Drug Detail:Gemtesa (Vibegron [ vye-beg-ron ])
Drug Class: Urinary antispasmodics
Highlights of Prescribing Information
GEMTESA (vibegron) tablets, for oral use
Initial U.S. Approval: 2020
Indications and Usage for Gemtesa
GEMTESA is a beta-3 adrenergic agonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults. (1)
Gemtesa Dosage and Administration
- The recommended dose is one 75 mg tablet once daily. (2.1)
- Swallow tablet whole with water. (2.1)
- Tablet may be crushed and mixed with applesauce. (2.1)
Dosage Forms and Strengths
Tablets: 75 mg (3)
Contraindications
Do not use if prior hypersensitivity reaction to vibegron or any components of the product. (4)
Warnings and Precautions
Urinary Retention: Monitor for urinary retention, especially in patients with bladder outlet obstruction and also in patients taking muscarinic antagonist medications for OAB, in whom the risk of urinary retention may be greater. If urinary retention develops, discontinue GEMTESA. (5.1)
Adverse Reactions/Side Effects
Most common adverse reactions (≥2%) reported with GEMTESA were headache, urinary tract infection, nasopharyngitis, diarrhea, nausea, and upper respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Urovant Sciences, Inc., at 1-833-876-8268 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Digoxin: Measure serum digoxin concentrations before initiating GEMTESA. Monitor serum digoxin concentrations to titrate digoxin dose to desired clinical effect. (7)
Use In Specific Populations
Pediatric use: Safety and effectiveness in pediatric patients have not been established. (8.4)
End-stage Renal Disease with or without Hemodialysis: Not recommended. (8.6)
Severe Hepatic Impairment: Not recommended. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2020
Related/similar drugs
Botox, solifenacin, VESIcare, darifenacin, vibegron, onabotulinumtoxinAFull Prescribing Information
1. Indications and Usage for Gemtesa
GEMTESA® is indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency in adults.
2. Gemtesa Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of GEMTESA is one 75 mg tablet orally, once daily with or without food. Swallow GEMTESA tablets whole with a glass of water.
In adults, GEMTESA tablets also may be crushed, mixed with a tablespoon (approximately 15 mL) of applesauce and taken immediately with a glass of water [see Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Tablets: 75 mg, oval, light green, film-coated, debossed with V75 on one side and no debossing on the other side.
4. Contraindications
GEMTESA is contraindicated in patients with known hypersensitivity to vibegron or any components of the product [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Urinary Retention
Urinary retention has been reported in patients taking GEMTESA. The risk of urinary retention may be increased in patients with bladder outlet obstruction and also in patients taking muscarinic antagonist medications for the treatment of OAB. Monitor patients for signs and symptoms of urinary retention, particularly in patients with bladder outlet obstruction and patients taking muscarinic antagonist medications for the treatment of OAB. Discontinue GEMTESA in patients who develop urinary retention [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reaction is described elsewhere in the labeling:
- Urinary retention [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of GEMTESA was evaluated in a 12-week, double-blind, placebo- and active-controlled study (Study 3003) in patients with OAB [see Clinical Studies (14)]. A total of 545 patients received GEMTESA. The majority of the patients were Caucasian (78%) and female (85%) with a mean age of 60 years (range 18 to 93 years).
Adverse reactions that were reported in Study 3003 at an incidence greater than placebo and in ≥2% of patients treated with GEMTESA are listed in Table 1.
| GEMTESA 75 mg n (%) | Placebo n (%) |
|
|---|---|---|
| Number of Patients | 545 | 540 |
| Headache | 22 (4.0) | 13 (2.4) |
| Nasopharyngitis | 15 (2.8) | 9 (1.7) |
| Diarrhea | 12 (2.2) | 6 (1.1) |
| Nausea | 12 (2.2) | 6 (1.1) |
| Upper respiratory tract infection | 11 (2.0) | 4 (0.7) |
Other adverse reactions reported in <2% of patients treated with GEMTESA included:
Gastrointestinal disorders: dry mouth, constipation
Investigations: residual urine volume increased
Renal and urinary disorders: urinary retention
Vascular disorders: hot flush
GEMTESA was also evaluated for long-term safety in an extension study (Study 3004) in 505 patients who completed the 12-week study (Study 3003). Of the 273 patients who received GEMTESA 75 mg once daily in the extension study, 181 patients were treated for a total of one year.
Adverse reactions reported in ≥2% of patients treated with GEMTESA 75 mg for up to 52 weeks in the long-term extension study, and not already listed above, were urinary tract infection (6.6%) and bronchitis (2.9%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of vibegron. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse events have been reported in association with vibegron use in worldwide postmarketing experience:
Urologic disorders: urinary retention
Skin and subcutaneous tissue disorders: pruritus, rash, drug eruption, eczema
Gastrointestinal disorders: constipation
7. Drug Interactions
Concomitant use of GEMTESA increases digoxin maximal concentrations (Cmax) and systemic exposure as assessed by area under the concentration-time curve (AUC) [see Clinical Pharmacology (12.3)]. Serum digoxin concentrations should be monitored before initiating and during therapy with GEMTESA and used for titration of the digoxin dose to obtain the desired clinical effect. Continue monitoring digoxin concentrations upon discontinuation of GEMTESA and adjust digoxin dose as needed.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of GEMTESA in pediatric patients have not been established.
8.5 Geriatric Use
Of 526 patients who received GEMTESA in the clinical studies for OAB with symptoms of urge urinary incontinence, urgency, and urinary frequency, 242 (46%) were 65 years of age or older, and 75 (14%) were 75 years of age or older [see Clinical Studies (14)]. No overall differences in safety or effectiveness of GEMTESA have been observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
No dosage adjustment for GEMTESA is recommended for patients with mild, moderate, or severe renal impairment (eGFR 15 to <90 mL/min/1.73 m2). GEMTESA has not been studied in patients with eGFR <15 mL/min/1.73 m2 (with or without hemodialysis) and is not recommended in these patients [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment for GEMTESA is recommended for patients with mild to moderate hepatic impairment (Child-Pugh A and B). GEMTESA has not been studied in patients with severe hepatic impairment (Child-Pugh C) and is not recommended in this patient population [see Clinical Pharmacology (12.3)].
10. Overdosage
There is no experience with inadvertent GEMTESA overdosage. In case of suspected overdose, treatment should be symptomatic and supportive.
11. Gemtesa Description
Vibegron is a selective beta-3 adrenergic agonist. The chemical name is (6S)-N-[4-[[(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl]methyl]phenyl]-4-oxo-7,8-dihydro-6H-pyrrolo[1,2-a]pyrimidine-6-carboxamide having a molecular formula of C26H28N4O3 and a molecular weight of 444.538 g/mol. The structural formula of vibegron is:
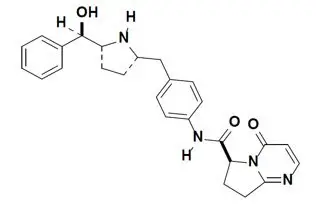
Vibegron is a crystalline, white to off-white to tan powder.
GEMTESA tablets, for oral administration contain 75 mg of vibegron and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, mannitol, and microcrystalline cellulose. The light green film coating contains FD&C Blue No. 2 - aluminum lake, hypromellose, iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin.
12. Gemtesa - Clinical Pharmacology
12.1 Mechanism of Action
Vibegron is a selective human beta-3 adrenergic receptor agonist. Activation of the beta-3 adrenergic receptor increases bladder capacity by relaxing the detrusor smooth muscle during bladder filling.
12.2 Pharmacodynamics
Vibegron's exposure-response relationship and the time course of pharmacodynamic response are not fully characterized.
12.3 Pharmacokinetics
Mean vibegron Cmax and AUC increased in a greater than dose-proportional manner up to 600 mg (8 times the approved recommended dosage). Steady state concentrations are achieved within 7 days of once daily dosing. The mean accumulation ratio (Rac) was 1.7 for Cmax and 2.4 for AUC0-24hr.
14. Clinical Studies
The efficacy of GEMTESA was evaluated in a 12-week, double-blind, randomized, placebo-controlled, and active-controlled trial (Study 3003, NCT03492281) in patients with OAB (urge urinary incontinence, urgency, and urinary frequency). Patients were randomized 5:5:4 to receive either GEMTESA 75 mg, placebo, or active control orally, once daily for 12 weeks. For study entry, patients had to have symptoms of OAB for at least 3 months with an average of 8 or more micturitions per day and at least 1 urge urinary incontinence (UUI) per day, or an average of 8 or more micturitions per day and an average of at least 3 urgency episodes per day. Urge urinary incontinence was defined as leakage of urine of any amount because the patient felt an urge or need to urinate immediately. The study population included OAB medication-naïve patients as well as patients who had received prior therapy with OAB medications.
The co-primary endpoints were change from baseline in average daily number of micturitions and average daily number of UUI episodes at week 12. Additional endpoints included change from baseline in average daily number of "need to urinate immediately" (urgency) episodes and average volume voided per micturition.
A total of 1,515 patients received at least one daily dose of placebo (n=540), GEMTESA 75 mg (n=545), or an active control treatment (n=430). The majority of patients were Caucasian (78%) and female (85%) with a mean age of 60 (range 18 to 93) years.
Table 2 shows changes from baseline at week 12 for average daily number of micturitions, average daily number of UUI episodes, average daily number of "need to urinate immediately" (urgency) episodes, and average volume voided per micturition.
| Parameter | GEMTESA 75 mg | Placebo |
|---|---|---|
|
||
| Average Daily Number of Micturitions | ||
| Baseline mean (n) | 11.3 (526) | 11.8 (520) |
| Change from Baseline* (n) | -1.8 (492) | -1.3 (475) |
| Difference from Placebo | -0.5 | |
| 95% Confidence Interval | -0.8, -0.2 | |
| p-value | <0.001 | |
| Average Daily Number of UUI Episodes | ||
| Baseline mean (n) | 3.4 (403) | 3.5 (405) |
| Change from Baseline* (n) | -2.0 (383) | -1.4 (372) |
| Difference from Placebo | -0.6 | |
| 95% Confidence Interval | -0.9, -0.3 | |
| p-value | <0.0001 | |
| Average Daily Number of "Need to Urinate Immediately" (Urgency) Episodes | ||
| Baseline mean (n) | 8.1 (526) | 8.1 (520) |
| Change from Baseline* (n) | -2.7 (492) | -2.0 (475) |
| Difference from Placebo | -0.7 | |
| 95% Confidence Interval | -1.1, -0.2 | |
| p-value | 0.002 | |
| Average Volume Voided (mL) per Micturition | ||
| Baseline mean (n) | 155 (524) | 148 (514) |
| Change from Baseline* (n) | 23 (490) | 2 (478) |
| Difference from Placebo | 21 | |
| 95% Confidence Interval | 14, 28 | |
| p-value | <0.0001 | |
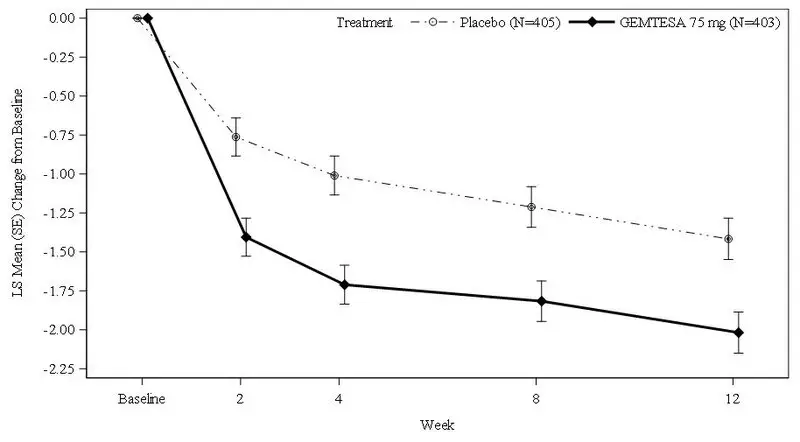
Figures 1 and 2 show the mean change from baseline over time in average daily number of micturitions and mean change from baseline over time in average daily number of UUI episodes, respectively.
Figure 1: Mean (SE) Change from Baseline in the Average Daily Number of Micturitions
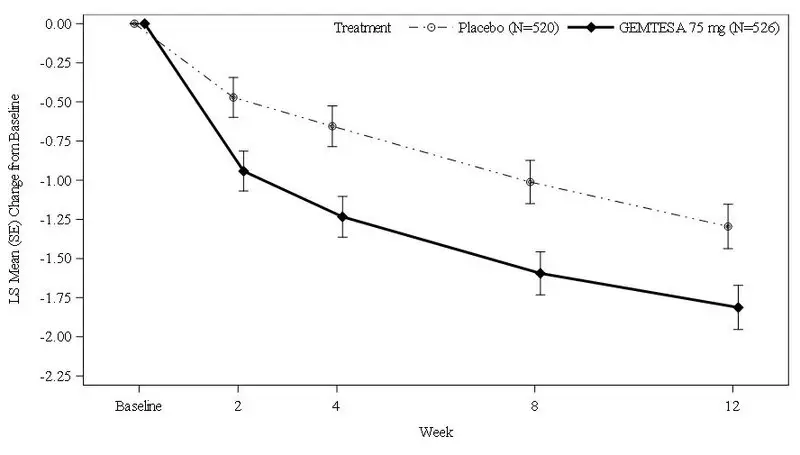
Figure 2: Mean (SE) Change from Baseline in the Average Daily Number of UUI Episodes in Patients with At Least 1 Average Daily UUI Episode at Baseline
16. How is Gemtesa supplied
GEMTESA 75 mg tablets are light green, oval, film-coated tablets, debossed with V75 on one side and no debossing on the other side.
GEMTESA is marketed in two packaging configurations:
Thirty (30) tablets in a 60 cc HDPE bottle with a child-resistant cap, NDC 73336-075-30
Ninety (90) tablets in a 60 cc HDPE bottle with a child-resistant cap, NDC 73336-075-90
| GEMTESA
vibegron tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Urovant Sciences, Inc. (081330666) |
| Registrant - Urovant Sciences, Inc. (081330666) |





