, discontinue the infusion and administer appropriate medical treatment.
5.4 Decrease in INR with Concomitant Warfarin
Coadministration of fosaprepitant with warfarin, a CYP2C9 substrate, may result in a clinically significant decrease in the International Normalized Ratio (INR) of prothrombin time [see Clinical Pharmacology ( 12.3)] . Monitor the INR in patients on chronic warfarin therapy in the 2-week period, particularly at 7 to 10 days, following initiation of FOCINVEZ with each chemotherapy cycle [see Drug Interactions ( 7.1)] .
5.5 Risk of Reduced Efficacy of Hormonal Contraceptives
Upon coadministration with fosaprepitant, the efficacy of hormonal contraceptives may be reduced during administration of and for 28 days following the last dose of fosaprepitant [see Clinical Pharmacology ( 12.3)] . Advise patients to use effective alternative or back-up methods of contraception during treatment with FOCINVEZ and for 1 month following administration of the last dose of fosaprepitant or oral aprepitant [see Drug Interactions ( 7.1), and Use in Specific Populations ( 8.3)] .
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions ( 5.2)]
- Infusion Site Reactions [see Warnings and Precautions ( 5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of FOCINVEZ has been established from adequate and well-controlled studies of another intravenous formulation of fosaprepitant [see Clinical Studies ( 14)] . Below is a display of the adverse reactions of fosaprepitant in these studies.
The overall safety of intravenous fosaprepitant was evaluated in approximately 1800 adult and pediatric patients.
Adverse Reactions in Adults for the Prevention of Nausea and Vomiting Associated with MEC
In an active-controlled clinical trial in patients receiving MEC, safety was evaluated in 504 patients receiving a single dose of intravenous fosaprepitant in combination with ondansetron and dexamethasone (intravenous fosaprepitant regimen) compared to 497 patients receiving ondansetron and dexamethasone alone (standard therapy). The most common adverse reactions are listed in Table 6.
| Intravenous fosaprepitant, ondansetron, and dexamethasone b(N=504) | Ondansetron and dexamethasone c(N=497) | |
|---|---|---|
| aReported in ≥2% of patients treated with the intravenous fosaprepitant regimen and at a greater incidence than standard therapy (ondansetron and dexamethasone alone). | ||
| bIntravenous fosaprepitant regimen | ||
| cStandard therapy | ||
| fatigue | 15% | 13% |
| diarrhea | 13% | 11% |
| neutropenia | 8% | 7% |
| asthenia | 4% | 3% |
| anemia | 3% | 2% |
| peripheral neuropathy | 3% | 2% |
| leukopenia | 2% | 1% |
| dyspepsia | 2% | 1% |
| urinary tract infection | 2% | 1% |
| pain in extremity | 2% | 1% |
Infusion-site reactions were reported in 2.2% of patients treated with the intravenous fosaprepitant regimen compared to 0.6% of patients treated with standard therapy. The infusion-site reactions included: infusion-site pain (1.2%, 0.4%), injection-site irritation (0.2%, 0.0%), vessel puncture-site pain (0.2%, 0.0%), and infusion-site thrombophlebitis (0.6%, 0.0%), reported in the intravenous fosaprepitant regimen compared to standard therapy, respectively.
Adverse Reactions in Adults for the Prevention of Nausea and Vomiting Associated with HEC
In an active-controlled clinical study in patients receiving HEC, safety was evaluated for 1143 patients receiving a single dose of intravenous fosaprepitant compared to 1169 patients receiving the 3-day regimen of oral aprepitant
[see Clinical Studies (
14.1)]
. The safety profile was generally similar to that seen in the MEC study with fosaprepitant and prior HEC studies with aprepitant. However, infusion-site reactions occurred at a higher incidence in patients in the fosaprepitant group (3.0%) compared to those in the aprepitant group (0.5%). The following additional infusion-site reactions occurred in the HEC study and were not reported in the MEC study described above: infusion-site erythema (0.5%, 0.1%), infusion-site pruritus (0.3%, 0.0%), and infusion-site induration (0.2%, 0.1%), reported in the fosaprepitant group compared to the aprepitant group, respectively.
Adverse Reactions in Pediatric Patients 6 Months to 17 Years of Age for the Prevention of Nausea and Vomiting Associated with HEC or MEC
Single-Dose Intravenous Fosaprepitant Regimen
The safety of a single dose of intravenous fosaprepitant in pediatric patients (6 months to 17 years) was evaluated in two active-controlled and a single-arm clinical study in patients who received either HEC or MEC. Patients also received ondansetron with or without dexamethasone. The adverse reaction profile was similar to adults. The safety analysis included 69 pediatric patients who received the recommended dose. An additional 70 patients received a single, higher-than-recommended dose. The most common adverse reactions that occurred in >15% of patients who received the recommended dose were anemia, neutropenia, thrombocytopenia, and febrile neutropenia.
3-Day Intravenous Fosaprepitant/Oral Aprepitant/Oral Aprepitant Regimen
In pediatric patients (12 to 17 years), the safety of the 3-day intravenous fosaprepitant/oral aprepitant/oral aprepitant regimen was evaluated in a single-arm clinical study including 12 patients who received a regimen of either HEC or MEC. In pediatric patients 6 months to 12 years of age, the safety of the 3-day regimen was not directly evaluated. The safety of a single-dose of intravenous fosaprepitant (3 mg/kg) administered on day 1 of the 3-day regimen was evaluated in one active-controlled and one single-arm study including 48 patients who received a regimen of either HEC or MEC.
In these clinical studies, pediatric patients also received ondansetron with or without dexamethasone. The adverse reaction profile was similar to adults and pediatric patients receiving a single dose of intravenous fosaprepitant.
Because fosaprepitant is converted to aprepitant, those adverse reactions associated with aprepitant might also be expected to occur with intravenous fosaprepitant. See the full prescribing information for aprepitant capsules for complete safety information regarding studies performed with oral aprepitant.
Additional pediatric use information is approved for Merck Sharp & Dohme LLC’s EMEND (fosaprepitant) for injection. However, due to Merck Sharp & Dohme LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fosaprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: pruritus, rash, urticaria, Stevens-Johnson syndrome/toxic epidermal necrolysis [see Warnings and Precautions ( 5.2)] .
Immune system disorders:hypersensitivity reactions including anaphylaxis and anaphylactic shock [see Contraindications ( 4), Warnings and Precautions ( 5.2)] .
Nervous system disorders:ifosfamide-induced neurotoxicity reported after fosaprepitant and ifosfamide coadministration.
7. Drug Interactions
7.1 Effect of Fosaprepitant/Aprepitant on the Pharmacokinetics of Other Drugs
When administered intravenously, fosaprepitant, a prodrug of aprepitant, is converted to aprepitant within 30 minutes. Therefore, drug interactions following administration of FOCINVEZ are likely to occur with drugs that interact with oral aprepitant.
Fosaprepitant, given as a single 150-mg dose, is a weak inhibitor of CYP3A4, and the weak inhibition of CYP3A4 continues for 2 days after single dose administration. Single dose fosaprepitant does not induce CYP3A4. Aprepitant is a substrate, an inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9 [see Clinical Pharmacology ( 12.3)] .
Some substrates of CYP3A4 are contraindicated with FOCINVEZ [see Contraindications ( 4)] . Dosage adjustment of some CYP3A4 and CYP2C9 substrates may be warranted, as shown in Table 7.
| CYP3A4 Substrates | |
| Pimozide | |
| Clinical Impact | Increased pimozide exposure |
| Intervention | FOCINVEZ is contraindicated [see Contraindications ( 4)] . |
| Benzodiazepines | |
| Clinical Impact | Increased exposure to midazolam or other benzodiazepines metabolized via CYP3A4 (alprazolam, triazolam) may increase the risk of adverse reactions [see Clinical Pharmacology ( 12.3)] . |
| Intervention | Monitor for benzodiazepine-related adverse reactions. |
| Dexamethasone | |
| Clinical Impact | Increased dexamethasone exposure [see Clinical Pharmacology ( 12.3)] . |
| Intervention | Reduce the dose of oral dexamethasone by approximately 50% [see Dosage and Administration( 2.1)]. |
| Methylprednisolone | |
| Clinical Impact | Increased methylprednisolone exposure [see Clinical Pharmacology ( 12.3)]. |
| Intervention | Reduce the dose of oral methylprednisolone by approximately 50% on Days 1 and 2 for patients receiving HEC and on Day 1 for patients receiving MEC.
Reduce the dose of intravenous methylprednisolone by 25% on Days 1 and 2 for patients receiving HEC and on Day 1 for patients receiving MEC. |
| Chemotherapeutic agents that are metabolized by CYP3A4 | |
| Clinical Impact | Increased exposure of the chemotherapeutic agent may increase the risk of adverse reactions [see Clinical Pharmacology ( 12.3)]. |
| Intervention | Vinblastine, vincristine, or ifosfamide or other chemotherapeutic agents
|
| Hormonal Contraceptives | |
| Clinical Impact | Decreased hormonal exposure during administration of and for 28 days after administration of the last dose of fosaprepitant [see Warnings and Precautions ( 5.5), Use in Specific Populations ( 8.3), and Clinical Pharmacology ( 12.3)] . |
| Intervention | Effective alternative or back-up methods of contraception (such as condoms and spermicides) should be used during treatment with FOCINVEZ and for 1 month following administration of the last dose of fosaprepitant or oral aprepitant. |
| Examples | birth control pills, transdermal systems, implants, and certain intrauterine systems |
| CYP2C9 Substrates | |
| Warfarin | |
| Clinical Impact | Decreased warfarin exposure and decreased prothrombin time (INR) [see Warnings and Precautions ( 5.4) and Clinical Pharmacology ( 12.3)] . |
| Intervention | In patients on chronic warfarin therapy, monitor the prothrombin time (INR) in the 2-week period, particularly at 7 to 10 days, following administration of FOCINVEZ with each chemotherapy cycle. |
| Other | |
| 5-HT 3Antagonists | |
| Clinical Impact | No change in the exposure of the 5-HT3 antagonist [see Clinical Pharmacology ( 12.3)]. |
| Intervention | No dosage adjustment needed |
| Examples | ondansetron, granisetron, dolasetron |
7.2 Effect of Other Drugs on the Pharmacokinetics of Fosaprepitant/Aprepitant
Aprepitant is a CYP3A4 substrate [see Clinical Pharmacology ( 12.3)] . Co-administration of FOCINVEZ with drugs that are inhibitors or inducers of CYP3A4 may result in increased or decreased plasma concentrations of aprepitant, respectively, as shown in Table 8.
| Moderate to Strong CYP3A4 Inhibitors | |
| Clinical Impact | Significantly increased exposure of aprepitant may increase the risk of adverse reactions associated with FOCINVEZ [see Adverse Reactions ( 6.1), Clinical Pharmacology ( 12.3)] . |
| Intervention | Avoid concomitant use of FOCINVEZ |
| Examples |
Moderateinhibitor:diltiazem Stronginhibitors:ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, nelfinavir |
| Strong CYP3A4 Inducers | |
| Clinical Impact | Substantially decreased exposure of aprepitant in patients chronically taking a strong CYP3A4 inducer may decrease the efficacy of FOCINVEZ [see Clinical Pharmacology ( 12.3)]. |
| Intervention | Avoid concomitant use of FOCINVEZ |
| Examples | rifampin, carbamazepine, phenytoin |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are insufficient data on use of fosaprepitant in pregnant women to identify a drug associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproduction studies, no adverse developmental effects were observed in rats or rabbits exposed during the period of organogenesis to systemic drug levels (AUC) approximately equivalent to the exposure at the recommended human dose (RHD) of 150 mg (see
Data).
The background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In embryofetal development studies in rats and rabbits, aprepitant was administered during the period of organogenesis at oral doses up to 1000 mg/kg twice daily (rats) and up to the maximum tolerated dose of 25 mg/kg/day (rabbits). No embryofetal lethality or malformations were observed at any dose level in either species. The exposures (AUC) in pregnant rats at 1000 mg/kg twice daily and in pregnant rabbits at 25 mg/kg/day were approximately equivalent to the exposure at the RHD of 150 mg. Aprepitant crosses the placenta in rats and rabbits.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted to assess the presence of aprepitant in human milk, the effects on the breastfed infant, or the effects on milk production. Aprepitant is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for FOCINVEZ and any potential adverse effects on the breastfed infant from FOCINVEZ or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Upon administration of FOCINVEZ, the efficacy of hormonal contraceptives may be reduced. Advise females of reproductive potential using hormonal contraceptives to use an effective alternative or back-up non-hormonal contraceptive (such as condoms and spermicides) during treatment with FOCINVEZ and for 1 month following the last dose of fosaprepitant or oral aprepitant
[see Drug Interactions (
7.1) and Clinical Pharmacology (
12.3)]
.
8.4 Pediatric Use
The safety and effectiveness of a single dose regimen of FOCINVEZ and a 3-day intravenous fosaprepitant/oral aprepitant/oral aprepitant regimen have been established in pediatric patients 6 months to 17 years for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of HEC and MEC.
Use of FOCINVEZ in this age group is supported by evidence from adequate and well-controlled studies of intravenous fosaprepitant in adults, with additional safety, efficacy and pharmacokinetic data in pediatric patients 6 months to 17 years. Efficacy and safety were also supported by data from an adequate and well controlled study of a 3-day oral aprepitant regimen in pediatric patients 6 months to 17 years. See the full prescribing information for aprepitant capsules for complete clinical information regarding studies performed with oral aprepitant. Adverse reactions were similar to those reported in adult patients. [See Dosage and Administration ( 2.2), Adverse Reactions ( 6.1), and Clinical Pharmacology ( 12.3)] .
The safety and effectiveness of FOCINVEZ for the prevention of nausea and vomiting associated with HEC or MEC have not been established in patients less than 6 months of age.
Juvenile Animal Toxicity Data
In juvenile dogs treated with fosaprepitant, changes in reproductive organs were observed. In juvenile rats treated with aprepitant, slight changes in sexual maturation were observed without an effect on reproduction. No effects on neurobehavior, sensory and motor function, or learning and memory were observed in rats.
In a toxicity study in juvenile dogs treated with fosaprepitant from postnatal day 14 (equivalent to a newborn human) to day 42 (approximately equivalent to a 2 year old human), decreased testicular weight and Leydig cell size were seen in the males at 6 mg/kg/day and increased uterine weight, hypertrophy of the uterus and cervix, and edema of vaginal tissues were seen in females from 4 mg/kg/day. A study was also conducted in young rats to evaluate the effects of aprepitant on growth and on neurobehavioral and sexual development. Rats were treated at oral doses up to the maximum feasible dose of 1000 mg/kg twice daily (providing exposure in male and female rats lower than the exposure at the recommended pediatric human dose) from the early postnatal period (Postnatal Day 10 (equivalent to a newborn human) through Postnatal Day 58 (approximately equivalent to a 15 year old human)). Slight changes in the onset of sexual maturation were observed in female and male rats; however, there were no effects on mating, fertility, embryonic-fetal survival, or histomorphology of the reproductive organs. There were no effects in neurobehavioral tests of sensory function, motor function, and learning and memory.
Additional pediatric use information is approved for Merck Sharp & Dohme LLC’s EMEND (fosaprepitant) for injection. However, due to Merck Sharp & Dohme LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Of the 1649 adult cancer patients treated with intravenous fosaprepitant in HEC and MEC clinical studies, 27% were aged 65 and over, while 5% were aged 75 and over. Other reported clinical experience with fosaprepitant has not identified differences in responses between
elderly and younger adult patients. No clinically meaningful differences in the pharmacokinetics of oral aprepitant were observed in healthy adult subjects 65 years of age and over compared to younger adult subjects
[see Clinical Pharmacology (
12.3)].
8.6 Patients with Hepatic Impairment
The pharmacokinetics of aprepitant in patients with mild and moderate hepatic impairment were similar to those of healthy subjects with normal hepatic function. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9). There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9). Therefore, additional monitoring for adverse reactions in these patients may be warranted when FOCINVEZ is administered [see Clinical Pharmacology ( 12.3)] .
10. Overdosage
There is no specific information on the treatment of overdosage with fosaprepitant or aprepitant.
In the event of overdose, FOCINVEZ should be discontinued and general supportive treatment and monitoring should be provided.
Aprepitant is not removed by hemodialysis.
11. Focinvez Injection Description
FOCINVEZ (fosaprepitant injection) is a sterile, ready-to-use, clear and colorless solution formulation containing fosaprepitant dimeglumine, a prodrug of aprepitant, a substance P/neurokinin-1 (NK1) receptor antagonist, an antiemetic agent, chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1H-1,2,4triazol-1-yl]phosphonate (2:1) (salt).
Its empirical formula is C 23H 22F 7N 4O 6P ⋅ 2(C 7H 17NO 5) and its structural formula is:
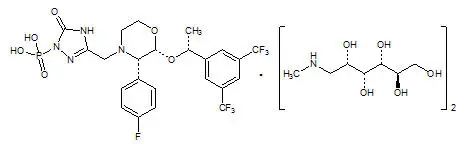
Fosaprepitant dimeglumine is a white to off-white amorphous powder with a molecular weight of 1004.83. It is freely soluble in water.
Each 50 mL vial of FOCINVEZ for administration as an intravenous infusion contains 150 mg of fosaprepitant (equivalent to 245.3 mg of fosaprepitant dimeglumine) and the following inactive ingredients: Betadex sulfobutyl ether sodium (8 g), edetate disodium (5.4 mg), sodium hydroxide (for pH adjustment) in water for injection.
12. Focinvez Injection - Clinical Pharmacology
12.1 Mechanism of Action
Fosaprepitant is a prodrug of aprepitant and accordingly, its antiemetic effects are attributable to aprepitant.
Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK 1) receptors. Aprepitant has little or no affinity for serotonin (5-HT 3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CINV). Aprepitant has been shown in animal models to inhibit emesis induced by cytotoxic chemotherapeutic agents, such as cisplatin, via central actions. Animal and human Positron Emission Tomography (PET) studies with aprepitant have shown that it crosses the blood brain barrier and occupies brain NK1 receptors. Animal and human studies have shown that aprepitant augments the antiemetic activity of the 5-HT 3-receptor antagonist ondansetron and the corticosteroid dexamethasone and inhibits both the acute and delayed phases of cisplatin-induced emesis.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a randomized, double-blind, positive-controlled, thorough QTc study, a single 200-mg dose of fosaprepitant (approximately 1.3 times the recommended dose) had no effect on the QTc interval.
12.3 Pharmacokinetics
Aprepitant after Fosaprepitant Administration
Following administration of a single intravenous 150-mg dose of fosaprepitant, a prodrug of aprepitant administered as a 20-minute infusion to healthy subjects, the mean AUC
0-∞of aprepitant was 37.4 (± 14.8) mcg•hr/mL and the mean maximal aprepitant concentration (C
max) was 4.2 (± 1.2) mcg/mL. Plasma concentrations of fosaprepitant are below the limits of quantification (10 ng/mL) within 30 minutes of the completion of infusion.
Distribution
Aprepitant is greater than 95% bound to plasma proteins. The mean apparent volume of distribution at steady state (Vd
ss) was approximately 70 L in humans.
Aprepitant crosses the blood brain barrier in humans
[see Clinical Pharmacology
(12.1)]
.
Elimination
Metabolism
Fosaprepitant is converted to aprepitant in
in vitroincubations with human liver preparations and in S9 preparations from multiple other human tissues including kidney, lung and ileum. Thus, it appears that the conversion of fosaprepitant to aprepitant can occur in multiple extrahepatic tissues in addition to the liver.
Aprepitant undergoes extensive metabolism. In vitrostudies using human liver microsomes indicate that aprepitant is metabolized primarily by CYP3A4 with minor metabolism by CYP1A2 and CYP2C19. Metabolism is largely via oxidation at the morpholine ring and its side chains. No metabolism by CYP2D6, CYP2C9, or CYP2E1 was detected.
In healthy young adults, aprepitant accounts for approximately 24% of the radioactivity in plasma over 72 hours following a single oral 300-mg dose of [ 14C]-aprepitant, indicating a substantial presence of metabolites in the plasma. Seven metabolites of aprepitant, which are only weakly active, have been identified in human plasma.
Excretion
Following administration of a single intravenous 100-mg dose of [
14C]-fosaprepitant to healthy subjects, 57% of the radioactivity was recovered in urine and 45% in feces.
Aprepitant is eliminated primarily by metabolism; aprepitant is not renally excreted. The apparent terminal half-life ranged from approximately 9 to 13 hours.
Specific Populations
Geriatric Patients
Following oral administration of a single 125-mg dose of aprepitant on Day 1 and 80 mg once daily on Days 2 through 5, the AUC
0-24hrof aprepitant was 21% higher on Day 1 and 36% higher on Day 5 in healthy elderly subjects (65 years and older) relative to younger adult subjects. The C
maxwas 10% higher on Day 1 and 24% higher on Day 5 in healthy elderly subjects relative to younger adult subjects. These differences are not considered clinically meaningful [
see Use in Specific Populations
(8.5)].
Pediatric Patients
Single-Dose Intravenous Fosaprepitant Regimen: Simulated systemic exposures of aprepitant in patients 2 years to less than 12 years and observed systemic exposures in patients 6 months to less than 2 years and 12 to 17 years are shown in Table 9, including AUC
0-24hr, peak plasma concentration (C
max) on Day 1 and concentrations at the end of Day 1 (C
24), Day 2 (C
48) and Day 3 (C
72).
| aND = Not Determined. Pharmacokinetic samples were not collected to support the parameter value of interest. | ||||||
| bNE = Not Estimated. The geometric mean could not be estimated due to values being below the limitation of quantification. | ||||||
| Population | Single-Dose of Intravenous Fosaprepitant Regimen | Geometric Mean | ||||
| AUC
0-24hr.
(mcg*hr/mL) | C
max
(mcg/mL) | C
24
(mcg/mL) | C
48
(mcg/mL) | C
72
(mcg/mL) |
||
| 12 Years to 17 Years | 150 mg | 29.4 | 3.4 | 0.7 | ND a | ND a |
| 6 Years to less than 12 Years | 4 mg/kg | 35.2 | 3.6 | 0.7 | 0.2 | 0.05 |
| 2 Years to less than 6 Years | 28.2 | 3.1 | 0.4 | 0.1 | 0.02 | |
| 6 Months to less than 2 Years | 5 mg/kg | 32.7 | 3.3 | 0.4 | NE b | ND a |
3-Day Intravenous fosaprepitant/Oral fosaprepitant/Oral aprepitant Regimen:Simulated aprepitant systemic exposures in patients 6 months to less than 12 years and observed systemic exposures in patients 12 to 17 years are shown in Table 10, including AUC 0-24hr, peak plasma concentration (C max) on Day 1 and concentrations at the end of Day 1 (C 24), Day 2 (C 48) and Day 3 (C 72).
| aIntravenous fosaprepitant on Day 1, oral aprepitant on Day 2, and oral aprepitant on Day 3 | ||||||
| bNE = Not Estimated. The geometric mean could not be estimated due to values being below the limitation of quantification | ||||||
| Population | 3-Day Dose of fosaprepitant/ aprepitant (IV/Oral/Oral a) | Geometric Mean | ||||
| AUC
0-24hr.
(mcg*hr/mL) | C
max
(mcg/mL) | C
24
(mcg/mL) | C
48
(mcg/mL) | C
72
(mcg/mL) |
||
| 12 Years to 17 Years | 115/80/80 mg | 18.0 | 3.0 | 0.4 | 0.2 | NE b |
| 6 Years to less than 12 Years | 3/2/2 mg/kg | 25.7 | 2.7 | 0.5 | 0.3 | 0.3 |
| 2 Years to less than 6 Years | 20.2 | 2.3 | 0.3 | 0.2 | 0.2 | |
| 6 Months to less than 2 Years | 16.6 | 1.9 | 0.2 | 0.1 | 0.1 | |
Plasma concentrations of fosaprepitant are negligible within 15-30 minutes after the completion of the infusion in pediatric patients.
Male and Female Patients
Following oral administration of a single dose of aprepitant, ranging from 40 mg to 375 mg, the AUC
0-24hrand C
maxare 9% and 17% higher in females as compared with males. The half-life of aprepitant is approximately 25% lower in females as compared with males and T
maxoccurs at approximately the same time. These differences are not considered clinically meaningful. A population pharmacokinetic analysis of aprepitant in pediatric patients (6 months to 17 years) suggests that sex has no clinically meaningful effect on the pharmacokinetics of aprepitant.
Racial and Ethnic Groups
Following oral administration of a single dose of aprepitant, ranging from 40 mg to 375 mg, the AUC
0-24hrand C
maxare approximately 27% and 19% higher in Hispanics as compared with Caucasians. The AUC
0-24hrand C
maxwere 74% and 47% higher in Asians as compared to Caucasians. There was no difference in AUC
0-24hror C
maxbetween Caucasians and Blacks. These differences are not considered clinically meaningful. A population pharmacokinetic analysis of aprepitant in pediatric patients (6 months to 17 years) suggests that race has no clinically meaningful effect on the pharmacokinetics of aprepitant.
Patients with Renal Impairment
A single 240-mg oral dose of aprepitant was administered to patients with severe renal impairment (creatinine clearance less than 30 mL/min/1.73 m
2as measured by 24-hour urinary creatinine clearance) and to patients with end stage renal disease (ESRD) requiring hemodialysis.
In patients with severe renal impairment, the AUC 0-∞of total aprepitant (unbound and protein bound) decreased by 21% and C maxdecreased by 32%, relative to healthy subjects (creatinine clearance greater than 80 mL/min estimated by Cockcroft-Gault method). In patients with ESRD undergoing hemodialysis, the AUC 0-∞of total aprepitant decreased by 42% and C maxdecreased by 32%. Due to modest decreases in protein binding of aprepitant in patients with renal disease, the AUC of pharmacologically active unbound drug was not significantly affected in patients with renal impairment compared with healthy subjects. Hemodialysis conducted 4 or 48 hours after dosing had no significant effect on the pharmacokinetics of aprepitant; less than 0.2% of the dose was recovered in the dialysate.
Patients with Hepatic Impairment
Fosaprepitant is metabolized in various extrahepatic tissues; therefore, hepatic impairment is not expected to alter the conversion of fosaprepitant to aprepitant.
Following administration of a single 125-mg oral dose of aprepitant on Day 1 and 80 mg once daily on Days 2 and 3 to patients with mild hepatic impairment (Child-Pugh score 5 to 6), the AUC 0-24hrof aprepitant was 11% lower on Day 1 and 36% lower on Day 3, as compared with healthy subjects given the same regimen. In patients with moderate hepatic impairment (Child-Pugh score 7 to 9), the AUC 0-24hrof aprepitant was 10% higher on Day 1 and 18% higher on Day 3, as compared with healthy subjects given the same regimen. These differences in AUC 0-24hrare not considered clinically meaningful.
There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9) [see Use in Specific Populations (8.6)].
Body Mass Index (BMI)
For every 5 kg/m
2increase in BMI, AUC
0-24hrand C
maxof aprepitant decrease by 9% and 10%. BMI of subjects in the analysis ranged from 18 kg/m
2to 36 kg/m
2. This change is not considered clinically meaningful.
Drug Interaction Studies
Fosaprepitant, given as a single 150-mg dose, is a weak inhibitor of CYP3A4, with no evidence of inhibition or induction of CYP3A4 observed on Day 4. The weak inhibition of CYP3A4 continues for 2 days after single dose administration of fosaprepitant. Aprepitant is a substrate, an inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9.
Fosaprepitant or aprepitant is unlikely to interact with drugs that are substrates for the Pglycoprotein transporter.
Effects of Fosaprepitant/Aprepitant on the Pharmacokinetics of Other Drugs
CYP3A4 Substrates
Midazolam:Fosaprepitant 150 mg administered as a single intravenous dose on Day 1 increased the AUC
0-∞of midazolam by approximately 1.8-fold on Day 1 and had no effect on Day 4 when midazolam was coadministered as a single oral dose of 2 mg on Days 1 and 4
[see Drug Interactions (
7.1)].
Corticosteroids:
Dexamethasone:Fosaprepitant administered as a single 150 mg intravenous dose on Day 1 increased the AUC
0-24hrof dexamethasone, administered as a single 8-mg oral dose on Days 1, 2, and 3, by approximately 2-fold on Days 1 and 2
[see Dosage and Administration (
2.1) and Drug Interactions (
7.1)].
Methylprednisolone:When oral aprepitant as a 3-day regimen (125-mg/80-mg/80-mg) was administered with intravenous methylprednisolone 125 mg on Day 1 and oral methylprednisolone 40 mg on Days 2 and 3, the AUC of methylprednisolone was increased by 1.34-fold on Day 1 and by 2.5-fold on Day 3 [see Drug Interactions ( 7.1)].
Chemotherapeutic agents:
Docetaxel:In a pharmacokinetic study, oral aprepitant administered as a 3-day regimen (125mg/80-mg/80-mg) did not influence the pharmacokinetics of docetaxel.
Vinorelbine:In a pharmacokinetic study, oral aprepitant administered as a 3-day regimen (125mg/80-mg/80-mg) did not influence the pharmacokinetics of vinorelbine to a clinically significant degree.
Oral contraceptives:When oral aprepitant was administered as a 3-day regimen (125-mg/80mg/80-mg) with ondansetron and dexamethasone, and coadministered with an oral contraceptive containing ethinyl estradiol and norethindrone, the trough concentrations of both ethinyl estradiol and norethindrone were reduced by as much as 64% for 3 weeks post-treatment [see Drug Interactions ( 7.1)] .
CYP2C9 substrates (Warfarin, Tolbutamide):
Warfarin:A single 125-mg dose of oral aprepitant was administered on Day 1 and 80 mg/day on Days 2 and 3 to subjects who were stabilized on chronic warfarin therapy. Although there was no effect of oral aprepitant on the plasma AUC of R(+) or S(-) warfarin determined on Day 3, there was a 34% decrease in S(-) warfarin trough concentration accompanied by a 14% decrease in the prothrombin time (reported as International Normalized Ratio or INR) 5 days after completion of dosing with oral aprepitant
[see Drug Interactions (
7.1)]
.
Tolbutamide:Oral aprepitant, when given as 125 mg on Day 1 and 80 mg/day on Days 2 and 3, decreased the AUC of tolbutamide by 23% on Day 4, 28% on Day 8, and 15% on Day 15, when a single dose of tolbutamide 500 mg was administered prior to the administration of the 3-day regimen of oral aprepitant and on Days 4, 8, and 15. This effect was not considered clinically important.
Other Drugs
P-glycoprotein substrates:Aprepitant is unlikely to interact with drugs that are substrates for the Pglycoprotein transporter, as demonstrated by the lack of interaction of oral aprepitant with digoxin in a clinical drug interaction study.
5-HT 3 antagonists:In clinical drug interaction studies, aprepitant did not have clinically important effects on the pharmacokinetics of ondansetron, granisetron, or hydrodolasetron (the active metabolite of dolasetron).
Effect of Other Drugs on the Pharmacokinetics of Fosaprepitant/Aprepitant
Rifampin:When a single 375-mg dose of oral aprepitant was administered on Day 9 of a 14-day regimen of 600 mg/day of rifampin, a strong CYP3A4 inducer, the AUC of aprepitant decreased approximately 11-fold and the mean terminal half-life decreased approximately 3-fold
[see Drug Interactions (
7.2)]
.
Ketoconazole:When a single 125-mg dose of oral aprepitant was administered on Day 5 of a 10-day regimen of 400 mg/day of ketoconazole, a strong CYP3A4 inhibitor, the AUC of aprepitant increased approximately 5-fold and the mean terminal half-life of aprepitant increased approximately 3-fold [see Drug Interactions ( 7.2)] .
Diltiazem:In a study in 10 patients with mild to moderate hypertension, administration of 100 mg of fosaprepitant as an intravenous infusion with 120 mg of diltiazem, a moderate CYP3A4 inhibitor administered three times daily, resulted in a 1.5-fold increase in the aprepitant AUC and a 1.4-fold increase in the diltiazem AUC.
When fosaprepitant was administered with diltiazem, the mean maximum decrease in diastolic blood pressure was significantly greater than that observed with diltiazem alone [24.3 ± 10.2 mm Hg with fosaprepitant versus 15.6 ± 4.1 mm Hg without fosaprepitant]. The mean maximum decrease in systolic blood pressure was also greater after co-administration of diltiazem with fosaprepitant than administration of diltiazem alone [29.5 ± 7.9 mm Hg with fosaprepitant versus 23.8 ± 4.8 mm Hg without fosaprepitant]. Co-administration of fosaprepitant and diltiazem; however, did not result in any additional clinically significant changes in heart rate or PR interval, beyond those changes observed with diltiazem alone
[see Drug Interactions (
7.2)].
Paroxetine:Coadministration of once daily doses of oral aprepitant 170 mg, with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and C maxby approximately 20% of both aprepitant and paroxetine. This effect was not considered clinically important.
Additional pediatric use information is approved for Merck Sharp & Dohme LLC’s EMEND (fosaprepitant) for injection. However, due to Merck Sharp & Dohme LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in Sprague-Dawley rats and in CD-1 mice for 2 years. In the rat carcinogenicity studies, animals were treated with oral doses ranging from 0.05 to 1000 mg/kg twice daily. The highest dose produced systemic exposures to aprepitant approximately equivalent to (female rats) or less than (male rats) the adult human exposure at the RHD of 150 mg. Treatment with aprepitant at doses of 5 to 1000 mg/kg twice daily caused an increase in the incidences of thyroid follicular cell adenomas and carcinomas in male rats. In female rats, it produced hepatocellular adenomas at 5 to 1000 mg/kg twice daily and hepatocellular carcinomas and thyroid follicular cell adenomas at 125 to 1000 mg/kg twice daily. In the mouse carcinogenicity studies, the animals were treated with oral doses ranging from 2.5 to 2000 mg/kg/day. The highest dose produced a systemic exposure approximately 2 times the adult human exposure at the RHD of 150 mg. Treatment with aprepitant produced skin fibrosarcomas at 125 and 500 mg/kg/day doses in male mice. Carcinogenicity studies were not conducted with fosaprepitant.
Mutagenesis
Aprepitant and fosaprepitant were not genotoxic in the Ames test, the human lymphoblastoid cell (TK6) mutagenesis test, the rat hepatocyte DNA strand break test, the Chinese hamster ovary (CHO) cell chromosome aberration test and the mouse micronucleus test.
Impairment of Fertility
Fosaprepitant, when administered intravenously, is rapidly converted to aprepitant. In the fertility studies conducted with fosaprepitant and aprepitant, the highest systemic exposures to aprepitant were obtained following oral administration of aprepitant. Oral aprepitant did not affect the fertility or general reproductive performance of male or female rats at doses up to the maximum feasible dose of 1000 mg/kg twice daily (providing exposure in male rats lower than the exposure at the recommended adult human dose of 150 mg and exposure in female rats approximately equivalent to the adult human exposure).
14. Clinical Studies
The safety and efficacy of FOCINVEZ have been established based on adequate and well-controlled adult studies of another intravenous formulation of fosaprepitant for the prevention and of chemotherapy induced nausea and vomiting. Below is a description of the results of these adequate and well-controlled studies of fosaprepitant.
14.1 Prevention of Nausea and Vomiting Associated with HEC in Adults
In a randomized, parallel, double-blind, active-controlled study, fosaprepitant 150 mg as a single intravenous infusion (N=1147) was compared to a 3-day oral aprepitant regimen (N=1175) in patients receiving a HEC regimen that included cisplatin (70 mg/m 2). All patients in both groups received dexamethasone and ondansetron (see Table 11). Patient demographics were similar between the two treatment groups. Of the total 2322 patients, 63% were men, 56% White, 26% Asian, 3% American Indian/Alaska Native, 2% Black, 13% Multi-Racial, and 33% Hispanic/Latino ethnicity. Patient ages ranged from 19 to 86 years of age, with a mean age of 56 years. Other concomitant chemotherapy agents commonly administered were fluorouracil (17%), gemcitabine (16%), paclitaxel (15%), and etoposide (12%).
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| aIntravenous fosaprepitant placebo, aprepitant capsules placebo and dexamethasone placebo (in the evenings on Days 3 and 4) were used to maintain blinding. | ||||
| bDexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. Dexamethasone was also administered in the evenings on Days 3 and 4. The 12 mg dose of dexamethasone on Day 1 and the 8 mg once daily dose on Day 2 reflects a dosage adjustment to account for a drug interaction with the intravenous fosaprepitant regimen [see Clinical Pharmacology ( 12.3)] . | ||||
| cOndansetron 32 mg intravenous was used in the clinical trials of intravenous fosaprepitant. Although this dose was used in clinical trials, this is no longer the currently recommended dose. Refer to the ondansetron prescribing information for the current recommended dose. | ||||
| dDexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. The 12 mg dose of dexamethasone on Day 1 and the 8 mg once daily dose on Days 2 through 4 reflects a dosage adjustment to account for a drug interaction with the oral aprepitant regimen [see Clinical Pharmacology ( 12.3)] . | ||||
| Fosaprepitant Regimen | ||||
| intravenous fosaprepitant | 150 mg intravenously over 20 to 30 minutes approximately 30 minutes prior to chemotherapy | none | none | none |
| Oral dexamethasone b | 12 mg | 8 mg | 8 mg twice daily | 8 mg twice daily |
| Ondansetron | Ondansetron c | none | none | none |
| Oral aprepitant Regimen | ||||
| Aprepitant capsules | 125 mg | 80 mg | 80 mg | none |
| Oral dexamethasone d | 12 mg | 8 mg | 8 mg | 8 mg |
| Ondansetron | Ondansetron c | none | none | none |
The efficacy of intravenous fosaprepitant was evaluated based on the primary and secondary endpoints listed in Table 12 and was shown to be non-inferior to that of the 3-day oral aprepitant regimen with regard to complete response in each of the evaluated phases. The pre-specified non-inferiority margin for complete response in the overall phase was 7%. The pre-specified non-inferiority margin for complete response in the delayed phase was 7.3%. The pre-specified non-inferiority margin for no vomiting in the overall phase was 8.2%.
| ENDPOINTS | Intravenous fosaprepitant Regimen
(N = 1106) a % | Oral aprepitant
Regimen (N = 1134) a % | Difference b(95% CI) |
|---|---|---|---|
| aN: Number of patients included in the primary analysis of complete response. | |||
| bDifference and Confidence interval (CI) were calculated using the method proposed by Miettinen and Nurminen and adjusted for Gender. | |||
| cComplete Response = no vomiting and no use of rescue therapy. | |||
| dOverall = 0 to 120 hours post-initiation of cisplatin chemotherapy. | |||
| eDelayed phase = 25 to 120 hours post-initiation of cisplatin chemotherapy. | |||
| PRIMARY ENDPOINT | |||
| Complete Response c | |||
| Overall d | 71.9 | 72.3 | -0.4 (-4.1, 3.3) |
| SECONDARY ENDPOINTS | |||
| Complete Response c | |||
| Delayed phase e | 74.3 | 74.2 | 0.1 (-3.5, 3.7) |
| No Vomiting | |||
| Overall d | 72.9 | 74.6 | -1.7 (-5.3, 2.0) |
14.2 Prevention of Nausea and Vomiting Associated with MEC in Adults
In a randomized, parallel, double-blind, active comparator-controlled study, intravenous fosaprepitant 150 mg as a single intravenous infusion (N=502) in combination with ondansetron and dexamethasone (intravenous fosaprepitant regimen) was compared with ondansetron and
dexamethasone alone (standard therapy) (N=498) (see Table 13) in patients receiving a MEC regimen. Patient demographics were similar between the two treatment groups. Of the total 1,000 patients included in the efficacy analysis, 41% were men, 84% White, 4% Asian, 1%
American Indian/Alaska Native, 2% Black, 10% Multi-Racial, and 19% Hispanic/Latino ethnicity. Patient ages ranged from 23 to 88 years of age, with a mean age of 60 years. The most commonly administered MEC chemotherapeutic agents were carboplatin (51%), oxaliplatin (24%), and cyclophosphamide (12%).
| aIntravenous fosaprepitant placebo and dexamethasone placebo (on Day 1) were used to maintain blinding. | |||
| bDexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1. The 12 mg dose reflects a dosage adjustment to account for a drug interaction with the intravenous fosaprepitant regimen [see Clinical Pharmacology ( 12.3)]. | |||
| cThe first ondansetron dose was administered 30 to 60 minutes prior to chemotherapy treatment on Day 1 and the second dose was administered 8 hours after first ondansetron dose. | |||
| Day 1 | Day 2 | Day 3 | |
| Intravenous fosaprepitant Regimen | |||
| Intravenous fosaprepitant | 150 mg intravenously over 20 to 30 minutes approximately 30 minutes prior to chemotherapy | none | none |
| Oral Dexamethasone b | 12 mg | none | none |
| Oral Ondansetron c | 8 mg for 2 doses | none | none |
| Standard Therapy | |||
| Oral Dexamethasone | 20 mg | none | none |
| Oral Ondansetron c | 8 mg for 2 doses | 8 mg twice daily | 8 mg twice daily |
The primary endpoint was complete response (defined as no vomiting and no rescue therapy) in the delayed phase (25 to 120 hours) of chemotherapy-induced nausea and vomiting. The results by treatment group are shown in Table 14.
| aN: Number of patients included in the intention to treat population. | ||||
| bComplete Response = no vomiting and no use of rescue therapy. | ||||
| cDelayed phase = 25 to 120 hours post-initiation of chemotherapy. | ||||
| ENDPOINTS | Intravenous fosaprepitant Regimen
(N = 502) a % | Standard Therapy
Regimen (N = 498) a % | P-Value | Treatment
Difference (95% CI) |
| PRIMARY ENDPOINT | ||||
| Complete Response b | ||||
| Delayed phase c | 78.9 | 68.5 | <0.001 | 10.4 (5.1, 15.9) |
16. How is Focinvez Injection supplied
FOCINVEZ (fosaprepitant injection) contains 150 mg/50 mL (3 mg/mL) of fosaprepitant as a clear and colorless ready-to-use injection solution in a single-dose vial. Supplied as follows:
NDC 82243-1001-1 1 vial per carton.
Storage
- Refrigerate FOCINVEZ at 2°C to 8°C (36°F to 46°F).
- FOCINVEZ vials, when kept in original carton, can remain at room temperature 20°C to 25°C (68°F to 77°F) for up to 90 days.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
Advise patients that hypersensitivity reactions, including anaphylaxis and anaphylactic shock, have been reported in patients taking fosaprepitant. Advise patients to seek immediate medical attention if they experience signs or symptoms of a hypersensitivity reaction, such as hives, rash and itching, skin peeling or sores, flushing, difficulty in breathing or swallowing, or dizziness, rapid or weak heartbeat or feeling faint
[see Warnings and Precautions (
5.2)]
.
Infusion Site Reactions
Advise patients to seek medical attention if they experience new or worsening signs or symptoms of an infusion site reaction, such as erythema, edema, pain, necrosis, vasculitis, or thrombophlebitis at or near the infusion sitee
[see Warnings and Precautions (
5.3)]
.
Drug Interactions
Advise patients to discuss all medications they are taking, including other prescription, nonprescription medication or herbal products
[see Contraindications (4), Warnings and Precautions (
5.1)]
.
Warfarin: Instruct patients on chronic warfarin therapy to follow instructions from their healthcare provider regarding blood draws to monitor their INR during the 2-week period, particularly at 7 to 10 days, following initiation of FOCINVEZ with each chemotherapy cycle [see Warnings and Precautions ( 5.4)] .
Hormonal Contraceptives:Advise patients that administration of FOCINVEZ may reduce the efficacy of hormonal contraceptives. Instruct patients to use effective alternative or back-up methods of contraception (such as condoms and spermicides) during treatment with FOCINVEZ and for 1 month following administration of the last dose of fosaprepitant or oral aprepitant [see Warnings and Precautions ( 5.5) and Use in Specific Populations( 8.3)] .
Manufactured for:
Spes Pharmaceuticals Inc., Plainsboro, NJ 08536, USA
Manufactured by:
Pharmaceutics International Inc., Hunt Valley, MD 21031, USA
Spes Pharmaceuticals Inc.
uspi-sp001-iv-2023r000
| Patient Information
FOCINVEZ (FOR sin vez) (fosaprepitant injection), for intravenous use |
|
| Read this Patient Information before you start receiving FOCINVEZ and each time you are scheduled to receive FOCINVEZ. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. | |
| What is FOCINVEZ?
FOCINVEZ is a prescription medicine used with other medicines that treat nausea and vomiting in patients 6 months of age and older to prevent nausea and vomiting caused by certain anti-cancer (chemotherapy) medicines.
|
|
| Who should not receive FOCINVEZ?
Do not receive FOCINVEZ if you:
|
|
| What should I tell my healthcare provider before receiving FOCINVEZ?
Before receiving FOCINVEZ, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
FOCINVEZ works, causing serious side effects. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine. |
|
| How will I receive FOCINVEZ?
Adults 18 years of age and older: FOCINVEZ will be given on Day 1 of chemotherapy treatment. It will be given to you by intravenous (IV) infusion in your vein about 50 to 60 minutes before you start your chemotherapy treatment. Children 6 months to 17 years of age: FOCINVEZ will be given to your child by intravenous (IV) infusion into a large vein through a type of IV line called a central venous catheter, about 1 hour to 1 ½ hours before the start of their chemotherapy treatment. Depending on the chemotherapy treatment, there are 2 ways that FOCINVEZ may be given:
|
|
| What are the possible side effects of FOCINVEZ?
FOCINVEZ may cause serious side effects, including:
In adults, the most common side effects of FOCINVEZ include: |
|
|
|
| In children 6 months to 17 years of age, the most common side effects of FOCINVEZ include: | |
|
|
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of FOCINVEZ. For more information ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
General information about the safe and effective use of FOCINVEZ. If you would like more information about FOCINVEZ, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about FOCINVEZ that is written for health professionals. For more information about FOCINVEZ call 1-732-354-3630. |
|
| What are the ingredients in FOCINVEZ?
Active ingredient:fosaprepitant Inactive ingredients: betadex sulfobutyl ether sodium, edetate disodium, sodium hydroxide (for pH adjustment) and water for injection Additional pediatric use information is approved for Merck Sharp & Dohme LLC’s EMEND (fosaprepitant) for injection. However, due to Merck Sharp & Dohme LLC’s marketing exclusivity rights, this drug product is not labeled with that information
|
|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Issue Date: 8/2023 |
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
NDC 82243-1001-1
FOCINVEZ (Fosaprepitant Injection) 150 mg/50 mL (3 mg/mL)
Rx only
Attention:This is a ready-to-use injection of fosaprepitant (3 mg/mL). Fosaprepitant is available in different concentrations.
FOR INTRAVENOUS USE
DO NOT USE WITH SOLUTIONS CONTAINING DIVALENT CATIONS (e.g., Ca 2+, Mg 2+) INCLUDING LACTATED RINGER’S SOLUTION AND HARTMANN’S SOLUTION
50 mL single-dose vial - discardunused portion
Recommended Dosage:See Prescribing Information
Storage
Must be refrigerated at 2 oC to 8 oC (36 oF to 46°F )
FOCINVEZ vials, when kept in original carton, can remain at room temperature 20°C to 25°C (68°F to 77°F) for up to 90 days.
Each vial contains: Fosaprepitant, 150 mg (equivalent to 245.3 mg of fosaprepitant dimeglumine)
Inactive ingredients: Betadex sulfobutylether sodium (8 g), edetate disodium (5.4mg), sodium hydroxide (for pHadjustment) in water for njection.
FOCINVEZ is preservative-free
Manufactured for:
Spes Pharmaceuticals Inc., Plainsboro, NJ 08536, USA
Manufactured by:
Pharmaceutics International Inc., Hunt Valley, MD 21031, USA

| FOCINVEZ
fosaprepitant injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Spes Pharmaceuticals Inc. (081511593) |
| Registrant - Spes Pharmaceuticals Inc. (081511593) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmaceutics International Inc. | 049185696 | manufacture(82243-1001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lianyungang Runzhong Pharmaceutical Co., Ltd. | 421265144 | api manufacture(82243-1001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nelson Laboratories LLC | 151663234 | analysis(82243-1001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Microbac Laboratories Inc. | 178463126 | analysis(82243-1001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmaceutics International Inc. | 878265586 | analysis(82243-1001) | |




