Drug Detail:Gastrocrom (Cromolyn sodium (oral) [ kro-moe-lin-soe-dee-um ])
Drug Class: Mast cell stabilizers
Gastrocrom Description
Each 5 mL ampule of GASTROCROM contains 100 mg cromolyn sodium, USP, in purified water. Cromolyn sodium is a hygroscopic, white powder having little odor. It may leave a slightly bitter aftertaste. GASTROCROM (cromolyn sodium, USP) Oral Concentrate is clear, colorless, and sterile. It is intended for oral use.
Chemically, cromolyn sodium is disodium 5,5’-[(2-hydroxytrimethylene)dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]. The empirical formula is C23H14Na2O11; the molecular weight is 512.34. Its chemical structure is:
Pharmacologic Category: Mast cell stabilizer
Therapeutic Category: Antiallergic
Gastrocrom - Clinical Pharmacology
In vitro and in vivo animal studies have shown that cromolyn sodium inhibits the release of mediators from sensitized mast cells. Cromolyn sodium acts by inhibiting the release of histamine and leukotrienes (SRS-A) from the mast cell.
Cromolyn sodium has no intrinsic vasoconstrictor, antihistamine, or glucocorticoid activity.
Cromolyn sodium is poorly absorbed from the gastrointestinal tract. No more than 1% of an administered dose is absorbed by humans after oral administration, the remainder being excreted in the feces. Very little absorption of cromolyn sodium was seen after oral administration of 500 mg by mouth to each of 12 volunteers. From 0.28 to 0.50% of the administered dose was recovered in the first 24 hours of urinary excretion in 3 subjects. The mean urinary excretion of an administered dose over 24 hours in the remaining 9 subjects was 0.45%.
Related/similar drugs
prednisone, omeprazole, dexamethasone, hydrocortisone, budesonide, Prilosec, lansoprazoleClinical Studies
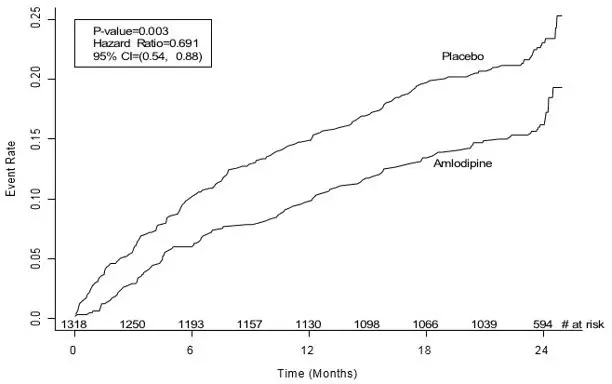
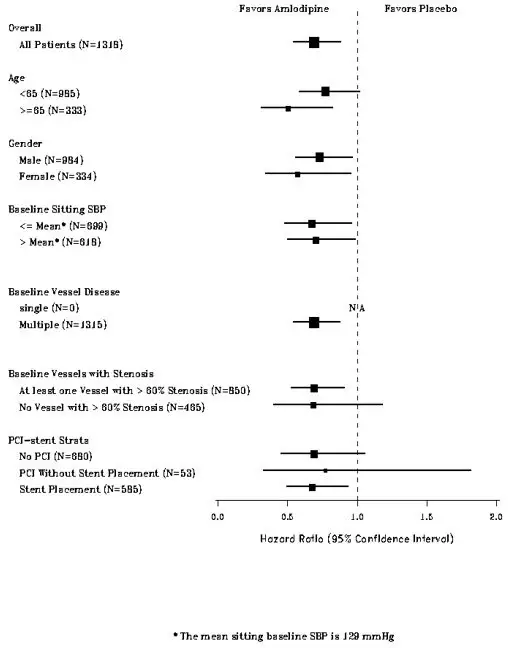
Four randomized, controlled clinical trials were conducted with GASTROCROM in patients with either cutaneous or systemic mastocytosis; two of which utilized a placebo-controlled crossover design, one utilized an active-controlled (chlorpheniramine plus cimetidine) crossover design, and one utilized a placebo-controlled parallel group design. Due to the rare nature of this disease, only 36 patients qualified for study entry, of whom 32 were considered evaluable. Consequently, formal statistical analyses were not performed. Clinically significant improvement in gastrointestinal symptoms (diarrhea, abdominal pain) were seen in the majority of patients with some improvement also seen for cutaneous manifestations (urticaria, pruritus, flushing) and cognitive function.
The benefit seen with GASTROCROM 200 mg QID was similar to chlorpheniramine (4 mg QID) plus cimetidine (300 mg QID) for both cutaneous and systemic symptoms of mastocytosis.
Clinical improvement occurred within 2-6 weeks of treatment initiation and persisted for 2-3 weeks after treatment withdrawal. GASTROCROM did not affect urinary histamine levels or peripheral eosinophilia, although neither of these variables appeared to correlate with disease severity. Positive clinical benefits were also reported for 37 of 51 patients who received GASTROCROM in United States and foreign humanitarian programs.
Indications and Usage for Gastrocrom
GASTROCROM is indicated in the management of patients with mastocytosis. Use of this product has been associated with improvement in diarrhea, flushing, headaches, vomiting, urticaria, abdominal pain, nausea, and itching in some patients.
Contraindications
GASTROCROM is contraindicated in those patients who have shown hypersensitivity to cromolyn sodium.
Warnings
The recommended dosage should be decreased in patients with decreased renal or hepatic function. Severe anaphylactic reactions may occur rarely in association with cromolyn sodium administration.
Precautions
In view of the biliary and renal routes of excretion of GASTROCROM, consideration should be given to decreasing the dosage of the drug in patients with impaired renal or hepatic function.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In carcinogenicity studies in mice, hamsters, and rats, cromolyn sodium had no neoplastic effects at intraperitoneal doses up to 150 mg/kg three days per week for 12 months in mice, at intraperitoneal doses up to 53 mg/kg three days per week for 15 weeks followed by 17.5 mg/kg three days per week for 37 weeks in hamsters, and at subcutaneous doses up to 75 mg/kg six days per week for 18 months in rats. These doses in mice, hamsters, and rats are less than the maximum recommended daily oral dose in adults and children on a mg/m2 basis.
Cromolyn sodium showed no mutagenic potential in Ames Salmonella/microsome plate assays, mitotic gene conversion in Saccharomyces cerevisiae and in an in vitro cytogenetic study in human peripheral lymphocytes.
In rats, cromolyn sodium showed no evidence of impaired fertility at subcutaneous doses up to 175 mg/kg in males (approximately equal to the maximum recommended daily oral dose in adults on a mg/m2 basis) and 100 mg/kg in females (less than the maximum recommended daily oral dose in adults on a mg/m2 basis).
Pregnancy
In reproductive studies in pregnant mice, rats, and rabbits, cromolyn sodium produced no evidence of fetal malformations at subcutaneous doses up to 540 mg/kg in mice (approximately equal to the maximum recommended daily oral dose in adults on a mg/m2 basis) and 164 mg/kg in rats (less than the maximum recommended daily oral dose in adults on a mg/m2 basis) or at intravenous doses up to 485 mg/kg in rabbits (approximately 4 times the maximum recommended daily oral dose in adults on a mg/m2 basis). There are, however, no adequate and well controlled studies in pregnant women.
Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Drug Interaction During Pregnancy
In pregnant mice, cromolyn sodium alone did not cause significant increases in resorptions or major malformations at subcutaneous doses up to 540 mg/kg (approximately equal to the maximum recommended daily oral dose in adults on a mg/m2 basis). Isoproterenol alone increased both resorptions and major malformations (primarily cleft palate) at a subcutaneous dose of 2.7 mg/kg (approximately 7 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). The incidence of major malformations increased further when cromolyn sodium at a subcutaneous dose of 540 mg/kg was added to isoproterenol at a subcutaneous dose of 2.7 mg/kg. No such interaction was observed in rats or rabbits.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when GASTROCROM is administered to a nursing woman.
Pediatric Use
In adult rats no adverse effects of cromolyn sodium were observed at oral doses up to 6144 mg/kg (approximately 25 times the maximum recommended daily oral dose in adults on a mg/m2 basis). In neonatal rats, cromolyn sodium increased mortality at oral doses of 1000 mg/kg or greater (approximately 9 times the maximum recommended daily oral dose in infants on a mg/m2 basis) but not at doses of 300 mg/kg or less (approximately 3 times the maximum recommended daily oral dose in infants on a mg/m2 basis). Plasma and kidney concentrations of cromolyn after oral administration to neonatal rats were up to 20 times greater than those in older rats. In term infants up to six months of age, available clinical data suggest that the dose should not exceed 20 mg/kg/day. The use of this product in pediatric patients less than two years of age should be reserved for patients with severe disease in which the potential benefits clearly outweigh the risks.
Geriatric Use
Clinical studies of GASTROCROM did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Adverse Reactions/Side Effects
Most of the adverse events reported in mastocytosis patients have been transient and could represent symptoms of the disease. The most frequently reported adverse events in mastocytosis patients who have received GASTROCROM during clinical studies were headache and diarrhea, each of which occurred in 4 of the 87 patients. Pruritus, nausea, and myalgia were each reported in 3 patients and abdominal pain, rash, and irritability in 2 patients each. One report of malaise was also recorded.
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-877-999-8406 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Other Adverse Events: Additional adverse events have been reported during studies in other clinical conditions and from worldwide postmarketing experience. In most cases the available information is incomplete and attribution to the drug cannot be determined. The majority of these reports involve the gastrointestinal system and include: diarrhea, nausea, abdominal pain, constipation, dyspepsia, flatulence, glossitis, stomatitis, vomiting, dysphagia, esophagospasm.
Other less commonly reported events (the majority representing only a single report) include the following:
Skin: pruritus, rash, urticaria/angioedema, erythema/ burning, photosensitivity
Musculoskeletal: arthralgia, myalgia, stiffness/weakness of legs
Neurologic: headache, dizziness, hypoesthesia, paresthesia, migraine, convulsions, flushing
Psychiatric: psychosis, anxiety, depression, hallucinations, behavior change, insomnia, nervousness
Heart Rate: tachycardia, premature ventricular contractions (PVCs), palpitations
Respiratory: pharyngitis, dyspnea
Miscellaneous: fatigue, edema, unpleasant taste, chest pain, postprandial lightheadedness and lethargy, dysuria, urinary frequency, purpura, hepatic function test abnormal, polycythemia, neutropenia, pancytopenia, tinnitus, lupus erythematosus (LE) syndrome
Gastrocrom Dosage and Administration
NOT FOR INHALATION OR INJECTION. SEE DIRECTIONS FOR USE.
The usual starting dose is as follows:
Adults and Adolescents (13 Years and Older)
Two ampules four times daily, taken one-half hour before meals and at bedtime.
Pediatric Patients Under 2 Years
Not recommended.
If satisfactory control of symptoms is not achieved within two to three weeks, the dosage may be increased but should not exceed 40 mg/kg/day.
Patients should be advised that the effect of GASTROCROM therapy is dependent upon its administration at regular intervals, as directed.
How is Gastrocrom supplied
GASTROCROM Oral Concentrate is an unpreserved, colorless solution supplied in a low density polyethylene plastic unit dose ampule with 8 ampules per foil pouch. Each 5 mL ampule contains 100 mg cromolyn sodium, USP, in purified water.
NDC 0037-0678-08 8 ampules x 5 mL
NDC 0037-0678-96 96 ampules x 5 mL
GASTROCROM Oral Concentrate should be stored between 15°-30°C (59°-86°F) and protected from light. Do not use if it contains a precipitate or becomes discolored. Keep out of the reach of children.
Store ampules in foil pouch until ready for use.
Distributed by:
Meda Pharmaceuticals®
Somerset, New Jersey 08873-4120
Gastrocrom is a registered trademark of Meda Pharma S.A.R.L., a Mylan Company
© 2018 Mylan Specialty LP
Rev. 10/2018
STW-ME7096-642R01
IN-0678-02
PATIENT INSTRUCTIONS
Gastrocrom®
(cromolyn sodium, USP)
Oral Concentrate
For Oral Use Only – Not for Inhalation or Injection.
How to Use GASTROCROM:
As with all prescription drugs, follow the directions for dosage that your physician recommends.
The effect of GASTROCROM therapy is dependent upon its administration at REGULAR intervals, for as long as recommended by your physician.
Usual Starting Dose:
Adults and Adolescents (13 Years and Older):
Two ampules four times daily, taken one-half hour before meals and at bedtime.
Children 2-12 Years:
One ampule four times daily, taken one-half hour before meals and at bedtime.
Note:
Your physician may decide to increase OR decrease your dosage to achieve optimum results with GASTROCROM. However, do not change your dose or stop taking GASTROCROM without first consulting your physician.
Care & Storage:
GASTROCROM Oral Concentrate should be stored between 15°-30°C (59°-86°F) and protected from light. Do not use if it contains a precipitate (particles or cloudiness) or becomes discolored. Keep out of the reach of children.
Store ampules in foil pouch until ready for use.
Recycling Information: GASTROCROM Oral Concentrate ampules are made with a low density polyethylene plastic (recycling material code:  LDPE).
LDPE).
Directions for Use:
1.Open foil pouch by tearing at serrated edge as shown.
2.Remove ampule(s) from the strip.
3.Open the ampule by twisting off the tabbed top section.
4.Squeeze liquid contents into a glass of water. Stir solution. Drink all of the liquid. Discard the empty ampule.
Distributed by:
Meda Pharmaceuticals®
Somerset, New Jersey 08873-4120
Gastrocrom is a registered trademark of Meda Pharma S.A.R.L., a Mylan Company
© 2018 Mylan Specialty LP
Rev. 10/2018
STW-ME7096-642R01
IN-0678-02
PRINCIPAL DISPLAY PANEL
NDC 0037-0678-96
Gastrocrom ®
(cromolyn sodium, USP)
Oral Concentrate
FOR ORAL USE ONLY –
NOT FOR INHALATION OR INJECTION.
Rx Only
100 mg/5 mL
96 Plastic Ampules
DESCRIPTION: Each 5 mL ampule
contains 100 mg cromolyn sodium, USP,
in purified water.
NOTE: See package circular for full
prescribing information including
contraindications, warnings and
precautions.
GASTROCROM Oral Concentrate should
be stored between 15º-30ºC (59º-86ºF)
and protected from light. Do not use if it
contains a precipitate or becomes
discolored. Keep out of the reach of
children.
Store ampules in foil pouch until ready
to use
Rev. 10/2018
STW-ME7096-442R01
UC-0678-03
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
Gastrocrom is a registered trademark of Meda
Pharma S.A.R.L., a Mylan Company.
© 2018 Mylan Specialty LP
| GASTROCROM
cromolyn sodium liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Meda Pharmaceuticals (051229602) |