Drug Detail:Haegarda (C1 esterase inhibitor subcutaneous (human) [ c1 es-ter-ase-in-hib-it-or ])
Drug Class: Hereditary angioedema agents
Highlights of Prescribing Information
HAEGARDA® (C1 Esterase Inhibitor Subcutaneous [Human])
For Subcutaneous Injection, Freeze-Dried Powder for Reconstitution
Initial U.S. Approval: 2017
Recent Major Changes
| Indications and Usage (1) | 09/2020 |
Indications and Usage for Haegarda
HAEGARDA is a plasma-derived concentrate of C1 Esterase Inhibitor (Human) (C1-INH) indicated for routine prophylaxis to prevent Hereditary Angioedema (HAE) attacks in patients 6 years of age and older. (1)
Haegarda Dosage and Administration
For subcutaneous use after reconstitution only.
- Administer 60 International Units per kg body weight twice weekly (every 3 or 4 days). (2)
- Reconstitute HAEGARDA prior to use using Sterile Water for Injection, USP. (2.1)
- Use a silicone-free syringe for reconstitution and administration. (2.1)
- Administer at room temperature within 8 hours after reconstitution. (2.1)
Dosage Forms and Strengths
HAEGARDA is available as a white lyophilized powder supplied in single-dose vials containing 2000 or 3000 International Units (IU) of C1-INH. (3)
Contraindications
Do not use in patients with a history of life-threatening immediate hypersensitivity reactions, including anaphylaxis to C1-INH preparations or its excipients. (4)
Warnings and Precautions
- Severe hypersensitivity reactions may occur. In case of severe hypersensitivity, discontinue HAEGARDA administration and institute appropriate treatment. Epinephrine should be immediately available for treatment of severe hypersensitivity reaction. (5.1)
- At the recommended subcutaneous (S.C.) dose, a causal relationship between thromboembolic events (TEEs) and the use of HAEGARDA has not been established. However, thrombosis has occurred in treatment attempts with high doses of C1-INH intravenous (I.V.) for prevention or therapy of capillary leak syndrome before, during or after cardiac surgery (unapproved indication and dose). (5.2)
- Because HAEGARDA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.3)
Adverse Reactions/Side Effects
- Adverse reactions occurring in more than 4% of subjects treated with HAEGARDA were injection site reactions, hypersensitivity, nasopharyngitis and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2022
Full Prescribing Information
1. Indications and Usage for Haegarda
HAEGARDA is a plasma-derived concentrate of C1 Esterase Inhibitor (Human) (C1-INH) indicated for routine prophylaxis to prevent Hereditary Angioedema (HAE) attacks in patients 6 years of age and older.
2. Haegarda Dosage and Administration
After reconstitution, for subcutaneous use only.
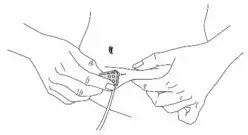
HAEGARDA is intended for self (or caregiver)-administration after reconstitution at a dose of 60 International Units (IU) per kg body weight by subcutaneous (S.C.) injection twice weekly (every 3 or 4 days). The patient or caregiver should be trained on how to administer HAEGARDA.
HAEGARDA is provided as a freeze-dried powder for reconstitution with Sterile Water for Injection, USP.
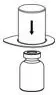
2.1 Preparation and Handling
- Check the expiration date on the product vial label. Do not use beyond the expiration date.
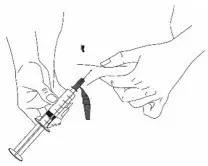
- Work on a clean surface and wash hands before performing the following procedures.
- Prepare and administer using aseptic techniques [see Dosage and Administration (2.2)].
- Use a silicone-free syringe for reconstitution and administration.
- Each vial of HAEGARDA is for single-dose only. Promptly use the reconstituted solution. The solution must be used within 8 hours. Discard partially used vials. HAEGARDA contains no preservative.
- Do not freeze the reconstituted solution.
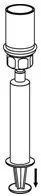
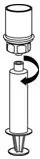
2.2 Reconstitution and Administration
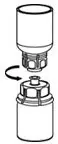
Use either the Mix2Vial® transfer set provided with HAEGARDA or a commercially available double-ended needle and vented filter spike [see How Supplied/Storage and Handling (16)].
3. Dosage Forms and Strengths
HAEGARDA is available as a white lyophilized powder supplied in single-dose vials containing 2000 or 3000 IU of C1-INH.
- The 2000 IU vial must be reconstituted with 4 mL of Sterile Water for Injection, USP.
- The 3000 IU vial must be reconstituted with 5.6 mL of Sterile Water for Injection, USP.
4. Contraindications
HAEGARDA is contraindicated in individuals who have experienced life-threatening hypersensitivity reactions, including anaphylaxis, to C1-INH preparations or its excipients [see Description (11)].
5. Warnings and Precautions
The physician should discuss the risks and benefits of this product with the patient before prescribing or administering it to the patient [see Patient Counseling Information (17)].
Initiate individualized treatment in case of an acute HAE attack.
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur. The signs and symptoms of hypersensitivity reactions may include hives (local and generalized), tightness of the chest, difficulty breathing, wheezing, hypotension, and/or anaphylaxis during or after injection of HAEGARDA. In case of severe hypersensitivity, discontinue HAEGARDA administration and institute appropriate treatment. Epinephrine should be immediately available for treatment of severe hypersensitivity reaction [see Patient Counseling Information (17)].
5.2 Thromboembolic Events
At the recommended subcutaneous dose, a causal relationship between thromboembolic events (TEEs) and the use of HAEGARDA has not been established [see Patient Counseling Information (17)]. Thrombosis has occurred in treatment attempts with high doses of C1-INH intravenous (I.V.) for prevention or therapy of capillary leak syndrome before, during or after cardiac surgery (unapproved indication and dose).
5.3 Transmissible Infectious Agents
Because HAEGARDA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by processes demonstrated to inactivate and/or remove certain viruses during manufacturing [see Description (11) and Patient Counseling Information (17)]. Despite these measures, such products may still contain human pathogenic agents, including those not yet known or identified. Thus, the risk of transmission of infectious agents cannot be totally eliminated.
All infections thought by a physician possibly to have been transmitted by HAEGARDA should be reported by lot number, by the physician or other healthcare provider, to the CSL Behring Pharmacovigilance Department at 1-866-915-6958.
6. Adverse Reactions/Side Effects
Adverse reactions occurring in more than 4% of subjects treated with HAEGARDA were injection site reactions, hypersensitivity, nasopharyngitis and dizziness.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Of the 90 subjects randomized in the double-blind, placebo-controlled, cross-over study (Study 1) [see Clinical Studies (14)], 86 subjects received at least one dose of HAEGARDA and 86 subjects received at least one dose of placebo (Table 1). A total of 5081 injections of HAEGARDA and placebo were administered over a range of 3 to 19 weeks (median of 16.6 weeks for HAEGARDA; median of 16.3 weeks for placebo). Eligible patients were also able to participate in a randomized, open-label, active treatment-controlled study (Study 2) for up to 140 weeks (n=120).
| MedDRA System Organ Class | Adverse Reaction | HAEGARDA | Placebo (N=86) |
||
|---|---|---|---|---|---|
| 60 IU/kg (N=43) | 40 IU/kg (N=43) |
Overall* (N=86) |
|||
| n (%) | n (%) | n (%) | n (%) | ||
| N = number of subjects receiving the treatment; n = number of subjects experiencing ≥1 event. | |||||
|
|||||
| General Disorders and Administration Site Conditions | Injection Site Reaction† | 15 (35) | 12 (28) | 27 (31) | 21 (24) |
| Immune System Disorders | Hypersensitivity‡ | 3 (7) | 2 (5) | 5 (6) | 1 (1) |
| Infections and Infestations | Nasopharyngitis | 8 (19) | 1 (2) | 9 (11) | 6 (7) |
| Nervous System Disorders | Dizziness | 0 (0) | 4 (9) | 4 (5) | 1 (1) |
Of the injection site reactions occurring after treatment with HAEGARDA, 95% were of mild intensity and 83% resolved within 1 day after onset.
Overall, safety data from the open-label study (Study 2), consisting of 59 patients who participated in Study 1 and 61 patients who did not participate in Study 1 (n=120), were consistent with the safety data from the randomized, double-blind, placebo-controlled, crossover, routine prophylaxis trial (Study 1).
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of HAEGARDA were evaluated in a subgroup of nine patients 8 to <17 years of age, in the randomized, double-blind, placebo-controlled, crossover, routine prophylaxis trial (Study 1) and the randomized, open-label, active treatment-controlled study (Study 2). Results of subgroup analysis by age were consistent with overall study results.
8.5 Geriatric Use
The safety and effectiveness of HAEGARDA were evaluated in a subgroup of nine subjects 65 to 72 years of age, eight subjects who received the high 60 IU/kg dose and one subject who received the 40 IU/kg dose, in the randomized, double-blind, placebo-controlled, crossover, routine prophylaxis trial (Study 1) and in the randomized, open-label, active treatment-controlled study (Study 2). Clinical studies of HAEGARDA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10. Overdosage
No case of overdose has been reported. Doses corresponding to up to 117 IU/kg S.C. have been administered twice weekly in a fixed-dose clinical study.
11. Haegarda Description
HAEGARDA is a human plasma-derived, purified, pasteurized, lyophilized concentrate of C1-INH to be reconstituted for S.C. administration. HAEGARDA is prepared from large pools of human plasma from U.S. donors. The potency of C1-INH is expressed in International Units (IU), which is related to the current WHO Standard for C1-INH products.
Reconstituted HAEGARDA has a concentration of 500 IU/mL C1-INH, 65 mg/mL total protein, 10 mg/mL glycine, 8.5 mg/mL sodium chloride and 2.7 mg/mL sodium citrate.
12. Haegarda - Clinical Pharmacology
12.1 Mechanism of Action
C1-INH is a normal constituent of human plasma and belongs to the group of serine protease inhibitors (serpins) that includes antithrombin III, alpha1-protease inhibitor, alpha2-antiplasmin, and heparin cofactor II. As with the other inhibitors in this group, C1-INH has an important inhibiting potential on several of the major human cascade systems, including the complement, fibrinolytic and coagulation systems. Regulation of these systems is performed through the formation of complexes between the protease and the inhibitor, resulting in inactivation of both and consumption of the C1-INH.
C1-INH, which is usually activated during the inflammatory process, inactivates its substrate by covalently binding to the reactive site. C1-INH is the only known inhibitor for the C1r and C1s subcomponents of complement component 1 (C1), coagulation factor XIIa, and plasma kallikrein. Additionally, C1-INH is the main inhibitor for coagulation factor XIa of the intrinsic coagulation cascade.
HAE patients have absence or low levels of endogenous or functional C1-INH. Although the events that cause attacks of angioedema in HAE patients are not well defined, it has been postulated that increased vascular permeability and the clinical manifestation of HAE attacks may be primarily mediated through contact system activation. Suppression of contact system activation by C1-INH through the inactivation of plasma kallikrein and factor XIIa is thought to modulate this vascular permeability by preventing the generation of bradykinin. Administration of HAEGARDA replaces the missing or malfunctioning C1-INH protein in patients with HAE.
12.2 Pharmacodynamics
In untreated patients, insufficient levels of functional C1-INH lead to increased activation of C1, which results in decreased levels of complement component 4 (C4). The administration of HAEGARDA increases plasma levels of C1-INH in a dose-dependent manner and subsequently increases plasma concentrations of C4. The C4 plasma concentrations after S.C. administration of 60 IU/kg HAEGARDA were in the normal range (16 to 38 mg/dL).
12.3 Pharmacokinetics
The pharmacokinetics (PK) of C1-INH were described using population PK analysis.
The PK parameters of C1-INH following twice weekly subcutaneous 60 IU/kg dosing are shown in Table 3.
| Parameter | Mean | 95% CI |
|---|---|---|
|
||
| CL (mL/hr/kg)* | 1.03 | 0.90-1.17 |
| Vd (L/kg)* | 0.05 | 0.04-0.06 |
| Bioavailability % | 42.7 | 35.2-50.2 |
| Cmax % | 60.7† | 31.8-128‡ |
| Ctrough % | 48.0† | 25.1-102‡ |
| Tmax (hr) | 59§ | 23-134‡ |
| Half-life (hr)¶ | 69§ | 24-251‡ |
The steady state PK of S.C. C1-INH is independent of dose between 20-80 IU/kg in HAE subjects.
Studies have not been conducted to evaluate the PK of C1-INH in specific patient populations stratified by gender, race, or the presence of renal or hepatic impairment. Body weight was included as covariate in the population PK analysis of C1-INH in the age range of 8-72 years while age was not a statistically significant covariate. The body weight adjusted clearance is 11% and 10 % higher in children (8 to < 12 years old) and adolescents (12 to < 18 years old) as compared to adult subjects (18 to 72 years), respectively.
13. Nonclinical Toxicology
14. Clinical Studies
The efficacy and safety of HAEGARDA for routine prophylaxis to prevent HAE attacks were demonstrated in a multicenter, randomized, double-blind, placebo-controlled, crossover study (Study 1), and in a multicenter, randomized, open-label, active treatment-controlled study (Study 2).
15. References
- Martinez-Saguer I, Rusicke E, Aygören-Pürsün E, et al. Characterization of acute hereditary angioedema attacks during pregnancy and breast-feeding and their treatment with C1 inhibitor concentrate. Am J Obstet Gynecol. 2010;203:131.e1-7.
- Fox J, Vegh AB, Martinez-Saguer I, et al. Safety of a C1-inhibitor concentrate in pregnant women with hereditary angioedema. Allergy Asthma Proc. 2017;38(3):216-221
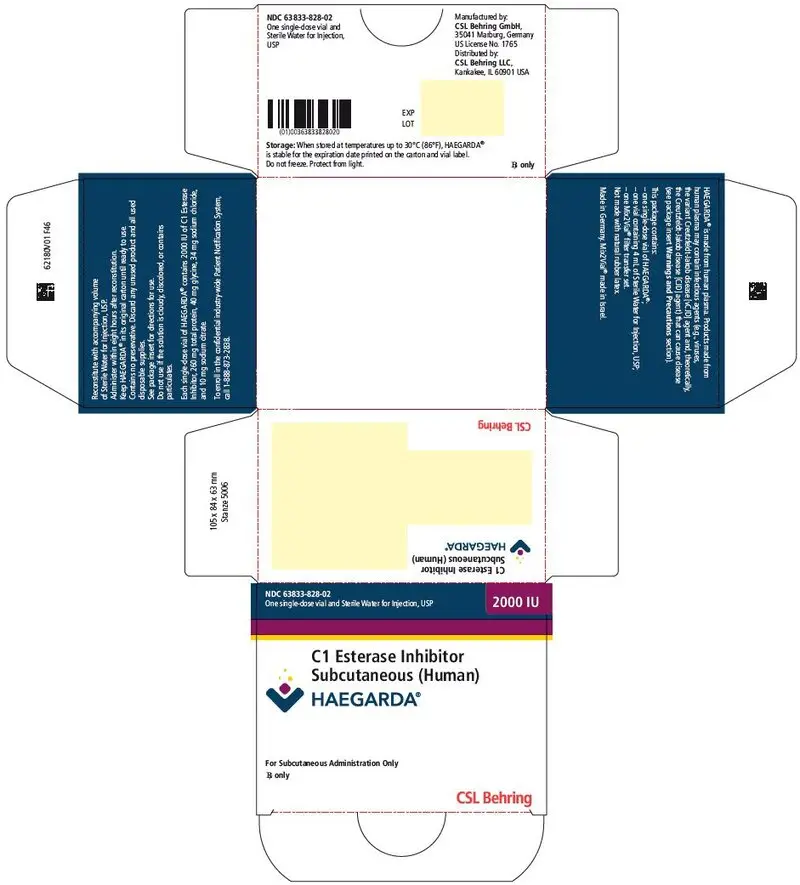
16. How is Haegarda supplied
HAEGARDA is supplied in a kit containing a lyophilized powder in a single-dose vial.
HAEGARDA is packaged with Sterile Water for Injection, USP (4 mL for reconstitution of 2000 IU or 5.6 mL for reconstitution of 3000 IU) and one Mix2Vial filter transfer set. Not made with natural rubber latex.
| Nominal Strength | Fill Size Color Indicator | Kit NDC |
|---|---|---|
| 2000 IU | Fuschia | 63833-828-02 |
| 3000 IU | Yellow | 63833-829-02 |
17. Patient Counseling Information
See FDA-approved patient labeling (Patient Product Information).
All risks and benefits of HAEGARDA should be discussed with the patient/caregiver before prescribing or administering it to the patient.
FDA-Approved Patient Labeling – Patient Product Information (PPI)
HAEGARDA (hay-GAR-duh)
C1 Esterase Inhibitor Subcutaneous (Human)
Freeze-Dried Powder for Reconstitution
This leaflet summarizes important information about HAEGARDA. Please read it carefully before using HAEGARDA and each time you get a refill. There may be new information provided. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about HAEGARDA. If you have any questions after reading this, ask your healthcare provider.
Do not attempt to self-administer unless you have been taught how by your healthcare provider.
What is HAEGARDA?
HAEGARDA is an injectable medicine used to prevent swelling and/or painful attacks in patients 6 years of age and older with Hereditary Angioedema (HAE). HAE is caused by the poor functioning or lack of a protein called C1 that is present in your blood and helps control inflammation (swelling) and parts of the immune system. HAEGARDA contains C1 esterase inhibitor (C1-INH), a protein that helps control C1.
HAEGARDA should not be used to treat an acute HAE attack. In case of an acute HAE attack, initiate individualized treatment as discussed with your prescribing health care professional.
Who should not use HAEGARDA?
You should not use HAEGARDA if you have experienced life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product.
What should I tell my healthcare provider before using HAEGARDA?
Tell your healthcare provider about all of your medical conditions, including if you:
- Are pregnant or planning to become pregnant. It is not known if HAEGARDA can harm your unborn baby.
- Are breastfeeding or plan to breastfeed. It is not known if HAEGARDA passes into your milk and if it can harm your baby.
- Have a history of blood clotting problems. Blood clots have occurred in patients receiving HAEGARDA. Very high doses of C1-INH could increase the risk of blood clots. Tell your healthcare provider if you have a history of heart or blood vessel disease, stroke, blood clots, or have thick blood, an indwelling catheter/access device in one of your veins, or have been immobile for some time. These things may increase your risk of having a blood clot after using HAEGARDA. Also, tell your healthcare provider what drugs you are using, as some drugs, such as birth control pills or certain androgens, may increase your risk of developing a blood clot.
Tell your healthcare provider and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines such as over-the-counter medicines, supplements, or herbal remedies.
What are the possible side effects of HAEGARDA?
Allergic reactions may occur with HAEGARDA. Call your healthcare provider or seek emergency support services right away if you have any of the following symptoms after using HAEGARDA:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- rash or hives
Signs of a blood clot include:
- pain and/or swelling of an arm or leg with warmth over the affected area
- discoloration of an arm or leg
- unexplained shortness of breath
- chest pain or discomfort that worsens on deep breathing
- unexplained rapid pulse
- numbness or weakness on one side of the body
Because HAEGARDA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
The most common side effects with HAEGARDA are injection site reactions (pain, redness, swelling), hypersensitivity (itching and rash), nasopharyngitis (runny or stuffy nose, sneezing, watery eyes) and dizziness.
These are not all the possible side effects of HAEGARDA.
Tell your healthcare provider about any side effect that bothers you or that does not go away. You can also report side effects to the FDA at 1-800-FDA-1088.
How should I store HAEGARDA?
- Keep the non-reconstituted HAEGARDA in its original carton to protect from light until ready to use.
- When stored at temperatures up to 30°C (86°F), HAEGARDA is stable for the period indicated by the expiration date on the carton and vial label.
- Do not freeze.
What else should I know about HAEGARDA?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use HAEGARDA for a condition for which it is not prescribed. Do not share HAEGARDA with other people, even if they have the same symptoms that you have.
This leaflet summarizes the most important information about HAEGARDA. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about HAEGARDA that was written for healthcare professionals. For more information, go to www.HAEGARDA.com or call 1-877-236-4423.
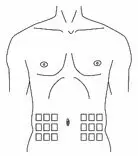
What should I know about self-administration?
- You should prepare the prescribed dose of HAEGARDA for self-administration as directed by your healthcare provider.
| HAEGARDA
C1 ESTERASE INHIBITOR SUBCUTANEOUS (HUMAN)
human c1-esterase inhibitor kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| HAEGARDA
C1 ESTERASE INHIBITOR SUBCUTANEOUS (HUMAN)
human c1-esterase inhibitor kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - CSL Behring GmbH (326530474) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring GmbH | 326530474 | MANUFACTURE | |