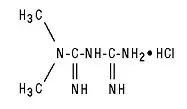
Drug Detail:Glucophage xr (Metformin [ met-for-min ])
Drug Class: Non-sulfonylureas
Glucophage XR - Clinical Pharmacology
GLUCOPHAGE
In a double-blind, placebo-controlled, multicenter US clinical trial involving obese patients with type 2 diabetes whose hyperglycemia was not adequately controlled with dietary management alone (baseline fasting plasma glucose [FPG] of approximately 240 mg/dL), treatment with GLUCOPHAGE (up to 2550 mg/day) for 29 weeks resulted in significant mean net reductions in fasting and postprandial plasma glucose (PPG) and hemoglobin A1c (HbA1c) of 59 mg/dL, 83 mg/dL, and 1.8%, respectively, compared to the placebo group (see Table 2).
| GLUCOPHAGE (n=141) | Placebo (n=145) | p-Value | |
|---|---|---|---|
| * All patients on diet therapy at Baseline | ** Not statistically significant | ||
| FPG (mg/dL)
Baseline Change at FINAL VISIT | 241.5 –53.0 | 237.7 6.3 | NS** 0.001 |
| Hemoglobin A1c (%)
Baseline Change at FINAL VISIT | 8.4 –1.4 | 8.2 0.4 | NS** 0.001 |
| Body Weight (lbs)
Baseline Change at FINAL VISIT | 201.0 –1.4 | 206.0 –2.4 | NS** NS** |
A 29-week, double-blind, placebo-controlled study of GLUCOPHAGE and glyburide, alone and in combination, was conducted in obese patients with type 2 diabetes who had failed to achieve adequate glycemic control while on maximum doses of glyburide (baseline FPG of approximately 250 mg/dL) (see Table 3). Patients randomized to the combination arm started therapy with GLUCOPHAGE 500 mg and glyburide 20 mg. At the end of each week of the first 4 weeks of the trial, these patients had their dosages of GLUCOPHAGE increased by 500 mg if they had failed to reach target fasting plasma glucose. After week 4, such dosage adjustments were made monthly, although no patient was allowed to exceed GLUCOPHAGE 2500 mg. Patients in the GLUCOPHAGE only arm (metformin plus placebo) followed the same titration schedule. At the end of the trial, approximately 70% of the patients in the combination group were taking GLUCOPHAGE 2000 mg/glyburide 20 mg or GLUCOPHAGE 2500 mg/glyburide 20 mg. Patients randomized to continue on glyburide experienced worsening of glycemic control, with mean increases in FPG, PPG, and HbA1c of 14 mg/dL, 3 mg/dL, and 0.2%, respectively. In contrast, those randomized to GLUCOPHAGE (up to 2500 mg/day) experienced a slight improvement, with mean reductions in FPG, PPG, and HbA1c of 1 mg/dL, 6 mg/dL, and 0.4%, respectively. The combination of GLUCOPHAGE and glyburide was effective in reducing FPG, PPG, and HbA1c levels by 63 mg/dL, 65 mg/dL, and 1.7%, respectively. Compared to results of glyburide treatment alone, the net differences with combination treatment were –77 mg/dL, –68 mg/dL, and –1.9%, respectively (see Table 3).
| p-values | ||||||
|---|---|---|---|---|---|---|
| Comb (n=213) | Glyb (n=209) | GLU (n=210) | Glyb vs Comb | GLU vs Comb | GLU vs Glyb |
|
| * All patients on glyburide, 20 mg/day, at Baseline | ** Not statistically significant | |||||
| Fasting Plasma Glucose (mg/dL) | ||||||
| Baseline Change at FINAL VISIT | 250.5 –63.5 | 247.5 13.7 | 253.9 –0.9 | NS** 0.001 | NS** 0.001 | NS** 0.025 |
| Hemoglobin A1c (%) | ||||||
| Baseline Change at FINAL VISIT | 8.8 –1.7 | 8.5 0.2 | 8.9 –0.4 | NS** 0.001 | NS** 0.001 | 0.007 0.001 |
| Body Weight (lbs) | ||||||
| Baseline Change at FINAL VISIT | 202.2 0.9 | 203.0 –0.7 | 204.0 –8.4 | NS** 0.011 | NS** 0.001 | NS** 0.001 |
The magnitude of the decline in fasting blood glucose concentration following the institution of GLUCOPHAGE (metformin hydrochloride) Tablets therapy was proportional to the level of fasting hyperglycemia. Patients with type 2 diabetes with higher fasting glucose concentrations experienced greater declines in plasma glucose and glycosylated hemoglobin.
In clinical studies, GLUCOPHAGE, alone or in combination with a sulfonylurea, lowered mean fasting serum triglycerides, total cholesterol, and LDL cholesterol levels, and had no adverse effects on other lipid levels (see Table 4).
| GLUCOPHAGE vs Placebo | Combined GLUCOPHAGE/Glyburide vs Monotherapy |
||||
|---|---|---|---|---|---|
| GLUCOPHAGE (n=141) | Placebo (n=145) | GLUCOPHAGE (n=210) | GLUCOPHAGE/ Glyburide (n=213) | Glyburide (n=209) |
|
| Total Cholesterol (mg/dL) | |||||
| Baseline Mean % Change at FINAL VISIT | 211.0 –5% | 212.3 1% | 213.1 –2% | 215.6 –4% | 219.6 1% |
| Total Triglycerides (mg/dL) | |||||
| Baseline Mean % Change at FINAL VISIT | 236.1 –16% | 203.5 1% | 242.5 –3% | 215.0 –8% | 266.1 4% |
| LDL-Cholesterol (mg/dL) | |||||
| Baseline Mean % Change at FINAL VISIT | 135.4 –8% | 138.5 1% | 134.3 –4% | 136.0 –6% | 137.5 3% |
| HDL-Cholesterol (mg/dL) | |||||
| Baseline Mean % Change at FINAL VISIT | 39.0 2% | 40.5 –1% | 37.2 5% | 39.0 3% | 37.0 1% |
In contrast to sulfonylureas, body weight of individuals on GLUCOPHAGE tended to remain stable or even decrease somewhat (see Tables 2 and 3).
A 24-week, double-blind, placebo-controlled study of GLUCOPHAGE plus insulin versus insulin plus placebo was conducted in patients with type 2 diabetes who failed to achieve adequate glycemic control on insulin alone (see Table 5). Patients randomized to receive GLUCOPHAGE plus insulin achieved a reduction in HbA1c of 2.10%, compared to a 1.56% reduction in HbA1c achieved by insulin plus placebo. The improvement in glycemic control was achieved at the final study visit with 16% less insulin, 93.0 U/day vs 110.6 U/day, GLUCOPHAGE plus insulin versus insulin plus placebo, respectively, p=0.04.
| GLUCOPHAGE/ Insulin (n=26) | Placebo/ Insulin (n=28) | Treatment Difference Mean± SE |
|
|---|---|---|---|
| a Statistically significant using analysis of covariance with baseline as covariate (p=0.04) Not significant using analysis of variance (values shown in table) |
|||
| b Statistically significant for insulin (p=0.04) | |||
| Hemoglobin A1c (%) | |||
| Baseline Change at FINAL VISIT | 8.95 –2.10 | 9.32 –1.56 |
–0.54 ± 0.43a |
| Insulin Dose (U/day) | |||
| Baseline Change at FINAL VISIT | 93.12 –0.15 | 94.64 15.93 |
–16.08± 7.77b |
A second double-blind, placebo-controlled study (n=51), with 16 weeks of randomized treatment, demonstrated that in patients with type 2 diabetes controlled on insulin for 8 weeks with an average HbA1c of 7.46 ± 0.97%, the addition of GLUCOPHAGE maintained similar glycemic control (HbA1c 7.15 ± 0.61 vs 6.97 ± 0.62 for GLUCOPHAGE plus insulin and placebo plus insulin, respectively) with 19% less insulin versus baseline (reduction of 23.68 ± 30.22 vs an increase of 0.43 ± 25.20 units for GLUCOPHAGE plus insulin and placebo plus insulin, p<0.01). In addition, this study demonstrated that the combination of GLUCOPHAGE plus insulin resulted in reduction in body weight of 3.11 ± 4.30 lbs, compared to an increase of 1.30 ± 6.08 lbs for placebo plus insulin, p=0.01.
GLUCOPHAGE XR
A 24-week, double-blind, placebo-controlled study of GLUCOPHAGE XR, taken once daily with the evening meal, was conducted in patients with type 2 diabetes who had failed to achieve glycemic control with diet and exercise (HbA1c 7.0%-10.0%, FPG 126-270 mg/dL). Patients entering the study had a mean baseline HbA1c of 8.0% and a mean baseline FPG of 176 mg/dL. After 12 weeks treatment, mean HbA1c had increased from baseline by 0.1% and mean FPG decreased from baseline by 2 mg/dL in the placebo group, compared with a decrease in mean HbA1c of 0.6% and a decrease in mean FPG of 23 mg/dL in patients treated with GLUCOPHAGE XR 1000 mg once daily. Subsequently, the treatment dose was increased to 1500 mg once daily if HbA1c was≥7.0% but <8.0% (patients with HbA1c ≥8.0% were discontinued from the study). At the final visit (24-week), mean HbA1c had increased 0.2% from baseline in placebo patients and decreased 0.6% with GLUCOPHAGE XR.
A 16-week, double-blind, placebo-controlled, dose-response study of GLUCOPHAGE XR, taken once daily with the evening meal or twice daily with meals, was conducted in patients with type 2 diabetes who had failed to achieve glycemic control with diet and exercise (HbA1c 7.0%-11.0%, FPG 126-280 mg/dL). Changes in glycemic control and body weight are shown in Table 6.
| GLUCOPHAGE XR | Placebo | |||||
|---|---|---|---|---|---|---|
| 500 mg Once Daily | 1000 mg Once Daily | 1500 mg Once Daily | 2000 mg Once Daily | 1000 mg Twice Daily |
||
| * All patients on diet therapy at Baseline | ||||||
| a All comparisons versus Placebo | ||||||
| ** Not statistically significant | ||||||
| Hemoglobin A1c (%) | (n=115) | (n=115) | (n=111) | (n=125) | (n=112) | (n=111) |
| Baseline | 8.2 | 8.4 | 8.3 | 8.4 | 8.4 | 8.4 |
| Change at FINAL VISIT | –0.4 | –0.6 | –0.9 | –0.8 | –1.1 | 0.1 |
| p-valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | – |
| FPG (mg/dL) | (n=126) | (n=118) | (n=120) | (n=132) | (n=122) | (n=113) |
| Baseline | 182.7 | 183.7 | 178.9 | 181.0 | 181.6 | 179.6 |
| Change at FINAL VISIT | –15.2 | –19.3 | –28.5 | –29.9 | –33.6 | 7.6 |
| p-valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | – |
| Body Weight (lbs) | (n=125) | (n=119) | (n=117) | (n=131) | (n=119) | (n=113) |
| Baseline | 192.9 | 191.8 | 188.3 | 195.4 | 192.5 | 194.3 |
| Change at FINAL VISIT | –1.3 | –1.3 | –0.7 | –1.5 | –2.2 | –1.8 |
| p-valuea | NS** | NS** | NS** | NS** | NS** | – |
Compared with placebo, improvement in glycemic control was seen at all dose levels of GLUCOPHAGE XR (metformin hydrochloride) Extended-Release Tablets and treatment was not associated with any significant change in weight (see DOSAGE AND ADMINISTRATION for dosing recommendations for GLUCOPHAGE and GLUCOPHAGE XR).
A 24-week, double-blind, randomized study of GLUCOPHAGE XR, taken once daily with the evening meal, and GLUCOPHAGE (metformin hydrochloride) Tablets, taken twice daily (with breakfast and evening meal), was conducted in patients with type 2 diabetes who had been treated with GLUCOPHAGE 500 mg twice daily for at least 8 weeks prior to study entry. The GLUCOPHAGE dose had not necessarily been titrated to achieve a specific level of glycemic control prior to study entry. Patients qualified for the study if HbA1c was≤8.5% and FPG was ≤200 mg/dL. Changes in glycemic control and body weight are shown in Table 7.
| GLUCOPHAGE
500 mg Twice Daily | GLUCOPHAGE XR | ||
|---|---|---|---|
| 1000 mg Once Daily | 1500 mg Once Daily |
||
| * All patients on GLUCOPHAGE 500 mg twice daily at Baseline | |||
| a n=68 | |||
| Hemoglobin A1c (%) | (n=67) | (n=72) | (n=66) |
| Baseline | 7.06 | 6.99 | 7.02 |
| Change at 12 Weeks | 0.14 | 0.23 | 0.04 |
| (95% CI) | (–0.03, 0.31) | (0.10, 0.36) | (–0.08, 0.15) |
| Change at FINAL VISIT | 0.14a | 0.27 | 0.13 |
| (95% CI) | (–0.04, 0.31) | (0.11, 0.43) | (–0.02, 0.28) |
| FPG (mg/dL) | (n=69) | (n=72) | (n=70) |
| Baseline | 127.2 | 131.0 | 131.4 |
| Change at 12 Weeks | 12.9 | 9.5 | 3.7 |
| (95% CI) | (6.5, 19.4) | (4.4, 14.6) | (–0.4, 7.8) |
| Change at FINAL VISIT | 14.0 | 11.5 | 7.6 |
| (95% CI) | (7.0, 21.0) | (4.4, 18.6) | (1.0, 14.2) |
| Body Weight (lbs) | (n=71) | (n=74) | (n=71) |
| Baseline | 210.3 | 202.8 | 192.7 |
| Change at 12 Weeks | 0.4 | 0.9 | 0.7 |
| (95% CI) | (–0.4, 1.5) | (0.0, 2.0) | (–0.4, 1.8) |
| Change at FINAL VISIT | 0.9 | 1.1 | 0.9 |
| (95% CI) | (–0.4, 2.2) | (–0.2, 2.4) | (–0.4, 2.0) |
After 12 weeks of treatment, there was an increase in mean HbA1c in all groups; in the GLUCOPHAGE XR 1000 mg group, the increase from baseline of 0.23% was statistically significant (see DOSAGE AND ADMINISTRATION).
Changes in lipid parameters in the previously described placebo-controlled dose-response study of GLUCOPHAGE XR are shown in Table 8.
| GLUCOPHAGE XR | Placebo | |||||
|---|---|---|---|---|---|---|
| 500 mg Once Daily | 1000 mg Once Daily | 1500 mg Once Daily | 2000 mg Once Daily | 1000 mg Twice Daily |
||
| * All patients on diet therapy at Baseline | ||||||
| Total Cholesterol (mg/dL) | (n=120) | (n=113) | (n=110) | (n=126) | (n=117) | (n=110) |
| Baseline | 210.3 | 218.1 | 214.6 | 204.4 | 208.2 | 208.6 |
| Mean % Change at FINAL VISIT | 1.0% | 1.7% | 0.7% | –1.6% | –2.6% | 2.6% |
| Total Triglycerides (mg/dL) | (n=120) | (n=113) | (n=110) | (n=126) | (n=117) | (n=110) |
| Baseline | 220.2 | 211.9 | 198.0 | 194.2 | 179.0 | 211.7 |
| Mean % Change at FINAL VISIT | 14.5% | 9.4% | 15.1% | 14.9% | 9.4% | 10.9% |
| LDL-Cholesterol (mg/dL) | (n=119) | (n=113) | (n=109) | (n=126) | (n=117) | (n=107) |
| Baseline | 131.0 | 134.9 | 135.8 | 125.8 | 131.4 | 131.9 |
| Mean % Change at FINAL VISIT | –1.4% | –1.6% | –3.5% | –3.3% | –5.5% | 3.2% |
| HDL-Cholesterol (mg/dL) | (n=120) | (n=108) | (n=108) | (n=125) | (n=117) | (n=108) |
| Baseline | 40.8 | 41.6 | 40.6 | 40.2 | 42.4 | 39.4 |
| Mean % Change at FINAL VISIT | 6.2% | 8.6% | 5.5% | 6.1% | 7.1% | 5.8% |
Changes in lipid parameters in the previously described study of GLUCOPHAGE and GLUCOPHAGE XR are shown in Table 9.
| GLUCOPHAGE | GLUCOPHAGE XR | ||
|---|---|---|---|
| 500 mg Twice Daily | 1000 mg Once Daily | 1500 mg Once Daily |
|
| * All patients on GLUCOPHAGE 500 mg twice daily at Baseline | |||
| Total Cholesterol (mg/dL) | (n=68) | (n=70) | (n=66) |
| Baseline | 199.0 | 201.9 | 201.6 |
| Mean % Change at FINAL VISIT | 0.1% | 1.3% | 0.1% |
| Total Triglycerides (mg/dL) | (n=68) | (n=70) | (n=66) |
| Baseline | 178.0 | 169.2 | 206.8 |
| Mean % Change at FINAL VISIT | 6.3% | 25.3% | 33.4% |
| LDL-Cholesterol (mg/dL) | (n=68) | (n=70) | (n=66) |
| Baseline | 122.1 | 126.2 | 115.7 |
| Mean % Change at FINAL VISIT | −1.3% | −3.3% | −3.7% |
| HDL-Cholesterol (mg/dL) | (n=68) | (n=70) | (n=65) |
| Baseline | 41.9 | 41.7 | 44.6 |
| Mean % Change at FINAL VISIT | 4.8% | 1.0% | –2.1% |
Pediatric Clinical Studies
In a double-blind, placebo-controlled study in pediatric patients aged 10 to 16 years with type 2 diabetes (mean FPG 182.2 mg/dL), treatment with GLUCOPHAGE (up to 2000 mg/day) for up to 16 weeks (mean duration of treatment 11 weeks) resulted in a significant mean net reduction in FPG of 64.3 mg/dL, compared with placebo (see Table 10).
| GLUCOPHAGE | Placebo | p-Value | |
|---|---|---|---|
| a Pediatric patients mean age 13.8 years (range 10-16 years) | |||
| * All patients on diet therapy at Baseline | |||
| ** Not statistically significant | |||
| FPG (mg/dL) | (n=37) | (n=36) | |
| Baseline Change at FINAL VISIT | 162.4 –42.9 | 192.3 21.4 |
<0.001 |
| Body Weight (lbs) | (n=39) | (n=38) | |
| Baseline Change at FINAL VISIT | 205.3 –3.3 | 189.0 –2.0 |
NS** |
Adverse Reactions/Side Effects
In a US double-blind clinical study of GLUCOPHAGE in patients with type 2 diabetes, a total of 141 patients received GLUCOPHAGE therapy (up to 2550 mg per day) and 145 patients received placebo. Adverse reactions reported in greater than 5% of the GLUCOPHAGE patients, and that were more common in GLUCOPHAGE- than placebo-treated patients, are listed in Table 11.
| Adverse Reaction | GLUCOPHAGE Monotherapy (n=141) | Placebo (n=145) |
|---|---|---|
| % of Patients | ||
| * Reactions that were more common in GLUCOPHAGE- than placebo-treated patients. | ||
| Diarrhea | 53.2 | 11.7 |
| Nausea/Vomiting | 25.5 | 8.3 |
| Flatulence | 12.1 | 5.5 |
| Asthenia | 9.2 | 5.5 |
| Indigestion | 7.1 | 4.1 |
| Abdominal Discomfort | 6.4 | 4.8 |
| Headache | 5.7 | 4.8 |
Diarrhea led to discontinuation of study medication in 6% of patients treated with GLUCOPHAGE. Additionally, the following adverse reactions were reported in ≥1.0% to ≤5.0% of GLUCOPHAGE patients and were more commonly reported with GLUCOPHAGE than placebo: abnormal stools, hypoglycemia, myalgia, lightheaded, dyspnea, nail disorder, rash, sweating increased, taste disorder, chest discomfort, chills, flu syndrome, flushing, palpitation.
In worldwide clinical trials over 900 patients with type 2 diabetes have been treated with GLUCOPHAGE XR in placebo- and active-controlled studies. In placebo-controlled trials, 781 patients were administered GLUCOPHAGE XR and 195 patients received placebo. Adverse reactions reported in greater than 5% of the GLUCOPHAGE XR patients, and that were more common in GLUCOPHAGE XR- than placebo-treated patients, are listed in Table 12.
| Adverse Reaction | GLUCOPHAGE XR (n=781) | Placebo (n=195) |
|---|---|---|
| % of Patients | ||
| * Reactions that were more common in GLUCOPHAGE XR- than placebo-treated patients. | ||
| Diarrhea | 9.6 | 2.6 |
| Nausea/Vomiting | 6.5 | 1.5 |
Diarrhea led to discontinuation of study medication in 0.6% of patients treated with GLUCOPHAGE XR. Additionally, the following adverse reactions were reported in ≥1.0% to ≤5.0% of GLUCOPHAGE XR patients and were more commonly reported with GLUCOPHAGE XR than placebo: abdominal pain, constipation, distention abdomen, dyspepsia/heartburn, flatulence, dizziness, headache, upper respiratory infection, taste disturbance.
Patient Information
Patient Information
GLUCOPHAGE®
(metformin hydrochloride) Tablets
and
GLUCOPHAGE® XR
(metformin hydrochloride) Extended-Release Tablets
Read this information carefully before you start taking this medicine and each time you refill your prescription. There may be new information. This information does not take the place of your doctor’s advice. Ask your doctor or pharmacist if you do not understand some of this information or if you want to know more about this medicine.
What are GLUCOPHAGE and GLUCOPHAGE XR?
GLUCOPHAGE and GLUCOPHAGE XR are used to treat type 2 diabetes. This is also known as non-insulin-dependent diabetes mellitus. People with type 2 diabetes are not able to make enough insulin or respond normally to the insulin their bodies make. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems including kidney damage, amputations, and blindness. Diabetes is also closely linked to heart disease. The main goal of treating diabetes is to lower your blood sugar to a normal level.
High blood sugar can be lowered by diet and exercise, by a number of medicines taken by mouth, and by insulin shots. Before you take GLUCOPHAGE or GLUCOPHAGE XR, try to control your diabetes by exercise and weight loss. While you take your diabetes medicine, continue to exercise and follow the diet advised for your diabetes. No matter what your recommended diabetes management plan is, studies have shown that maintaining good blood sugar control can prevent or delay complications of diabetes, such as blindness.
GLUCOPHAGE and GLUCOPHAGE XR have the same active ingredient. However, GLUCOPHAGE XR works longer in your body. Both of these medicines help control your blood sugar in a number of ways. These include helping your body respond better to the insulin it makes naturally, decreasing the amount of sugar your liver makes, and decreasing the amount of sugar your intestines absorb. GLUCOPHAGE and GLUCOPHAGE XR do not cause your body to make more insulin. Because of this, when taken alone, they rarely cause hypoglycemia (low blood sugar), and usually do not cause weight gain. However, when they are taken with a sulfonylurea or with insulin, hypoglycemia is more likely to occur, as is weight gain.
WARNING: A small number of people who have taken GLUCOPHAGE have developed a serious condition called lactic acidosis. Lactic acidosis is caused by a buildup of lactic acid in the blood. This happens more often in people with kidney problems. Most people with kidney problems should not take GLUCOPHAGE or GLUCOPHAGE XR. (See "What are the side effects of GLUCOPHAGE and GLUCOPHAGE XR?")
Who should not take GLUCOPHAGE or GLUCOPHAGE XR?
Some conditions increase your chance of getting lactic acidosis, or cause other problems if you take either of these medicines. Most of the conditions listed below can increase your chance of getting lactic acidosis.
Do not take GLUCOPHAGE or GLUCOPHAGE XR if you:
- have kidney problems
- have liver problems
- have heart failure that is treated with medicines, such as Lanoxin® (digoxin) or Lasix® (furosemide)
- drink a lot of alcohol. This means you binge drink for short periods or drink all the time
- are seriously dehydrated (have lost a lot of water from your body)
- are going to have an x-ray procedure with injection of dyes (contrast agents)
- are going to have surgery
- develop a serious condition, such as heart attack, severe infection, or a stroke
- are 80 years or older and you have NOT had your kidney function tested
Tell your doctor if you are pregnant or plan to become pregnant. GLUCOPHAGE and GLUCOPHAGE XR may not be right for you. Talk with your doctor about your choices. You should also discuss your choices with your doctor if you are nursing a child.
Can GLUCOPHAGE or GLUCOPHAGE XR be used in children?
GLUCOPHAGE has been shown to effectively lower glucose levels in children (ages 10-16 years) with type 2 diabetes. GLUCOPHAGE has not been studied in children younger than 10 years old. GLUCOPHAGE has not been studied in combination with other oral glucose-control medicines or insulin in children. If you have any questions about the use of GLUCOPHAGE in children, talk with your doctor or other healthcare provider.
GLUCOPHAGE XR has not been studied in children.
How should I take GLUCOPHAGE or GLUCOPHAGE XR?
Your doctor will tell you how much medicine to take and when to take it. You will probably start out with a low dose of the medicine. Your doctor may slowly increase your dose until your blood sugar is better controlled. You should take GLUCOPHAGE or GLUCOPHAGE XR with meals.
Your doctor may have you take other medicines along with GLUCOPHAGE or GLUCOPHAGE XR to control your blood sugar. These medicines may include insulin shots. Taking GLUCOPHAGE or GLUCOPHAGE XR with insulin may help you better control your blood sugar while reducing the insulin dose.
Continue your exercise and diet program and test your blood sugar regularly while taking GLUCOPHAGE or GLUCOPHAGE XR. Your doctor will monitor your diabetes and may perform blood tests on you from time to time to make sure your kidneys and your liver are functioning normally. There is no evidence that GLUCOPHAGE or GLUCOPHAGE XR causes harm to the liver or kidneys.
Tell your doctor if you:
- have an illness that causes severe vomiting, diarrhea or fever, or if you drink a much lower amount of liquid than normal. These conditions can lead to severe dehydration (loss of water in your body). You may need to stop taking GLUCOPHAGE or GLUCOPHAGE XR for a short time.
- plan to have surgery or an x-ray procedure with injection of dye (contrast agent). You may need to stop taking GLUCOPHAGE or GLUCOPHAGE XR for a short time.
- start to take other medicines or change how you take a medicine. GLUCOPHAGE and GLUCOPHAGE XR can affect how well other drugs work, and some drugs can affect how well GLUCOPHAGE and GLUCOPHAGE XR work. Some medicines may cause high blood sugar.
GLUCOPHAGE XR must be swallowed whole and never crushed or chewed. Occasionally, the inactive ingredients of GLUCOPHAGE XR may be eliminated as a soft mass in your stool that may look like the original tablet; this is not harmful and will not affect the way GLUCOPHAGE XR works to control your diabetes.
What should I avoid while taking GLUCOPHAGE or GLUCOPHAGE XR?
Do not drink a lot of alcoholic drinks while taking GLUCOPHAGE or GLUCOPHAGE XR. This means you should not binge drink for short periods, and you should not drink a lot of alcohol on a regular basis. Alcohol can increase the chance of getting lactic acidosis.
What are the side effects of GLUCOPHAGE and GLUCOPHAGE XR?
Lactic Acidosis.In rare cases, GLUCOPHAGE and GLUCOPHAGE XR can cause a serious side effect called lactic acidosis. This is caused by a buildup of lactic acid in your blood. This buildup can cause serious damage. Lactic acidosis caused by GLUCOPHAGE and GLUCOPHAGE XR is rare and has occurred mostly in people whose kidneys were not working normally. Lactic acidosis has been reported in about one in 33,000 patients taking GLUCOPHAGE over the course of a year. Although rare, if lactic acidosis does occur, it can be fatal in up to half the people who develop it.
It is also important for your liver to be working normally when you take GLUCOPHAGE or GLUCOPHAGE XR. Your liver helps remove lactic acid from your blood.
Make sure you tell your doctor before you use GLUCOPHAGE or GLUCOPHAGE XR if you have kidney or liver problems. You should also stop using GLUCOPHAGE or GLUCOPHAGE XR and call your doctor right away if you have signs of lactic acidosis. Lactic acidosis is a medical emergency that must be treated in a hospital.
Signs of lactic acidosis are:
- feeling very weak, tired, or uncomfortable
- unusual muscle pain
- trouble breathing
- unusual or unexpected stomach discomfort
- feeling cold
- feeling dizzy or lightheaded
- suddenly developing a slow or irregular heartbeat
If your medical condition suddenly changes, stop taking GLUCOPHAGE or GLUCOPHAGE XR and call your doctor right away. This may be a sign of lactic acidosis or another serious side effect.
Other Side Effects. Common side effects of GLUCOPHAGE and GLUCOPHAGE XR include diarrhea, nausea, and upset stomach. These side effects generally go away after you take the medicine for a while. Taking your medicine with meals can help reduce these side effects. Tell your doctor if the side effects bother you a lot, last for more than a few weeks, come back after they’ve gone away, or start later in therapy. You may need a lower dose or need to stop taking the medicine for a short period or for good.
About 3 out of every 100 people who take GLUCOPHAGE or GLUCOPHAGE XR have an unpleasant metallic taste when they start taking the medicine. It lasts for a short time.
GLUCOPHAGE and GLUCOPHAGE XR rarely cause hypoglycemia (low blood sugar) by themselves. However, hypoglycemia can happen if you do not eat enough, if you drink alcohol, or if you take other medicines to lower blood sugar.
General advice about prescription medicines
If you have questions or problems, talk with your doctor or other healthcare provider. You can ask your doctor or pharmacist for the information about GLUCOPHAGE and GLUCOPHAGE XR that is written for healthcare professionals. Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use GLUCOPHAGE or GLUCOPHAGE XR for a condition for which it was not prescribed. Do not share your medicine with other people.
GLUCOPHAGE® is a registered trademark of Merck Santé S.A.S., an associate of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
Other brands listed are the trademarks of their respective owners.
Distributed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
1125493A8
Rev January 2009
| GLUCOPHAGE XR
metformin hydrochloride tablet, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Caremark L.L.C. (858733918) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Caremark L.L.C. | 858733918 | repack | |