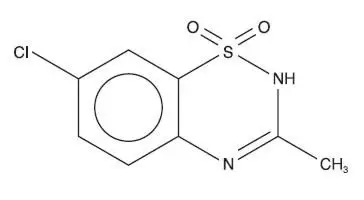
Drug Detail:Diazoxide (Diazoxide (oral) [ dye-az-ox-ide ])
Drug Class: Agents for hypertensive emergencies Glucose elevating agents
Hyperstat - Clinical Pharmacology
HYPERSTAT I.V. Injection produces a prompt reduction of blood pressure in man by relaxing smooth muscle in the peripheral arterioles. Cardiac output is increased as blood pressure is reduced. Studies in animals demonstrate that coronary blood flow is maintained, while renal blood flow is increased after an initial decrease.
Transient hyperglycemia occurs in the majority of patients treated with HYPERSTAT, but usually requires treatment only in patients with diabetes mellitus. It will respond to the usual management measures, including insulin.
Blood glucose levels should be monitored, especially in patients with diabetes and in those requiring multiple injections of diazoxide. Cataracts have been observed in a few animals receiving repeated daily doses of intravenous diazoxide.
Since diazoxide causes sodium retention, repeated injections may precipitate edema and congestive heart failure. Increased volume of extracellular fluid may be a cause of treatment failure in nonresponsive patients. The increase in fluid volume characteristically responds to diuretic agents if adequate renal function exists. Concurrently administered thiazide diuretics may be expected to potentiate the antihypertensive and hyperuricemic actions of diazoxide. (See Drug Interactions.)
Diazoxide is extensively bound to serum protein (>90%). The plasma half-life is 28 ± 8.3 hours; however, the duration of its antihypertensive effect is variable, generally lasting less than 12 hours.
Indications and Usage for Hyperstat
HYPERSTAT I.V. Injection is indicated for short-term use in the emergency reduction of blood pressure in severe, nonmalignant and malignant hypertension in hospitalized adults; and in acute severe hypertension in hospitalized children, when prompt and urgent decrease of diastolic pressure is required. Treatment with orally effective antihypertensive agents should not be instituted until blood pressure has stabilized. The use of HYPERSTAT I.V. Injection for longer than 10 days is not recommended.
HYPERSTAT I.V. Injection is ineffective against hypertension due to pheochromocytoma.
Precautions
Drug Interactions
Diazoxide is highly bound to serum protein. It can be expected to displace other substances which are also bound to protein, such as bilirubin or coumarin and its derivatives; resulting in higher blood levels of these substances.
An undesirable hypotension may result when diazoxide is administered to patients who have received other antihypertensive medication within 6 hours.
One patient in a clinical study exhibited excessive hypotension after concomitant administration of HYPERSTAT with hydralazine and methyldopa. An episode of maternal hypotension and fetal bradycardia occurred in a patient in labor who received both reserpine and hydralazine prior to administration of diazoxide. Neonatal hyperglycemia following intrapartum administration of HYPERSTAT I.V. Injection has also been reported.
HYPERSTAT I.V. Injection should not be administered within 6 hours of the administration of: hydralazine, reserpine, alphaprodine, methyldopa, beta-blockers, prazosin, minoxidil, the nitrites and other papaverine-like compounds.
Concomitant administration with thiazides or other commonly used diuretics may be expected to potentiate the hyperuricemic and antihypertensive effects of diazoxide.
| HYPERSTAT
diazoxide injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Schering Corporation |