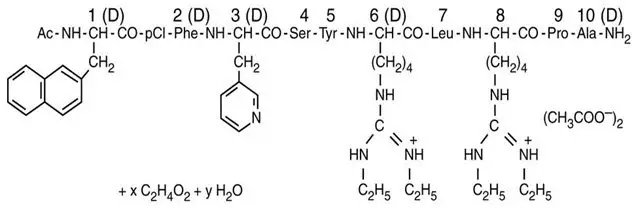
Drug Detail:Ganirelix (Ganirelix [ ga-ni-rel-ix ])
Drug Class: Gonadotropin-releasing hormone antagonists
Fyremadel - Clinical Pharmacology
The pulsatile release of GnRH stimulates the synthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The frequency of LH pulses in the mid and late follicular phase is approximately 1 pulse per hour. These pulses can be detected as transient rises in serum LH. At midcycle, a large increase in GnRH release results in an LH surge. The midcycle LH surge initiates several physiologic actions including: ovulation, resumption of meiosis in the oocyte, and luteinization. Luteinization results in a rise in serum progesterone with an accompanying decrease in estradiol levels.
Ganirelix acetate acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and subsequent transduction pathway. It induces a rapid, reversible suppression of gonadotropin secretion. The suppression of pituitary LH secretion by ganirelix acetate is more pronounced than that of FSH. An initial release of endogenous gonadotropins has not been detected with ganirelix acetate, which is consistent with an antagonist effect. Upon discontinuation of ganirelix acetate, pituitary LH and FSH levels are fully recovered within 48 hours.
Pharmacokinetics
The pharmacokinetic parameters of single and multiple injections of ganirelix acetate injection in healthy adult females are summarized in Table I. Steady-state serum concentrations are reached after 3 days of treatment. The pharmacokinetics of ganirelix acetate are dose-proportional in the dose range of 125 mcg to 500 mcg.
| tmax
h | t1/2
h | Cmax
ng/mL | AUC ng∙h/mL | CL/F L/h | Vd/F L |
|
|---|---|---|---|---|---|---|
| tmax Time to maximum concentration t1/2 Elimination half-life Cmax Maximum serum concentration AUC Area under the curve; Single dose: AUC0–∞; multiple dose: AUC0–24 Vd Volume of distribution CL Clearance = Dose/AUC0–∞ F Absolute bioavailability |
||||||
|
||||||
| Ganirelix Acetate single dose | 1.1 (0.3) | 12.8 (4.3) | 14.8 (3.2) | 96 (12) | 2.4 (0.2)* | 43.7 (11.4)* |
| Ganirelix Acetate multiple dose | 1.1 (0.2) | 16.2 (1.6) | 11.2 (2.4) | 77.1 (9.8) | 3.3 (0.4) | 76.5 (10.3) |
Clinical Studies
The efficacy of ganirelix acetate injection was established in two adequate and well-controlled clinical studies which included women with normal endocrine and pelvic ultrasound parameters. The studies intended to exclude subjects with polycystic ovary syndrome (PCOS) and subjects with low or no ovarian reserve. One cycle of study medication was administered to each randomized subject. For both studies, the administration of exogenous recombinant FSH [Follistim®1 (follitropin beta for injection)] 150 IU daily was initiated on the morning of Day 2 or 3 of a natural menstrual cycle. Ganirelix acetate injection was administered on the morning of Day 7 or 8 (Day 6 of recombinant FSH administration). The dose of recombinant FSH administered was adjusted according to individual responses starting on the day of initiation of ganirelix acetate. Both recombinant FSH and ganirelix acetate were continued daily until at least three follicles were 17 mm or greater in diameter at which time hCG [Pregnyl®1 (chorionic gonadotropin for injection, USP)] was administered. Following hCG administration, ganirelix acetate and recombinant FSH administration were discontinued. Oocyte retrieval, followed by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), was subsequently performed.
In a multicenter, double-blind, randomized, dose-finding study, the safety and efficacy of ganirelix acetate injection were evaluated for the prevention of LH surges in women undergoing COH with recombinant FSH. Ganirelix acetate injection doses ranging from 62.5 mcg to 2,000 mcg and recombinant FSH were administered to 332 patients undergoing COH for IVF (see TABLE II). Median serum LH on the day of hCG administration decreased with increasing doses of ganirelix acetate. Median serum E2 (17β-estradiol) on the day of hCG administration was 1,475; 1,110; and 1,160 pg/mL for the 62.5 mcg, 125 mcg, and 250 mcg doses, respectively. Lower peak serum E2 levels of 823, 703, and 441 pg/mL were seen at higher doses of ganirelix acetate 500 mcg, 1,000 mcg, and 2,000 mcg, respectively. The highest pregnancy and implantation rates were achieved with the 250 mcg dose of ganirelix acetate injection as summarized in Table II.
| Daily Dose (mcg) of Ganirelix Acetate Injection | ||||||
|---|---|---|---|---|---|---|
| 62.5 mcg | 125 mcg | 250 mcg | 500 mcg | 1,000 mcg | 2,000 mcg | |
| (Protocol 38602) | ||||||
|
||||||
| No. subjects receiving ganirelix acetate | 31 | 66 | 70 | 69 | 66 | 30 |
| No. subjects with ET* | 27 | 61 | 62 | 54 | 61 | 27 |
| No. of subjects with LH rise ≥ 10 mIU/mL† | 4 | 6 | 1 | 0 | 0 | 0 |
| Serum LH (mIU/mL) on day of hCG‡ 5th to 95th percentiles | 3.6 0.6 to 19.9 | 2.5 0.6 to 11.4 | 1.7 < 0.25 to 6.4 | 1 0.4 to 4.7 | 0.6 < 0.25 to 2.2 | 0.3 < 0.25 to 0.8 |
| Serum E2 (pg/mL) on day of hCG‡ 5th to 95th percentiles | 1,475 645 to 3,720 | 1,110 424 to 3,780 | 1,160 384 to 3,910 | 823 279 to 2,720 | 703 284 to 2,360 | 441 166 to 1,940 |
| Vital pregnancy rate§ | ||||||
| per attempt, n (%) | 7 (22.6) | 17 (25.8) | 25 (35.7) | 8 (11.6) | 9 (13.6) | 2 (6.7) |
| per transfer, n (%) | 7 (25.9) | 17 (27.9) | 25 (40.3) | 8 (14.8) | 9 (14.8) | 2 (7.4) |
| Implantation rate (%)¶ | 14.2 (26.8) | 16.3 (30.5) | 21.9 (30.6) | 9 (23.7) | 8.5 (21.7) | 4.9 (20.1) |
Transient LH rises alone were not deleterious to achieving pregnancy with ganirelix acetate at doses of 125 mcg (3/6 subjects) and 250 mcg (1/1 subjects). In addition, none of the subjects with LH rises ≥ 10 mIU/mL had premature luteinization indicated by a serum progesterone above 2 ng/mL.
A multicenter, open-label, randomized study was conducted to assess the efficacy and safety of ganirelix acetate injection in women undergoing COH. Follicular phase treatment with ganirelix acetate 250 mcg was studied using a luteal phase GnRH agonist as a reference treatment. A total of 463 subjects were treated with ganirelix acetate by subcutaneous injection once daily starting on Day 6 of recombinant FSH treatment. Recombinant FSH was maintained at 150 IU for the first 5 days of ovarian stimulation and was then adjusted by the investigator on the sixth day of gonadotropin use according to individual responses. The results for the ganirelix acetate arm are summarized in Table III.
| Ganirelix Acetate 250 mcg | |
|---|---|
| (Protocol 38607) | |
|
|
| No. subjects treated | 463 |
| Duration of GnRH analog (days)*† | 5.4 (2) |
| Duration of recombinant FSH (days)*† | 9.6 (2) |
| Serum E2 (pg/mL) on day of hCG‡ 5th to 95th percentiles | 1,190 373 to 3,105 |
| Serum LH (mIU/mL) on day of hCG‡ 5th to 95th percentiles | 1.6 0.6 to 6.9 |
| No. of subjects with LH rise ≥ 10 mIU/mL§ | 13 |
| No. of follicles > 11 mm*† | 10.7 (5.3) |
| No. of subjects with oocyte retrieval | 440 |
| No. of oocytes† | 8.7 (5.6) |
| Fertilization rate | 62.1% |
| No. subjects with ET¶ | 399 |
| No. of embryos transferred† | 2.2 (0.6) |
| No. of embryos† | 6 (4.5) |
| Ongoing pregnancy rate#* | |
| per attempt, n (%)Þ | 94 (20.3) |
| per transfer, n (%) | 93 (23.3) |
| Implantation rate (%)† | 15.7 (29) |
Some centers were limited to the transfer of ≤ 2 embryos based on local practice standards The mean number of days of ganirelix acetate treatment was 5.4 (2 to 14).
- 1
- All trademark names are the property of their respective owners.
Contraindications
Ganirelix acetate injection is contraindicated under the following conditions:
- Known hypersensitivity to ganirelix acetate or to any of its components.
- Known hypersensitivity to GnRH or any other GnRH analog.
- Known or suspected pregnancy (see PRECAUTIONS).
Precautions
General
Special care should be taken in women with signs and symptoms of active allergic conditions.
Cases of hypersensitivity reactions, including anaphylactoid reactions, have been reported, as early as with the first dose, during postmarketing surveillance (see ADVERSE REACTIONS). In the absence of clinical experience, ganirelix acetate treatment is not advised in women with severe allergic conditions.
The packaging of this product contains natural rubber latex which may cause allergic reactions (see HOW SUPPLIED).
Adverse Reactions/Side Effects
The safety of ganirelix acetate injection was evaluated in two randomized, parallel-group, multicenter controlled clinical studies. Treatment duration for ganirelix acetate ranged from 1 to 14 days. Table IV represents adverse events (AEs) from first day of ganirelix acetate administration until confirmation of pregnancy by ultrasound at an incidence of ≥ 1% in ganirelix acetate-treated subjects without regard to causality.
| Adverse Events Occurring in ≥ 1% | Ganirelix Acetate N=794 % (n) |
|---|---|
| Abdominal Pain (gynecological) | 4.8 (38) |
| Death Fetal | 3.7 (29) |
| Headache | 3 (24) |
| Ovarian Hyperstimulation Syndrome | 2.4 (19) |
| Vaginal Bleeding | 1.8 (14) |
| Injection Site Reaction | 1.1 (9) |
| Nausea | 1.1 (9) |
| Abdominal Pain (gastrointestinal) | 1 (8) |
During postmarketing surveillance, rare cases of hypersensitivity reactions, including anaphylactoid reactions, have been reported, as early as with the first dose (see PRECAUTIONS).
How is Fyremadel supplied
FYREMADEL (ganirelix acetate) injection is supplied in:
Disposable, ready for use, single-dose, sterile, prefilled 1 mL glass syringes containing 250 mcg/0.5 mL aqueous solution of ganirelix acetate closed with a rubber piston that does not contain latex. Each ganirelix acetate sterile, prefilled syringe is affixed with a 27 gauge × ½- inch needle closed by a needle shield of natural rubber latex. (See PRECAUTIONS, General.)
| Single syringe | NDC 55566-1010-1 |
| FYREMADEL
ganirelix acetate injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Ferring Pharmaceuticals Inc. (103722955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sun Pharmaceuticals Industries Limited | 725959238 | MANUFACTURE(55566-1010) , ANALYSIS(55566-1010) | |