Drug Detail:Gammagard s/d (igiv) (Immune globulin (igiv) (intravenous) [ im-myoon-glob-yoo-lin ])
Drug Class: Immune globulins
Highlights of Prescribing Information
GAMMAGARD S/D, Immune Globulin Intravenous (Human)
IgA less than 1 microgram per mL in a 5% Solution
Initial U.S. Approval: 1994
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE
See full prescribing information for complete boxed warning.
- Thrombosis may occur with immune globulin products, including GAMMAGARD S/D. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors. (5.3)
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients with immune globulin intravenous (IGIV) products, including GAMMAGARD S/D. Renal dysfunction and acute failure occur more commonly with IGIV products containing sucrose. GAMMAGARD S/D does not contain sucrose. (5.2)
- For patients at risk of thrombosis, administer GAMMAGARD S/D at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity. (5.3)
Indications and Usage for Gammagard S/D
GAMMAGARD S/D is an Immune Globulin Intravenous (Human) indicated for:
- Treatment of primary immunodeficiency (PI) in adults and pediatric patients two years of age or older. (1)
- Prevention of bacterial infections in hypogammaglobulinemia and/or recurrent bacterial infections associated with B-cell chronic lymphocytic leukemia (CLL). (1)
- Prevention and/or control of bleeding in adult chronic idiopathic thrombocytopenic purpura (ITP) patients. (1)
- Prevention of coronary artery aneurysms associated with Kawasaki syndrome in pediatric patients. (1)
Gammagard S/D Dosage and Administration
Intravenous Use Only
| Indication | Recommended Dosage | Duration |
|---|---|---|
|
||
| PI (2.2) | 300-600 mg/kg | Every 3 to 4 weeks |
| CLL (2.2) | 400 mg/kg | Every 3 to 4 weeks |
| ITP (2.2) | 1g/kg | Maximal 3 doses on alternate days |
| Kawasaki Syndrome (2.2) | Single 1g/kg or 400 mg/kg for 4 consecutive days | Begin within 7 days of onset of fever* |
Dosage Forms and Strengths
Freeze-dried preparation containing 5 g or 10 g IgG. (3)
Contraindications
- History of anaphylactic or severe systemic hypersensitivity reactions to the administration of GAMMAGARD S/D <1µg/mL IgA in a 5 % solution. (4)
Warnings and Precautions
- IgA-deficient patients with antibodies to IgA are at greater risk of severe hypersensitivity reactions and anaphylactic reactions. (5.1)
- Monitor renal function, including blood urea nitrogen, serum creatinine, and urine output in patients at risk of acute renal failure. (5.2)
- Thrombosis may occur. Monitor for signs and symptorms of thrombosis and assess blood viscosity for those at risk for hyperviscosity. (5.3)
- Aseptic Meningitis Syndrome (AMS) has been reported (5.4)
- Hemolytic anemia can develop. Monitor patients for clinical signs and symptoms of hemolysis and hemolytic anemia. (5.5)
- Monitor patients for pulmonary adverse reactions (transfusion-related acute lung injury, TRALI). (5.6)
- GAMMAGARD S/D is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.7)
- Hyperproteinemia, increased serum viscosity, and alterations in serum sodium levels may occur. (5.8)
Adverse Reactions/Side Effects
- The most common adverse reactions observed in ≥ 5% of patients during the clinical trials were headache, nausea, chills, fatigue, pyrexia, upper abdominal pain, diarrhea, back pain, infusion site pain, hyperhidrosis and flushing. (6.1)
- The most serious adverse reactions reported postmarketing include renal failure, thrombotic events (myocardial infarction, cerebrovascular accidents, and pulmonary embolism), anaphylactic shock, aseptic meningitis and hemolysis. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
Passive transfer of antibodies may interfere with the immune response to live vaccines, such as measles, mumps, and rubella. (7)
Use In Specific Populations
- Geriatric: Do not exceed the recommended dose. Infuse GAMMAGARD S/D at the minimum infusion rate practicable. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
Full Prescribing Information
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE
- ■
- Thrombosis may occur with immune globulin products, including GAMMAGARD S/D. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. [See Warnings and Precautions (5.3), Patient Counseling Information (17)]
- ●
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients who receive immune globulin intravenous (IGIV) products, including GAMMAGARD S/D. Patients predisposed to renal dysfunction include those with any degree of pre-existing renal insufficiency, diabetes mellitus, age above 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV product containing sucrose. GAMMAGARD S/D does not contain sucrose. (5.2)
- ●
- For patients at risk of thrombosis, administer GAMMAGARD S/D at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity. [See Dosage and Administration (2.3) and Warnings and Precautions (5.3)]
2. Gammagard S/D Dosage and Administration
For Intravenous Use Only
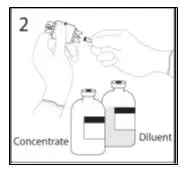
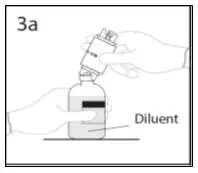
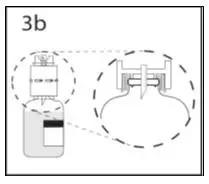
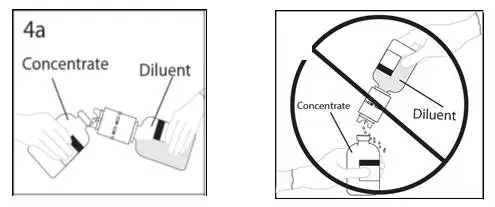
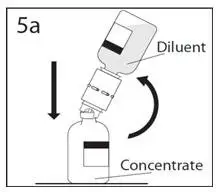
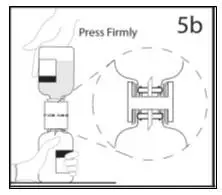
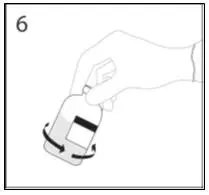
2.1 Preparation and Handling
2.3 Administration
Administer GAMMAGARD S/D as soon after reconstitution as possible and administer the reconstituted material at room temperature.
The recommended initial 5% solution infusion rate is 0.5 mL/kg/hour. The infusion rate may be gradually increased to a maximum rate of 4 mL/kg/hour as tolerated for patients with no history of adverse reactions to IGIV and no significant risk factors for renal dysfunction or thrombotic complications. Patients who tolerate the 5% concentration at 4 mL/kg/hour can be infused with the 10% concentration starting at 0.5 mL/kg/hour. The rate can be increased gradually up to a maximum of 8 mL/kg/hour if no adverse effects occur.9
Monitor patient vital signs throughout the infusion. Certain adverse reactions such as headaches, flushing and changes in pulse rate and blood pressure may be related to the rate of infusion. Slow or stop the infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a lower rate that does not result in reoccurrence of the symptoms.
It is recommended that, if possible, the antecubital veins are used, especially for 10% solutions, to reduce the likelihood of discomfort at the infusion site. [See Adverse Reactions (6)]
Adverse reactions may occur more frequently in patients who receive human IGIV for the first time, upon switching brands, or if there has been a long hiatus since the previous infusion. In such cases, start at a lower rate and gradually increase as tolerated.
There are no prospective studies demonstrating that any concentration or rate of infusion is completely safe. However, the risk may be decreased at lower rates of infusion.10 For patients who are judged to be at risk of renal dysfunction or thrombotic complications, administer GAMMAGARD S/D at the minimum practicable rate of infusion and gradually titrate up to a more conservative maximal rate of less than 3.3 mg/kg/min (< 2mL/kg/hour of a 10% or < 4mL/kg/hour of a 5% solution). [See Warnings and Precautions (5.3)]
3. Dosage Forms and Strengths
GAMMAGARD S/D with an IgA concentration of less than 1 µg/mL is a freeze-dried preparation containing 5 g or 10 g IgG in a single-use vial.
4. Contraindications
GAMMAGARD S/D is contraindicated in patients who have a history of anaphylactic or severe systemic hypersensitivity reactions to the administration of GAMMAGARD S/D with <1µg/mL IgA in a 5 % solution.
5. Warnings and Precautions
5.1 Hypersensitivity
Severe hypersensitivity reactions and anaphylactic reactions with a fall in blood pressure have occurred in patients receiving GAMMAGARD S/D, including patients who tolerated previous treatments with GAMMAGARD S/D, even though it contains low levels of IgA. If hypersensitivity reaction develops, discontinue GAMMAGARD S/D infusion immediately and institute appropriate treatment.
This product has an IgA concentration less than 1 µg/mL. Preparations depleted of IgA (0.4 to 2.9 µg/mL) were shown to be better tolerated by a limited number of patients who reacted to IGIV preparations with higher IgA concentrations. However, the concentration of IgA that will not provoke a reaction is not known, and therefore all IGIV preparations carry the risk of inducing an anaphylactic reaction to IgA. In such instances, a risk of anaphylaxis may exist despite the fact that GAMMAGARD S/D, IgA < 1 µg/mL, contains < 1 µg/mL IgA.
5.2 Renal Dysfunction/Failure
Acute renal failure has been reported in association with GAMMAGARD S/D. Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis and death have been reported in patients receiving IGIV, particularly those products containing sucrose.10 GAMMAGARD S/D does not contain sucrose.
Ensure that patients are not volume depleted prior to the initiation of the infusion of GAMMAGARD S/D. In patients who are at risk of developing renal dysfunction, because of pre-existing renal insufficiency or predisposition to acute renal failure (such as diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs, etc.), administer GAMMAGARD S/D at an infusion rate less than 4 mL/kg/hour (< 3.3 mg IG/kg/min) for a 5% solution or at a rate less than 2 mL/kg/hour (< 3.3 mg IG/kg/min) for a 10 % solution. [See Dosage and Administration (2)]
Monitor renal function and urine output in patients judged to be at increased risk for developing acute renal failure. Assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of GAMMAGARD S/D and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuation of GAMMAGARD S/D.
5.3 Thrombosis
Thrombosis may occur following treatment with immune globulin products, including GAMMAGARD S/D. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens,indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients at risk of thrombosis, administer GAMMAGARD S/D at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [See Boxed Warning, Dosage and Administration (2.3), Patient Counseling Information (17)]
5.4 Aseptic Meningitis Syndrome (AMS)
AMS has been reported to occur in association with immune globulin therapy, including GAMMAGARD S/D. AMS may occur more frequently in female patients. Discontinuation of immune globulin treatment has resulted in remission of AMS within several days without sequelae. The syndrome of AMS usually begins within several hours to two days following immune globulin treatment.
AMS is characterized by the following symptoms and signs: severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea, and vomiting. Cerebrospinal fluid (CSF) studies are frequently positive with pleocytosis up to several thousand cells per cubic mm, predominantly from the granulocytic series, and with elevated protein levels up to several hundred mg/dL, but negative culture results. Conduct a thorough neurological examination on patients exhibiting such symptoms and signs, including CSF studies, to rule out other causes of meningitis.
5.5 Hemolysis
Hemolytic anemia can develop subsequent to IGIV therapy, including GAMMAGARD S/D11. [See Adverse Reactions (6.2)] GAMMAGARD S/D contains blood group antibodies which may act as hemolysins and induce in vivo coating of red blood cells (RBC) with immunoglobulin, causing a positive direct antiglobulin reaction and, rarely, hemolysis. Acute intravascular hemolysis has been reported, and delayed hemolytic anemia can develop subsequent to IGIV therapy due to enhanced RBC sequestration. [See Adverse Reactions (6.2)]
Monitor patients for clinical signs and symptoms of hemolysis. If signs or symptoms of hemolysis are present after GAMMAGARD S/D infusion, perform appropriate confirmatory laboratory testing.
5.6 Transfusion-Related Acute Lung Injury (TRALI)
Non-cardiogenic pulmonary edema (TRALI) has been reported in patients following the administration of immunoglobulin products, including GAMMAGARD S/D therapy. [See Adverse Reactions (6.2)] TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically occur within 1 to 6 hours after treatment.
Monitor patients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil and anti-HLA antibodies in both the product and patient serum. TRALI may be managed using oxygen therapy with adequate ventilatory support.
5.7 Transmissible Infectious Agents
Because GAMMAGARD S/D is made from human blood and may carry a risk of transmitting infectious agents, e.g., viruses the variant Creutzfeldt-Jakob disease (vCJD) agent and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens.
All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327)(in the U.S.) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. The physician should discuss the risks and benefits of this product with the patient.
5.8 Hyperproteinemia, Increased Serum Viscosity, and Alterations in Serum Sodium Levels
Hyperproteinemia and increased serum viscosity may occur in patients receiving GAMMAGARD S/D. Alterations in serum sodium levels, such as hypernatremia acutely, or pseudohyponatemia after equilibration of the sodium, may occur with the administration of GAMMAGARD S/D.
The amount of sodium in the product may add materially to the recommended daily allowance of dietary sodium for patients on a low sodium diet. In these patients, calculate the amount of sodium from the product and use it when determining dietary sodium intake. GAMMAGARD S/D contains approximately 0.85% NaCl or approximately 3340 mg sodium/liter at a 5% concentration. A 70 kg patient receiving 1g/kg (1.4 L) of the product would receive 4676 mg of sodium.
5.9 Monitoring: Laboratory Tests
- Monitor renal function and urine output in patients at increased risk of developing acute renal failure. Assess renal function, including measurement of BUN and serum creatinine, before the initial infusion of GAMMAGARD S/D and at appropriate intervals thereafter.10
- Assess baseline blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies because of the potentially increased risk of thrombosis.
- If signs or symptoms of hemolysis are present after an infusion of GAMMAGARD S/D, perform appropriate laboratory testing for confirmation.
- If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies and anti-HLA antibodies in both the product and patient's serum.
5.10 Interference with Laboratory Tests
- After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient's blood may yield false positive serological testing results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs') test.
- Administration of GAMMAGARD S/D can lead to false positive readings in assays that depend on detection of beta-D-glucans for diagnosis of fungal infections; this may persist during the weeks following infusion of the product.
6. Adverse Reactions/Side Effects
The most common adverse reactions reported in ≥ 5% of clinical trial subjects occurring during or within 48 hours of an infusion were headache, nausea, chills, asthenia (fatigue), pyrexia, upper abdominal pain, diarrhea, back pain, hyperhidrosis, and flushing.
There were no serious adverse events that were attributed to GAMMAGARD S/D in the clinical trials.
In postmarketing surveillance, serious adverse reactions reported with GAMMAGARD S/D were anaphylaxis, acute renal failure, myocardial infarction, cerebral vascular accident, transient ischemic attack, deep vein thrombosis, pulmonary embolism, aseptic meningitis, acute hemolysis, and TRALI.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily post-approval from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
The following adverse reactions have been reported during postmarketing use of GAMMAGARD S/D (Table 5).
| Blood and Lymphatic System Disorders | Anemia, hemolysis, lymphadenopathy, thrombocytopenia |
| Immune System Disorders | Anaphylactic shock, anaphylactic/anaphylactoid reaction, hypersensitivity |
| Psychiatric Disorders | Restlessness |
| Nervous System Disorders | Aseptic meningitis, cerebrovascular accident, transient ischemic attack, seizures, dizziness, migraine, paresthesia, syncope, tremor |
| Eye Disorders | Retinal vein thrombosis, eye pain, photophobia, visual disturbance |
| Cardiac Disorders | Myocardial infarction, cyanosis, tachycardia, bradycardia |
| Vascular Disorders | Vena cava thrombosis, arterial thrombosis, deep vein thrombosis, hypotension, hypertension, pallor, thrombophlebitis |
| Respiratory, Thoracic And Mediastinal Disorders | Pulmonary embolism, pulmonary edema, bronchospasm, wheezing, cough, hyperventilation, hypoxia, throat tightness |
| Gastrointestinal Disorders | Abdominal pain, dyspepsia |
| Hepatobiliary Disorders | Non-infectious hepatitis |
| Skin and Subcutaneous Tissue Disorders | Angioedema, dermatitis, erythema, rash |
| Musculoskeletal And Connective Tissue Disorders | Arthralgia, myalgia |
| Renal and Urinary Disorders | Renal failure |
| General Disorders And Administration-Site Conditions | Infusion site reaction, asthenia, edema, rigors |
| Investigations | Positive direct Coombs test |
In addition to the events listed above, the following events have been identified for IGIV products in general:
| Renal | Osmotic nephrosis |
| Respiratory | Cyanosis, apnea, acute respiratory distress syndrome (ARDS) |
| Integumentary | Bullous dermatitis, epidermolysis, erythema multiforme, Stevens-Johnson syndrome |
| Cardiovascular | Cardiac arrest, vascular collapse |
| Neurological | Coma, loss of consciousness |
| Hematologic | Pancytopenia |
| Gastrointestinal | Hepatic dysfunction |
7. Drug Interactions
Admixtures of GAMMAGARD S/D with other drugs and intravenous solutions have not been evaluated. It is recommended that GAMMAGARD S/D be administered separately from other drugs or medications which the patient may be receiving. Do not mix the product with human IGIV products from other manufacturers.
Passive transfer of antibodies may transiently impair the immune responses to live attenuated vaccines, such as measles, mumps, rubella, and varicella. Inform the immunizing physician of recent therapy with GAMMAGARD S/D so that appropriate precautions can be taken.
8. Use In Specific Populations
8.2 Lactation
Risk Summary
There is no information regarding the presence of GAMMAGARD S/D in human milk, its effects on the breastfed infant or its effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for GAMMAGARD S/D and any potential adverse effects on the breastfed infant from GAMMAGARD S/D or from the underlying maternal condition.
8.4 Pediatric Use
Clinical studies for the treatment of PI did not include sufficient numbers of subjects aged 16 and younger to determine whether they respond differently from adults. Five children under the age of 16 were treated in the initial trial of GAMMAGARD. The mean age of subjects in the phase 4 study was 17.8 years (range 1.7 to 55.3).
Efficacy and safety of GAMMAGARD S/D in pediatric patients with chronic ITP has not been established.
Efficacy and safety of GAMMAGARD S/D in pediatric patients with Kawasaki disease has been established. Virtually all patients treated for Kawasaki disease were less than 5 years of age, with approximately 20% under the age of 1 year.
8.5 Geriatric Use
Limited information is available for the geriatric use. Clinical studies of GAMMAGARD S/D for the treatment of PI did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. When administering GAMMAGARD S/D to patients age 65 and over who are at increased risk for developing thromboembolic events or renal insufficiency, do not exceed the recommended dose and administer GAMMAGARD S/D at the minimum infusion rate practicable. [See Boxed Warning, Warnings and Precautions(5.2, 5.4) and Dosage and Administation (2.2)]
10. Overdosage
Overdose may lead to fluid overload or hyperviscosity. Patients at particular risk for complications of fluid overload or hyperviscosity include elderly patients and patients with cardiac or renal impairment.
11. Gammagard S/D Description
GAMMAGARD S/D, Immune Globulin Intravenous (Human), IgA less than 1 µg/mL in a 5% Solution (IgA < 1 µg/mL), is GAMMAGARD S/D selected to have an IgA concentration of less than 1 µg/mL of IgA in a 5% solution. GAMMAGARD S/D is a solvent/detergent treated, sterile, freeze-dried preparation of purified immunoglobulin G (IgG) derived from large pools of human plasma. IgG preparations are purified from plasma pools using a modified Cohn-Oncley cold ethanol fractionation process, as well as cation and anion exchange chromatography steps. The distribution of IgG subclasses present in this product is similar to that in normal plasma. The Fc portion is maintained intact. When reconstituted with the total volume of diluent (Sterile Water for Injection, USP) supplied and reconstituted to 5% solution, the product contains approximately 50 mg/mL of protein, of which at least 90% is gamma globulin. GAMMAGARD S/D contains < 1 µg/mL of IgA in a 5% solution and IgM is present in trace amounts. GAMMAGARD S/D contains all of the IgG antibody activities which are present in the donor population.
The product, after reconstituted to 5% solution, contains a physiological concentration of sodium chloride (approximately 8.5 mg/mL) and has a pH of 6.8 ± 0.4. Stabilizing agents and additional components are present in the following maximum amounts for a 5% solution: 3 mg/mL Albumin (Human), 22.5 mg/mL glycine, 20 mg/mL glucose, 2 mg/mL polyethylene glycol (PEG), 1 µg/mL tri-n-butyl phosphate, 1 µg/mL octoxynol 9, and 100 µg/mL polysorbate 80. GAMMAGARD S/D contains no preservative.
To prepare a 10% (100 mg/mL) solution for infusion, add half the volume of diluent, as described in Dosage and Administration (2.1). The content of the stabilizing agents and other components, including IgA, for the 10% solution will be doubled compared to the 5% solution.
Screening against potentially infectious agents in the product begins with the donor selection process and continues throughout plasma collection and plasma preparation. Each individual plasma donation used in the manufacture of GAMMAGARD S/D is collected only at FDA approved blood establishments and is tested by FDA licensed serological tests for hepatitis B surface antigen (HBsAg), and for antibodies to human immunodeficiency virus (HIV-1/HIV-2) and hepatitis C virus (HCV) in accordance with U.S. regulatory requirements. As an additional safety measure, mini-pools of the plasma are tested for the presence of HIV-1 and HCV by FDA licensed nucleic acid testing (NAT) and found negative.
The manufacturing process includes treatment with an organic solvent/detergent mixture12,13, composed of tri-n-butyl phosphate, octoxynol 9 and polysorbate 80. The GAMMAGARD S/D manufacturing process provides for viral reduction in in vitro studies. These studies, summarized in Table 6 demonstrate virus clearance during GAMMAGARD S/D manufacturing using human immunodeficiency virus, Type 1 (HIV-1), as the relevant virus and model for HIV-2; bovine viral diarrhea virus (BVDV), a model virus for enveloped RNA viruses such as hepatitis C virus (HCV); pseudorabies virus (PRV), a generic model virus for enveloped DNA viruses such as hepatitis B virus (HBV); encephalomyocarditis virus (EMCV), a model for non-enveloped RNA viruses such as hepatitis A virus (HAV); HAV; and mice minute virus (MMV), a model for small non-enveloped DNA viruses such as human parvovirus B19 (B19V). These reductions are achieved through partitioning and inactivation during cold ethanol fractionation, and the solvent/detergent treatment.
| Process Step Evaluated | Virus Clearance (log10) | ||||||
|---|---|---|---|---|---|---|---|
| Enveloped Viruses | Non- Enveloped Viruses | ||||||
| HIV-1 | BVDV | PRV | EMCV | HAV | MMV | ||
| NA Not Applicable. Solvent/detergent treatment does not affect non- enveloped viruses. NT Not Tested. |
|||||||
|
|||||||
| Step 1: | Processing of Cryo-Poor Plasma to Fraction I+II+III Precipitate | 5.6 | 0.6* | 1.0* | NT | 0.5* | NT |
| Step 2-3: | Processing of Resuspended Suspension A Precipitate to Suspension B Cuno 70 Filtrate | > 5.7 | 2.6 | > 5.2 | 5.0 | > 5.2 | > 5.3 |
| Step 4: | Solvent/Detergent Treatment | >5.5 | >6.2 | >4.9 | NA | NA | NA |
| Cumulative Reduction of Virus (log10) | >16.8 | >8.8 | >10.1 | 5.0 | > 5.2 | > 5.3 | |
12. Gammagard S/D - Clinical Pharmacology
12.1 Mechanism of Action
GAMMAGARD S/D, Immune Globulin Intravenous (Human), supplies a broad spectrum of opsonizing and neutralizing IgG antibodies against a wide variety of bacterial and viral agents. GAMMAGARD S/D also contains a spectrum of antibodies capable of reacting with cells such as erythrocytes. The role of these antibodies and the mechanisms of action of IgG in GAMMAGARD S/D have not been fully elucidated.
12.3 Pharmacokinetics
Following infusion, IGIV products show a biphasic decay curve. The initial (α) phase is characterized by an immediate post-infusion peak in serum IgG and is followed by rapid decay due to equilibration between the plasma and extravascular fluid compartments. The second (β) phase is characterized by a slower and constant rate of decay. As a class, IgG survives longer in vivo than other serum proteins. Peak levels of IgG are reached within 30 minutes after an intravenous infusion of GAMMAGARD S/D. In previous studies, where radio-labeled IgG was injected to subjects, the IgG half-life was 21 to 25 days in healthy individuals or 17.7 to 37.6 days in immunodeficient patients. The half-life of IgG can vary considerably from person to person, however. In particular, high serum concentrations of IgG and hypermetabolism associated with fever and infection have been seen to coincide with a shortened half-life of IgG.
The pharmacokinetics of GAMMAGARD S/D were evaluated in 15 subjects with PI, 10 of whom were previously treated. In the previously treated subjects, the half-life of GAMMAGARD S/D was approximately 37.7 ± 15 days compared to 34.1 ± 15.7 days for GAMMAGARD. The half-lives of the IgG subclasses were similar, ranging from 28.1 ± 11.2 days for IgG4 to 42.3 ± 26.6 days for IgG1. The half-life of pneumococcal antibody in these subjects was 41.4 ± 28.5 days. Pharmacokinetics did not differ between the previously licensed IGIV and GAMMAGARD S/D formulations administered to the previously treated patients. The pharmacokinetics of the GAMMAGARD S/D formulation in previously untreated patients were not significantly different from the results obtained in previously treated patients. The mean trough IgG concentration in the previously untreated patients was 1186 ± 614 mg/dL and the peak post-infusion concentration was 1859 ± 872 mg/dL. The mean dose was 460 ± 194 mg/kg.
14. Clinical Studies
Clinical studies were conducted with lots of GAMMAGARD S/D containing IgA < 2.2 µg/mL. No clinical studies have been specifically conducted using only lots with IgA content of < 1 µg/mL.
15. References
- Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson Jr. RP, Patel DD, Secord E, Sorenson RU, Wasserman RL, Cunningham-Rundles C, Use of Intravenous Immunoglobulin in Human Disease: A Review of Evidence by Members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol 2006; 117:S525-53.
- Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005; 94(suppl 1):S1-63.
- Ochs HD, Lee ML, Fischer SH, et al. Efficacy of a New Intravenous Immunoglobulin Preparation in Primary Immunodeficient Patients. Clinical Therapeutics. 1987; 9:512-522.
- Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia: Intravenous immunoglobulin for the prevention of infection in Chronic Lymphocytic Leukemia: A randomized, controlled clinical trial. N Eng J Med. 1988; 319:902-907.
- Newburger J, Takahashi M, Burns JG, et al. The Treatment of Kawasaki Syndrome with Intravenous Gamma Globulin. New England Journal of Medicine. 1986; 315:341-347.
- Eijkhout HW, Der Meer JW, Kallenbert CG, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001; 135:165-174.
- Barron KS, Murphy DJ, Siverman ED, Ruttenberg HD, Wright GB, Franklin W, Goldberg SJ, Higashino SM, Cox DG, Lee M. Treatment of Kawasaki syndrome: a comparison of two dosage regimens of intravenously administered immune globulin. J Pediatr. 1990; 117:638-644.
- Engle MA, Fatica NS, Bussel JB, O'Laughlin JE, Snyder MS, Lesser ML. Clinical Trial of Single-Dose Intravenous Gammaglobulin in Acute Kawasaki Disease. AJDC. 1989; 143:1300-1304.
- Polmar SH, Smith TF, Pirofsky B, Cox DG, Rechtman D. Rapid infusion of intravenous immunoglobulin in patients with primary immunodeficiency disease. J Allergy Clin Immunol. 1992; 69:166.
- Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Transfusion Med Rev. 2003; 17:241-251.
- Daw Z, Padmore R, Neurath D, Cober N, Tokessy M, Desjardins D, et al. Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. Transfusion 2008; 48:1598-601.
- Horowitz B, Wiebe ME, Lippin A, et al. Inactivation of viruses in labile blood derivatives: I. Disruption of lipid enveloped viruses by tri-n-butyl phosphate detergent combinations. Transfusion. 1985;25:516-522.
- Prince AM, Horowitz B, Brotman B. Sterilisation of hepatitis and HTLV-III viruses by exposure to tri-n-butyl phosphate and sodium cholate. Lancet. 1986;1:706-710.
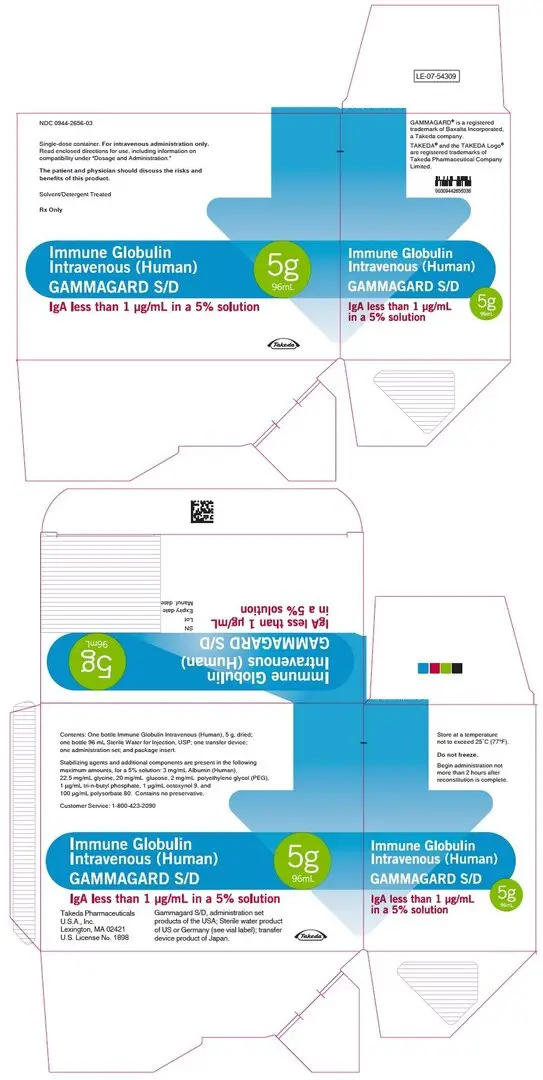
16. How is Gammagard S/D supplied
GAMMAGARD S/D is supplied in single use bottles containing the labeled amount of functionally active IgG. The following presentation of GAMMAGARD S/D is available:
| Grams Protein | Kit NDC |
|---|---|
| 5 g | 0944-2656-03 |
| 10 g | 0944-2658-04 |
Each bottle of GAMMAGARD S/D is furnished with a suitable volume of Sterile Water for Injection, USP, a transfer device and an administration set which contains an integral airway and a 15 micron filter.
17. Patient Counseling Information
Inform patients to immediately report the following signs and symptoms to their healthcare provider:
- Decreased urine output, sudden weight gain, fluid retention/edema, or shortness of breath. [See Warnings and Precautions (5.2)]
- Instruct patients to immediately report symptoms of thrombosis. These symptoms may include pain or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body. [See Warnings and Precautions (5.3)]
- Severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting. [See Warnings and Precautions (5.4)]
- Increased heart rate, fatigue, yellowing of the skin or eyes, and dark-colored urine. [See Warnings and Precautions (5.5)]
- Trouble breathing, chest pain, blue lips or extremities, fever. [See Warnings and Precautions (5.6)]
- Inform patients that GAMMAGARD S/D is made from human blood and may contain infectious agents that can cause disease agents e.g., viruses, the variant Creutzfelt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk of GAMMAGARD S/D transmitting an infectious agent has been reduced by screening plasma donors for prior exposure, testing donated plasma, and inactivating or removing certain viruses during manufacturing. Patients should report any symptoms that concern them or that might be caused by infections. [See Warnings and Precautions (5.7)]
- Inform patients that GAMMAGARD S/D can interfere with their immune response to live viral vaccines such as measles, mumps, and rubella. Inform patients to notify their healthcare professional of this potential interaction when they are receiving vaccinations. [See Drug Interactions (7)]
To enroll in the confidential, industry-wide Patient Notification System, call 1-888-873-2838.
| GAMMAGARD
S/D
immune globulin intravenous (human) kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GAMMAGARD
S/D
immune globulin intravenous (human) kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxalta Belgium Manufacturing SA | 370634700 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Corporation | 001728059 | MANUFACTURE | |