Drug Detail:Halcion (Triazolam [ trye-ay-zoe-lam ])
Drug Class: Benzodiazepines
Highlights of Prescribing Information
HALCION® (triazolam) tablets, for oral use, CIV
Initial U.S. Approval: 1982
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
See full prescribing information for complete boxed warning.
- •
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (5.1, 7.1).
- •
- The use of benzodiazepines, including Halcion, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing Halcion and throughout treatment, assess each patient's risk for abuse, misuse, and addiction (5.2).
- •
- Abrupt discontinuation or rapid dosage reduction of Halcion after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Halcion or reduce the dosage (2.3, 5.3).
Recent Major Changes
|
Warnings and Precautions (5.10) |
1/2023 |
Indications and Usage for Halcion
Halcion is a benzodiazepine indicated for the short-term treatment of insomnia (generally 7 to 10 days) in adults. (1)
Halcion Dosage and Administration
- •
- Adults: Recommended dosage is 0.25 mg once daily before bedtime. Maximum recommended dosage is 0.5 mg once daily (2.1)
- •
- Geriatric patients: Reduce starting dosage to 0.125 mg once daily. May increase to 0.25 mg if no response. Geriatric patients should not exceed 0.25 mg once daily (2.2, 8.5)
- •
- Halcion should not be prescribed in quantities exceeding a 1-month supply (2.1)
Dosage Forms and Strengths
Scored tablets: 0.25 mg (3)
Contraindications
- •
- Known hypersensitivity to Halcion or other benzodiazepines (4)
- •
- Concomitant use with medications that significantly impair the oxidative metabolism mediated by cytochrome P450 3A (CYP 3A) including ketoconazole, itraconazole, nefazodone, and several human immunodeficiency virus (HIV) protease inhibitors (4, 5.8, 17)
Warnings and Precautions
- •
- Persistent or Worsening Insomnia: Since sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. (5.4)
- •
- "Sleep-driving" and Other Complex Behaviors: Complex behaviors such as "sleep-driving" have been reported. The use of alcohol and other central nervous system (CNS) depressants with sedative-hypnotics appears to increase the risk, as well as doses exceeding the maximum recommended dose. (5.5)
- •
- CNS Manifestations: An increase in daytime anxiety, abnormal thinking, and behavioral changes have been reported. Emergence of any new behavioral changes require careful and immediate evaluation. (5.6)
- •
- Effects on Driving and Operating Heavy Machinery: Patients receiving triazolam should be cautioned against driving or operating heavy machinery, as well as avoiding concomitant use with alcohol and other CNS depressant drugs. (5.7)
- •
- Patients with Depression: Caution should be exercised in patients with signs or symptoms of depression that could be intensified by hypnotic drugs. Prescribe the least number of tablets feasible to avoid intentional overdose. (5.9)
- •
- Neonatal Sedation and Withdrawal Syndrome: Halcion use during pregnancy can result in neonatal sedation and/or neonatal withdrawal. (5.10, 8.1)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥4% and twice placebo) are drowsiness, dizziness, light-headedness, and coordination disorder/ataxia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Use with Opioids: Increase the risk of respiratory depression (7.1)
- •
- Use with Other CNS Depressants: Produces additive CNS depressant effects (7.1)
- •
- Use with CYP 3A4 Inhibitors: Increased risk of adverse reactions (4, 5.8, 7.1)
Use In Specific Populations
Lactation: A lactating woman may pump and discard breast milk during treatment and for 28 hours after HALCION administration (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2023
Full Prescribing Information
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- •
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- •
- The use of benzodiazepines, including Halcion, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing Halcion and throughout treatment, assess each patient's risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- •
- The continued use of benzodiazepines, including Halcion, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of Halcion after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Halcion or reduce the dosage [see Dosage and Administration (2.3), Warnings and Precautions (5.3)].
1. Indications and Usage for Halcion
Halcion is indicated for the short-term treatment of insomnia (generally 7 to 10 days) in adults.
2. Halcion Dosage and Administration
2.1 Dosing Information
The recommended dosage is 0.25 mg once daily before bedtime. A dosage of 0.125 mg once daily may be sufficient for some patients (e.g., patients with low body weight). A dosage of 0.5 mg should be used only for patients who do not respond adequately to a trial of a lower dose. The maximum recommended dosage is 0.5 mg once daily.
Use the lowest effective dose for the patient as there are significant dose related adverse reactions.
Use of Halcion for more than 3 weeks requires evaluation of the patient for a primary psychiatric or medical condition [see Warnings and Precautions (5.4, 5.6)].
Prescriptions for Halcion should be written for short-term use (7 to 10 days) and it should not be prescribed in quantities exceeding a 1-month supply.
2.2 Use in Geriatric Patients
In geriatric patients, the recommended dosage is 0.125 mg to 0.25 mg once daily. Initiate therapy at 0.125 mg once daily. The 0.25 mg dose should be used only for patients who do not respond to a trial of the lower dose. The maximum recommended dosage is 0.25 mg once daily. Elderly patients have an increased risk of dose related adverse reactions [see Use in Specific Populations (8.5)].
2.3 Discontinuation or Dosage Reduction of Halcion
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Halcion or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
3. Dosage Forms and Strengths
Halcion is available as 0.25 mg tablets. The tablets are powder blue, elliptical, scored, and imprinted with HALCION 0.25.
4. Contraindications
Halcion is contraindicated in:
- •
- Patients with known hypersensitivity to triazolam, any of component of Halcion, or other benzodiazepines. Reactions consistent with angioedema (involving the tongue, glottis, or larynx), dyspnea, and throat closing have been reported and may be fatal.
- •
- Concomitant administration of strong cytochrome P450 (CYP 3A) enzyme inhibitors (e.g., ketoconazole, itraconazole, nefazodone, lopinavir, ritonavir) [see Warnings and Precautions (5.8), Drug Interactions (7.1)].
5. Warnings and Precautions
5.1 Risks From Concomitant Use With Opioids
Concomitant use of benzodiazepines, including Halcion, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe Halcion concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of Halcion than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking Halcion, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when Halcion is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined [see Drug Interactions (7.1)].
5.2 Abuse, Misuse, and Addiction
The use of benzodiazepines, including Halcion, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death [see Drug Abuse and Dependence (9.2)].
Before prescribing Halcion and throughout treatment, assess each patient's risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of Halcion, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of Halcion along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
5.3 Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Halcion or reduce the dosage (a patient-specific plan should be used to taper the dose) [see Dosage and Administration (2.3)].
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including Halcion, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of Halcion after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) [see Drug Abuse and Dependence (9.3)].
5.4 Persistent or Worsening Insomnia
Since sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs.
5.5 "Sleep-driving" and Other Complex Behaviors
Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported with Halcion use. These events can occur in sedative-hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with sedative-hypnotics alone at recommended dosages, the use of alcohol and other central nervous system (CNS) depressants with sedative-hypnotics appears to increase the risk of such behaviors, as does the use of sedative-hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of sedative-hypnotics should be strongly considered for patients who report a "sleep-driving" episode.
Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic, including Halcion. As with sleep-driving, patients usually do not remember these events.
5.6 Central Nervous System Manifestations
An increase in daytime anxiety has been reported for Halcion after as few as 10 days of continuous use. In some patients this may be a manifestation of interdose withdrawal. If increased daytime anxiety is observed during treatment, discontinuation of treatment may be advisable.
A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of benzodiazepine hypnotics including Halcion. Some of these changes may be characterized by decreased inhibition, e.g., aggressiveness and extroversion that seem excessive, similar to that seen with alcohol and other CNS depressants (e.g., sedative/hypnotics). Other kinds of behavioral changes have also been reported, for example, bizarre behavior, agitation, hallucinations, depersonalization. In primarily depressed patients, the worsening of depression, including suicidal thinking, has been reported in association with the use of benzodiazepines [see Warnings and Precautions (5.9)].
Some adverse reactions reported in association with the use of Halcion such as drowsiness, dizziness, light-headedness, and amnesia appear to be dose related. More serious behavioral phenomena such as confusion, bizarre or abnormal behavior, agitation, and hallucinations may also be dose related, but this evidence is inconclusive. Therapy should be initiated at the lowest effective dose [see Dosage and Administration (2.1)].
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Anterograde amnesia of varying severity and paradoxical reactions have been reported following recommended dosages of Halcion. Data from several sources suggest that anterograde amnesia may occur at a higher rate with Halcion than with other benzodiazepine hypnotics. Because HALCION can cause drowsiness and a decreased level of consciousness, patients, particularly the elderly, are at higher risk of falls.
Cases of "traveler's amnesia" have been reported by individuals who have taken Halcion to induce sleep while traveling, such as during an airplane flight. In some of these cases, insufficient time was allowed for the sleep period prior to awakening and before beginning activity. Also, the concomitant use of alcohol may have been a factor in some cases.
5.7 Effects on Driving and Operating Heavy Machinery
Due to its depressant CNS effects, patients receiving Halcion should be cautioned against engaging in hazardous occupations requiring complete mental alertness such as operating machinery or driving a motor vehicle. For the same reason, patients should be cautioned about the concomitant use of alcohol and other CNS depressant drugs during treatment with Halcion.
5.8 Triazolam Interaction With Drugs That Inhibit Metabolism via Cytochrome P450 3A
The initial step in triazolam metabolism is hydroxylation catalyzed by CYP 3A. Drugs that inhibit this metabolic pathway may have a profound effect on the clearance of triazolam.
Strong CYP 3A Inhibitors
Halcion is contraindicated in patients receiving strong inhibitors of CYP 3A such as ketoconazole, itraconazole, nefazodone, ritonavir, indinavir, nelfinavir, saquinavir, and lopinavir [see Contraindications (4), Drug Interactions (7.1)].
Moderate and Weak CYP 3A Inhibitors
Halcion should be used with caution in patients receiving moderate or weak inhibitors of CYP 3A. If coadministered, consider dose reduction of Halcion.
Macrolide Antibiotics
Coadministration of erythromycin increased the maximum plasma concentration, decreased clearance and increased half-life of triazolam [see Drug Interactions (7.1), Clinical Pharmacology (12.3)]; caution and consideration of appropriate triazolam dose reduction are recommended. Similar caution should be observed during coadministration with clarithromycin and other macrolide antibiotics.
5.9 Patients With Depression
Benzodiazepines may worsen depression. Consequently, appropriate precautions (e.g., limiting the total prescription size and increased monitoring for suicidal ideation) should be considered in patients with depression.
5.10 Neonatal Sedation and Withdrawal Syndrome
Use of Halcion late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [see Use in Specific Populations (8.1)]. Monitor neonates exposed to HALCION during pregnancy or labor for signs of sedation and monitor neonates exposed to HALCION during pregnancy for signs of withdrawal; manage these neonates accordingly.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections:
- •
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- •
- Abuse, Misuse, and Addiction [see Warnings and Precautions (5.2)]
- •
- Dependence and Withdrawal Reactions [see Warnings and Precautions (5.3)]
- •
- Persistent or Worsening Insomnia [see Warnings and Precautions (5.4)]
- •
- "Sleep-driving" and Other Complex Behaviors [see Warnings and Precautions (5.5)]
- •
- Central Nervous System Manifestations [see Warnings and Precautions (5.6)]
- •
- Effects on Driving and Operating Heavy Machinery [see Warnings and Precautions (5.7)]
- •
- Patients with Depression [see Warnings and Precautions (5.9)]
- •
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.10)]
- •
- Compromised Respiratory Function [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The incidences cited below are estimates of clinical reactions among 1003 subjects who participated in the short term (duration of 1 to 42 days) placebo-controlled clinical trials of Halcion.
Adverse reactions leading to discontinuation in two multi-dose placebo controlled clinical trials include coordination disorders, drowsiness, grogginess, somnolence, depression, restlessness, dizziness, lightheadedness, headache, nausea, visual disturbance, nervousness, abdominal distress, bladder trouble, aching limbs, backache, and blepharitis.
| Event | Halcion
(N=1003) % Patients Reporting | Placebo
(N=997) % Patients Reporting |
|---|---|---|
|
Central Nervous System | ||
|
Drowsiness |
14.0 |
6.4 |
|
Headache |
9.7 |
8.4 |
|
Dizziness |
7.8 |
3.1 |
|
Nervousness |
5.2 |
4.5 |
|
Light-headedness |
4.9 |
0.9 |
|
Coordination disorders/ataxia |
4.6 |
0.8 |
|
Gastrointestinal | ||
|
Nausea/vomiting |
4.6 |
3.7 |
In addition to the common reactions enumerated above in Table1, the following adverse reactions have been reported at an incidence of 0.9% to 0.5%: euphoria, tachycardia, tiredness, confusional states/memory impairment, cramps/pain, depression, and visual disturbances.
Adverse reactions reported at an incidence less than 0.5% include: constipation, taste alterations, diarrhea, dry mouth, dermatitis/allergy, dreaming/nightmares, insomnia, paresthesia, tinnitus, dysesthesia, weakness, congestion, and death from hepatic failure in a patient also receiving diuretic drugs.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Halcion. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders and administration site conditions: Paradoxical drug reaction, chest pain and fatigue
Gastrointestinal disorders: Tongue discomfort, glossitis, stomatitis
Hepatobiliary disorders: Jaundice
Injury, poisoning and procedural complications: Fall
Metabolism and nutrition disorders: Anorexia
Nervous system disorders: Anterograde amnesia, altered state of consciousness, dystonia, sedation, syncope, dysarthria and muscle spasticity
Psychiatric disorders: Confusional state (disorientation, derealisation, depersonalization), mania, agitation, restlessness, irritability, sleep disorder and libido disorder, hallucination, delusion, aggression, somnambulism, and abnormal behavior
Renal and urinary disorders: Urinary retention and urinary incontinence
Reproductive system and breast disorders: Menstruation irregular
Skin and subcutaneous tissue disorders: Pruritis
7. Drug Interactions
7.1 Drugs Having Clinically Important Interactions With Halcion
Table 2 includes clinically significant drug interactions with Halcion [see Clinical Pharmacology (12.3)].
|
Opioids |
|
|
Clinical implication |
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. |
|
Prevention or management |
Limit dosage and duration of concomitant use of Halcion and opioids, and monitor patients closely for respiratory depression and sedation [see Warnings and Precautions (5.1)]. |
|
CNS Depressants |
|
|
Clinical implication |
Triazolam produces additive CNS depressant effects when co-administered with other CNS depressants. |
|
Prevention or management |
Limit dosage and duration of Halcion during concomitant use with CNS depressants. |
|
Strong Inhibitors of CYP 3A |
|
|
Clinical implication |
Concomitant use of Halcion with strong CYP3A inhibitors has a profound effect on the clearance of Halcion, resulting in increased concentrations of triazolam and increased risk of adverse reactions [see Clinical Pharmacology (12.3)]. |
|
Prevention or management |
Do not administer Halcion with a strong CYP3A4 inhibitor [see Contraindications (4), Warnings and Precautions (5.8)]. |
|
Moderate and Weak Inhibitors of CYP 3A |
|
|
Clinical implication |
Concomitant use of Halcion with moderate or weak inhibitors of CYP3A inhibitors may increase the concentrations of Halcion, resulting in increased risk of adverse reactions [see Clinical Pharmacology (12.3)]. |
|
Prevention or management |
Use with caution and consider appropriate dose reduction of HALCION when coadministered with moderate and weak CYP3A inhibitors [see Warnings and Precautions (5.8)]. |
|
Strong Inducers of CYP 3A |
|
|
Clinical implication |
Coadministration of triazolam with strong inducers of CYP3A4 can significantly decrease the plasma concentration of triazolam and may decrease effectiveness of triazolam. |
|
Prevention or management |
Caution is recommended during coadministration of Halcion with strong inducers of CYP3A4. |
|
Interactions Based on Experience with Other Benzodiazepines or in vitro Studies with Triazolam |
|
|
Clinical implication |
Available data from clinical studies of benzodiazepines other than triazolam, from in vitro studies with triazolam, or from in vitro studies with benzodiazepines other than triazolam suggest a possible drug interaction with triazolam [see Clinical Pharmacology (12.3)]. |
|
Prevention or management |
Caution is recommended during coadministration of Halcion with any of these drugs. [see Warnings and Precautions (5.8)]. |
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including Halcion, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/
Risk Summary
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia, and sedation in neonates. Monitor neonates exposed to Halcion during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to HALCION during pregnancy for signs of withdrawal. Manage these neonates accordingly [see Warnings and Precautions (5.10)].
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data
Oral administration of triazolam to pregnant rats and rabbits during the period of organogenesis caused skeletal developmental changes (variations and malformations) at maternally toxic doses in rats and at doses in rats and rabbits which are approximately equal to or greater than 200 times the maximum recommended human dose (MRHD) of 0.5 mg/day based on mg/m2 body surface area. Oral administration of triazolam to male and female rats before mating, and continuing during gestation and lactation did not result in embryotoxicity at doses up to approximately 100 times the MRHD based on mg/m2 body surface area, but did cause an increase in the number of stillbirths and postnatal pup mortalities at doses greater than or equal to approximately 40 times the MRHD based mg/m2 body surface area. 14C-triazolam was administered orally to pregnant mice. Drug-related material appeared uniformly distributed in the fetus with 14C concentrations approximately the same as in the brain of the mother.
8.2 Lactation
Risk Summary
There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. There are no data on the presence of triazolam in human milk or the effects on milk production. Triazolam and its metabolites are present in the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for HALCION and any potential adverse effects on the breastfed infant from HALCION or from the underlying maternal condition.
Clinical Considerations
Infants exposed to HALCION through breast milk should be monitored for sedation, poor feeding and poor weight gain. A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk during treatment and for 28 hours (approximately 5 elimination half-lives) after HALCION administration in order to minimize drug exposure to a breast fed infant.
8.4 Pediatric Use
Safety and effectiveness of Halcion have not been established in pediatric patients.
8.5 Geriatric Use
Elderly patients exhibit higher plasma triazolam concentrations due to reduced clearance as compared with younger subjects at the same dose. Because elderly patients are especially susceptible to dose related adverse reactions and to minimize oversedation, the smallest effective dose should be used [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
9. Drug Abuse and Dependence
9.2 Abuse
Halcion is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders [see Warnings and Precautions (5.2)].
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
9.3 Dependence
Physical Dependence
Halcion may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use [see Warnings and Precautions (5.3)].
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Halcion or reduce the dosage [see Dosage and Administration (2.3), Warnings and Precautions (5.3)].
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to Halcion may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of Halcion may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
10. Overdosage
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help line at (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
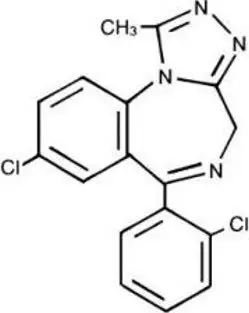
11. Halcion Description
Halcion Tablets contains triazolam, a triazolobenzodiazepine.
Triazolam is a white crystalline powder, soluble in alcohol and poorly soluble in water. It has a molecular weight of 343.21.
The chemical name for triazolam is 8-chloro-6-(o-chlorophenyl)-1-methyl-4H-s-triazolo-[4,3-α] [1,4] benzodiazepine.
The structural formula is represented below:
Each Halcion tablet, for oral administration, contains 0.25 mg of triazolam.
Inactive ingredients: cellulose, corn starch, docusate sodium, FD&C Blue No. 2, lactose, magnesium stearate, silicon dioxide, sodium benzoate.
12. Halcion - Clinical Pharmacology
12.1 Mechanism of Action
Triazolam is a benzodiazepine. Triazolam exerts its effect for the short-term treatment of insomnia through binding to the benzodiazepine site of the gamma-aminobutyric acid-A (GABAA) receptors in the brain and enhances GABA-mediated synaptic inhibition.
12.3 Pharmacokinetics
Absorption
Peak plasma levels of triazolam are reached within 2 hours following oral administration. Following recommended doses of Halcion, triazolam peak plasma levels in the range of 1 to 6 ng/mL are seen. The plasma levels achieved are proportional to the dose given. In normal subjects treated for 7 days with four times the recommended dosage, there was no evidence of altered systemic bioavailability, rate of elimination, or accumulation.
Distribution
Extremely high concentrations of triazolam do not displace bilirubin bound to human serum albumin in vitro.
Elimination
Triazolam has a mean plasma elimination half-life in the range of 1.5 to 5.5 hours.
Metabolism
The initial step in triazolam metabolism is cytochrome P450 3A (CYP 3A)-mediated hydroxylation to form 1-hydroxytriazolam and 4-hydroxytriazolam, which are subsequently conjugated to form glucuronides.
Excretion
Triazolam and its metabolites, principally as conjugated glucuronides which are presumably inactive, are excreted primarily in the urine. Only small amounts of unmetabolized triazolam appear in the urine. The two primary metabolites accounted for 79.9% of urinary excretion. Urinary excretion appeared to be biphasic in its time course.
Specific Populations
Geriatric Patients
In a study of elderly (62 to 83 years old) versus younger subjects (21 to 41 years old) who received triazolam at the same dose levels (0.125 mg and 0.25 mg), the elderly experienced both greater sedation and impairment of psychomotor performance. These effects resulted largely from higher plasma concentrations of triazolam in the elderly.
Drug Interaction Studies
The effect of other drugs on triazolam:
Macrolide Antibiotics
Coadministration of erythromycin increased the maximum plasma concentration of triazolam by 46%, decreased clearance by 53%, and increased half-life by 35%.
Cimetidine
Coadministration of cimetidine increased the maximum plasma concentration of triazolam by 51%, decreased clearance by 55%, and increased half-life by 68%.
Isoniazid
Coadministration of isoniazid increased the maximum plasma concentration of triazolam by 20%, decreased clearance by 42%, and increased half-life by 31%.
Oral Contraceptives
Coadministration of oral contraceptives increased maximum plasma concentration by 6%, decreased clearance by 32%, and increased half-life by 16%.
Grapefruit Juice
Coadministration of grapefruit juice increased the maximum plasma concentration of triazolam by 25%, increased the area under the concentration curve by 48%, and increased half-life by 18%.
Ranitidine
Coadministration of ranitidine increased the maximum plasma concentration of triazolam by 30%, increased the area under the concentration curve by 27%, and increased half-life by 3.3%. Caution is recommended during coadministration with triazolam. Available data from clinical studies of benzodiazepines other than triazolam suggest a possible drug interaction with triazolam for the following: fluvoxamine, diltiazem, and verapamil. Data from in vitro studies of triazolam suggest a possible drug interaction with triazolam for the following: sertraline and paroxetine. Data from in vitro studies of benzodiazepines other than triazolam suggest a possible drug interaction with triazolam for the following: ergotamine, cyclosporine, amiodarone, nicardipine, and nifedipine.
The effect of triazolam on other drugs:
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenic potential was observed in rats or mice administered triazolam in the diet for 24-months at doses greater than or equal to 900 times the MRHD of 0.5 mg, based on mg/m2 body surface area.
Mutagenesis
Triazolam was not mutagenic in the in vitro Ames bacterial reverse mutation assay, and no DNA damage was observed in an in vitro alkaline elution assay in Chinese hamster lung fibroblast cells.
Impairment of Fertility
Female rats were administered triazolam in the diet for 14 days before cohabitation, during gestation, and until 21 days post parturition, and male rats for 60 days before cohabitation. No effects on mating or fertility were observed in rats up to 5 mg/kg/day which is approximately 100 times the MRHD of 0.5 mg/day, based on mg/m2 body surface area.
16. How is Halcion supplied
Halcion is supplied as a powder blue, elliptical, scored tablet in the following strengths and package configurations:
| Package Configuration | Tablet Strength (mg) | NDC | |
|---|---|---|---|
|
Reverse numbered |
0.25 mg |
NDC 0009-0017-55 |
HALCION 0.25 |
|
Bottles of 10 |
0.25 mg |
NDC 0009-0017-58 |
HALCION 0.25 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risks from Concomitant Use with Opioids
Advise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when Halcion is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Abuse, Misuse, and Addiction
Inform patients that the use of Halcion, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug [see Warnings and Precautions (5.2), Drug Abuse and Dependence (9.2)].
Withdrawal Reactions
Inform patients that the continued use of Halcion may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of Halcion may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of Halcion may require a slow taper [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
"Sleep-driving" and Other Complex Behaviors
There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. Advise patients to report similar experiences to their healthcare provider immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other CNS depressants [see Warnings and Precautions (5.5)]. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative hypnotic. As with sleep-driving, patients usually do not remember these events.
Advise patients that increased drowsiness and decreased consciousness may increase the risk of falls in some patients.
Effects on Driving and Operating Heavy Machinery
Caution patients against driving a motor vehicle or operating heavy machinery until the effects of taking Halcion are determined due to its CNS depressant effects. Also advise patients to avoid the use of alcohol or other CNS depressants while taking Halcion [see Warnings and Precautions (5.7)].
Patients with Depression
Advise patients, their families and caregivers to look out for any signs of suicidality or worsening depression, and to inform the patient's prescriber or healthcare provider immediately [see Warnings and Precautions (5.9)].
Concomitant Medications
Advise patients to inform their healthcare provider of all medicines they take, including prescription and nonprescription medicines, vitamins and herbal supplements [see Drug Interactions (7.1)].
Grapefruit Juice
Advise patients to avoid eating grapefruit or drinking grapefruit juice while taking Halcion [see Drug Interactions (7.1)].
Pregnancy
Advise pregnant females that use of HALCION late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns [see Warnings and Precautions (5.10), Use in Specific Populations (8.1)]. Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to HALCION during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients using HALCION to monitor infants for excessive sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs. A lactating woman may consider pumping and discarding breastmilk during treatment and for 28 hours after Halcion administration to minimize drug exposure to a breastfed infant [see Use in Specific Populations (8.2)].
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
LAB-0666-13.0
| MEDICATION GUIDE
HALCION (HAL-cee-on) (triazolam) tablets, CIV |
||
|---|---|---|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised 1/2023 |
|
|
What is the most important information I should know about HALCION?
|
||
|
|
|
|
Call your healthcare provider right away if you find out that you have done any of the above activities after taking HALCION. |
||
|
What is HALCION? HALCION is a prescription medicine used in adults for the short-term treatment of a sleep problem called insomnia. HALCION is usually taken for 7 to 10 days.
|
||
|
Do not take HALCION if you:
|
||
|
Before you take HALCION, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking HALCION with certain other medicines can cause side effects or affect how well HALCION or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider. |
||
|
How should I take HALCION?
|
||
|
What are the possible side effects of HALCION? HALCION may cause serious side effects, including:
|
||
|
The most common side effects of HALCION include: |
||
|
|
|
|
Elderly people have an increased risk of dose related side effects during treatment with HALCION. These are not all the possible side effects of HALCION. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store HALCION?
|
||
|
General information about the safe and effective use of HALCION. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use HALCION for a condition for which it was not prescribed. Do not give HALCION to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about HALCION that is written for healthcare professionals. |
||
|
What are the ingredients in HALCION? Active ingredient: triazolam Inactive ingredients: cellulose, corn starch, docusate sodium, FD&C Blue No. 2, lactose, magnesium stearate, silicon dioxide, sodium benzoate.  If you would like more information, call 1-800-438-1985 or visit www.pfizer.com. LAB-0259-18.0 |
||
PRINCIPAL DISPLAY PANEL - 0.25 mg Tablet Blister Pack
Halcion®
triazolam tablets, USP
CIV
0.25 mg
Distributed by
Pharmacia & Upjohn Co
Div of Pfizer Inc,
NY, NY 10017
EXP &
LOT AREA
| HALCION
triazolam tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Pharmaceuticals LLC | 829084552 | PACK(0009-0017) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-0017) , API MANUFACTURE(0009-0017) , PACK(0009-0017) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Italia S.r.l. | 458521908 | ANALYSIS(0009-0017) , MANUFACTURE(0009-0017) , PACK(0009-0017) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Pharmaceuticals LLC | 829084545 | ANALYSIS(0009-0017) , MANUFACTURE(0009-0017) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 986019327 | ANALYSIS(0009-0017) , MANUFACTURE(0009-0017) | |