Drug Detail:Hysingla er (Hydrocodone (oral) [ hye-droe-koe-done ])
Drug Class: Opioids (narcotic analgesics)
Highlights of Prescribing Information
HYSINGLA® ER (hydrocodone bitartrate) extended-release tablets, for oral use, CII
Initial U.S. Approval: 1943
WARNING: ADDICTION, ABUSE, AND MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; NEONATAL OPIOID WITHDRAWAL SYNDROME; CYTOCHROME P450 3A4 INTERACTION; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
See full prescribing information for complete boxed warning.
- HYSINGLA ER exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient’s risk before prescribing, and monitor regularly for these behaviors and conditions. (5.1)
- To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products. (5.2)
- Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially upon initiation or following a dose increase. Instruct patients to swallow HYSINGLA ER whole to avoid exposure to a potentially fatal dose of hydrocodone. (5.3)
- Accidental ingestion of HYSINGLA ER, especially by children, can result in fatal overdose of hydrocodone. (5.3)
- Prolonged use of HYSINGLA ER during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. (5.4)
- Concomitant use with CYP3A4 inhibitors (or discontinuation of CYP3A4 inducers) can result in a fatal overdose of hydrocodone. (5.5, 7, 12.3)
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation. (5.6, 7)
Recent Major Changes
Indications and Usage for Hysingla ER
HYSINGLA ER is an opioid agonist indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. (1)
Limitations of Use
- Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, and because of the greater risks of overdose and death with extended-release opioid formulations, reserve HYSINGLA ER for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or immediate-release opioids) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain. (1)
- HYSINGLA ER is not indicated as an as-needed (prn) analgesic. (1)
Hysingla ER Dosage and Administration
- To be prescribed only by healthcare providers knowledgeable in use of potent opioids for management of chronic pain. (2.1)
- Daily doses of HYSINGLA ER greater than or equal to 80 mg are only for use in patients in whom tolerance to an opioid of comparable potency has been established.
- Patients considered opioid-tolerant are those taking, for one week or longer, at least 60 mg oral morphine per day, 25 mcg transdermal fentanyl per hour, 30 mg oral oxycodone per day, 8 mg oral hydromorphone per day, 25 mg oral oxymorphone per day, 60 mg oral hydrocodone per day, or an equianalgesic dose of another opioid. (2.1)
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals (2.1).
- Individualize dosing based on the severity of pain, patient response, prior analgesic experience, and risk factors for addiction, abuse, and misuse. (2.1)
- Instruct patients to swallow HYSINGLA ER intact, and not to crush, chew, or dissolve the tablets (risk of potentially fatal overdose). (2.1, 5.1)
- Instruct patients to take tablets one at a time, with enough water to ensure complete swallowing immediately after placing in the mouth (2.1, 5.12)
- Discuss availability of naloxone with the patient and caregiver and assess each patient’s need for access to naloxone, both when initiating and renewing treatment with HYSINGLA ER. Consider prescribing naloxone based on the patient’s risk factors for overdose (2.2, 5.1, 5.3, 5.6).
- For opioid-naïve patients, initiate with 20 mg tablets orally every 24 hours. (2.3)
- To convert to HYSINGLA ER from another opioid, follow the conversion instructions to obtain an estimated dose. (2.3)
- Dose titration of HYSINGLA ER may occur every 3 to 5 days (2.4)
- Patients with Severe Hepatic Impairment: Initiate dosing with one half of the recommended starting dosage and titrate carefully. Monitor for respiratory depression, sedation, and hypotension. (2.5)
- Patients with Moderate to Severe Renal Impairment and End-Stage Renal Disease: Initiate dosing at one half the recommended starting dosage and titrate carefully. Monitor for signs of respiratory depression, sedation, and hypotension. (2.6)
- Do not abruptly discontinue HYSINGLA ER in a physically dependent patient because rapid discontinuation of opioid analgesics has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. (2.7)
Dosage Forms and Strengths
Extended-release tablets: 20, 30, 40, 60, 80, 100, and 120 mg (3)
Contraindications
- Significant respiratory depression (4)
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment (4)
- Known or suspected gastrointestinal obstruction, including paralytic ileus (4)
- Hypersensitivity to hydrocodone or to any other components of HYSINGLA ER (4)
Warnings and Precautions
- Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration. (5.7)
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. (5.8)
- Severe Hypotension: Monitor during dosage initiation and titration. Avoid use of HYSINGLA ER in patients with circulatory shock. (5.9)
- QTc Prolongation: Avoid use in patients with congenital long QTc syndrome. In patients who develop QTc prolongation, consider reducing the dose. (5.10,12.2)
- Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. Avoid use of HYSINGLA ER in patients with circulatory shock. (5.11)
- Risk of Obstruction in Patients who have Difficulty Swallowing or have Underlying GI Disorders that may Predispose them to Obstruction: Consider use of an alternative analgesic. (5.12, 5.13)
Adverse Reactions/Side Effects
Most common treatment-emergent adverse events (incidence ≥ 5%) are constipation, nausea, vomiting, fatigue, upper respiratory tract infection, dizziness, headache, and somnolence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Purdue Pharma L.P. at 1-888-726-7535 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Serotonergic Drugs: Concomitant use may result in serotonin syndrome. Discontinue HYSINGLA ER if serotonin syndrome is suspected. (7)
- Mixed Agonists/Antagonists and Partial Agonist Opioid Analgesics: Avoid use with HYSINGLA ER because they may reduce analgesic effect of HYSINGLA ER or precipitate withdrawal symptoms. (7)
- Monoamine Oxidase Inhibitors (MAOIs): Can potentiate the effects of hydrocodone. Avoid concomitant use in patients receiving MAOIs or within 14 days of stopping an MAOI. (7)
Use In Specific Populations
- Pregnancy: May cause fetal harm. (8.1)
- Lactation: Not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2021
Full Prescribing Information
WARNING: ADDICTION, ABUSE, AND MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; NEONATAL OPIOID WITHDRAWAL SYNDROME; CYTOCHROME P450 3A4 INTERACTION; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS):
- complete a REMS-compliant education program,
- counsel patients and/or their caregivers, with every prescription, on safe use, serious risks, storage, and disposal of these products,
- emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist, and
- consider other tools to improve patient, household, and community safety.
- Reserve concomitant prescribing of HYSINGLA ER and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
1. Indications and Usage for Hysingla ER
- Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, and because of the greater risks of overdose and death with extended-release opioid formulations [see Warnings and Precautions (5.1)], reserve HYSINGLA ER for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or immediate-release opioids) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain.
- HYSINGLA ER is not indicated as an as-needed (prn) analgesic.
2. Hysingla ER Dosage and Administration
2.1 Important Dosage and Administration Information
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
- Initiate the dosing regimen for each patient individually; taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1)].
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with HYSINGLA ER and adjust the dosage accordingly [see Warnings and Precautions (5.3)].
2.3 Initial Dosage
Use of HYSINGLA ER as the First Opioid Analgesic (opioid-naïve patients)
Initiate therapy with HYSINGLA ER 20 mg orally every 24 hours.
Consider the following when using the information found in Table 1.
- This is not a table of equianalgesic doses.
- The conversion factors in this table are only for the conversion from one of the listed oral opioid analgesics to HYSINGLA ER.
- The table cannot be used to convert from HYSINGLA ER to another opioid. Doing so will result in an over-estimation of the dose of the new opioid and may result in fatal overdose
| Opioid | Oral dose (mg) | Approximate oral conversion factor |
| Codeine | 133 | 0.15 |
| Hydromorphone | 5 | 4 |
| Methadone | 13.3 | 1.5 |
| Morphine | 40 | 0.5 |
| Oxycodone | 20 | 1 |
| Oxymorphone | 10 | 2 |
| Tramadol | 200 | 0.1 |
To calculate the estimated total hydrocodone daily dose using Table 1:
- For patients on a single opioid, sum the current total daily dose of the opioid and then multiply the total daily dose by the approximate oral conversion factor to calculate the approximate oral hydrocodone daily dose.
- For patients on a regimen of more than one opioid, calculate the approximate oral hydrocodone dose for each opioid and sum the totals to obtain the approximate oral hydrocodone daily dose.
- For patients on a regimen of fixed-ratio opioid/non-opioid analgesic products, use only the opioid component of these products in the conversion.
- Reduce the calculated daily oral hydrocodone dose by 25%
3. Dosage Forms and Strengths
- 20 mg film-coated extended-release tablets (round, green-colored, bi-convex tablets printed with “HYD 20”)
- 30 mg film-coated extended-release tablets (round, yellow-colored, bi-convex tablets printed with “HYD 30”)
- 40 mg film-coated extended-release tablets (round, grey-colored, bi-convex tablets printed with “HYD 40”)
- 60 mg film-coated extended-release tablets (round, beige-colored, bi-convex tablets printed with “HYD 60”)
- 80 mg film-coated extended-release tablets (round, pink-colored, bi-convex tablets printed with “HYD 80”)
- 100 mg film-coated extended-release tablets (round, blue-colored, bi-convex tablets printed with “HYD 100”)
- 120 mg film-coated extended-release tablets (round, white-colored, bi-convex tablets printed with “HYD 120”)
4. Contraindications
HYSINGLA ER is contraindicated in patients with:
- Significant respiratory depression [see Warnings and Precautions (5.3)]
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see Warnings and Precautions (5.7)]
- Known or suspected gastrointestinal obstruction, including paralytic ileus [see Warnings and Precautions (5.12,5.13)]
- Hypersensitivity to hydrocodone or any component of HYSINGLA ER.
5. Warnings and Precautions
5.2 Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
- Complete a REMS-compliant education program offered by an accredited provider of continuing education (CE) or another education program that includes all the elements of the FDA Education Blueprint for Health Care Providers Involved in the Management or Support of Patients with Pain.
- Discuss the safe use, serious risks, and proper storage
and disposal of opioid analgesics with patients and/or their caregivers
every time these medicines are prescribed. The Patient Counseling
Guide (PCG) can be obtained at this link:
www.fda.gov/OpioidAnalgesicREMSPCG - Emphasize to patients and their caregivers the importance of reading the Medication Guide that they will receive from their pharmacist every time an opioid analgesic is dispensed to them.
- Consider using other tools to improve patient, household, and community safety, such as patient-prescriber agreements that reinforce patient-prescriber responsibilities.
5.3 Life-Threatening Respiratory Depression
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose:
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver and assess the potential need for access to naloxone, both when initiating and renewing treatment with HYSINGLA ER. Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program). Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help, even if naloxone is administered [see Patient Counseling Information (17)].
5.7 Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
5.11 Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in the labeling:
- Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)]
- Life-Threatening Respiratory Depression [see Warnings and Precautions (5.3)]
- Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions (5.4)]
- Interactions with Benzodiazepine or Other CNS Depressants [see Warnings and Precautions (5.6)]
- Adrenal Insufficiency [see Warnings and Precautions (5.8)]
- Severe Hypotension [see Warnings and Precautions (5.9)]
- Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.12, 5.13)]
- Seizures [see Warnings and Precautions (5.14)]
- Withdrawal [see Warnings and Precautions (5.15)]
6.1 Clinical Trial Experience
| Open-label Titration Period | Double-blind Treatment Period |
|||
| MedDRA Preferred Term | (N=905) (%) | Placebo (N=292) (%) | HYSINGLA ER (N=296) (%) |
|
| Nausea | 16 | 5 | 8 | |
| Constipation | 9 | 2 | 3 | |
| Vomiting | 7 | 3 | 6 | |
| Dizziness | 7 | 2 | 3 | |
| Headache | 7 | 2 | 2 | |
| Somnolence | 5 | 1 | 1 | |
| Fatigue | 4 | 1 | 1 | |
| Pruritus | 3 | <1 | 0 | |
| Tinnitus | 2 | 1 | 2 | |
| Insomnia | 2 | 2 | 3 | |
| Decreased appetite | 1 | 1 | 2 | |
| Influenza | 1 | 1 | 3 | |
| Ear and labyrinth disorders | tinnitus |
| Gastrointestinal disorders | abdominal pain, abdominal pain upper, diarrhea, dry mouth, dyspepsia, gastroesophageal reflux disease |
| General disorders and administration site conditions | chest pain, chills, edema peripheral, pain, pyrexia |
| Infections and infestations | bronchitis, gastroenteritis, gastroenteritis viral, influenza, nasopharyngitis, sinusitis, urinary tract infection |
| Injury, poisoning and procedural complications | fall, muscle strain |
| Metabolism and nutrition disorders | decreased appetite |
| Musculoskeletal and connective tissue disorders | arthralgia, back pain, muscle spasms, musculoskeletal pain, myalgia, pain in extremity |
| Nervous system disorders | lethargy, migraine, sedation |
| Psychiatric disorders | anxiety, depression, insomnia |
| Respiratory, thoracic and mediastinal disorders | cough, nasal congestion, oropharyngeal pain |
| Skin and subcutaneous tissue disorders | hyperhidrosis, pruritus, rash |
| Vascular disorders | hot flush, hypertension |
7. Drug Interactions
Table 3 includes clinically significant drug interactions with HYSINGLA ER.
| Inhibitors of CYP3A4 | |
| Clinical Impact: | The concomitant use of HYSINGLA
ER and CYP3A4 inhibitors can increase the plasma concentration of
hydrocodone, resulting in increased or prolonged opioid effects. These
effects could be more pronounced with concomitant use of HYSINGLA
ER and CYP3A4 inhibitors, particularly when an inhibitor is added
after a stable dose of HYSINGLA ER is achieved [see Warnings
and Precautions (5.5)]. After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the hydrocodone plasma concentration will decrease [see Clinical Pharmacology (12.3)], resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to hydrocodone. |
| Intervention: | If concomitant use is necessary,
consider dosage reduction of HYSINGLA ER until stable drug effects
are achieved. Monitor patients for respiratory depression and sedation
at frequent intervals. If a CYP3A4 inhibitor is discontinued, consider increasing the HYSINGLA ER dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. |
| Examples | Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g. ketoconazole), protease inhibitors (e.g., ritonavir) |
| CYP3A4 Inducers | |
| Clinical Impact: | The concomitant use of HYSINGLA
ER and CYP3A4 inducers can decrease the plasma concentration of hydrocodone [see Clinical Pharmacology (12.3)], resulting in decreased efficacy or onset of a withdrawal
syndrome in patients who have developed physical dependence to hydrocodone [see Warnings and Precautions (5.5)]. After stopping a CYP3A4 inducer, as the effects of the inducer decline, the hydrocodone plasma concentration will increase [see Clinical Pharmacology (12.3)], which could increase or prolong both the therapeutic effects and adverse reactions, and may cause serious respiratory depression. |
| Intervention: | If concomitant use is necessary, consider increasing the HYSINGLA ER dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider HYSINGLA ER dosage reduction and monitor for signs of respiratory depression. |
| Examples: | Rifampin, carbamazepine, phenytoin |
| Benzodiazepines and Other Central Nervous System (CNS) Depressants | |
| Clinical Impact: | Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, can increase the risk of hypotension, respiratory depression, profound sedation, coma, and death. |
| Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation. If concomitant use is warranted, consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration (2.2), Warnings and Precautions (5.1, 5.3, 5.6)]. |
| Examples: | Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol. |
| Serotonergic Drugs | |
| Clinical Impact: | The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. |
| Intervention: | If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue HYSINGLA ER if serotonin syndrome is suspected. |
| Examples: | Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). |
| Monoamine Oxidase Inhibitors (MAOIs) | |
| Clinical Impact: | MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.3)]. |
| Intervention: | The use of HYSINGLA ER is not recommended for patients taking MAOIs or within 14 days of stopping such treatment. |
| Examples: | Phenelzine, tranylcypromine, linezolid |
| Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics | |
| Clinical Impact: | May reduce the analgesic effect of HYSINGLA ER and/or precipitate withdrawal symptoms. |
| Intervention: | Avoid concomitant use. |
| Examples: | butorphanol, nalbuphine, pentazocine, buprenorphine |
| Muscle Relaxants | |
| Clinical Impact: | Hydrocodone may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. |
| Intervention: | Monitor patients for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of HYSINGLA ER and/or the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration (2.2), Warnings and Precautions (5.3, 5.6)]. |
| Example: | Cyclobenzaprine, metaxalone |
| Diuretics | |
| Clinical Impact: | Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. |
| Intervention: | Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. |
| Anticholinergic Drugs | |
| Clinical Impact: | The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. |
| Intervention: | Monitor patients for signs of urinary retention or reduced gastric motility when HYSINGLA ER is used concomitantly with anticholinergic drugs. |
| Strong Laxatives | |
| Clinical Impact: | Concomitant use of HYSINGLA ER with strong laxatives that rapidly increase gastrointestinal motility, may decrease hydrocodone absorption and result in decreased hydrocodone plasma levels. |
| Intervention: | If HYSINGLA ER is used in these patients, closely monitor for the development of adverse events as well as changing analgesic requirements. |
| Example: | Lactulose |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of HYSINGLA ER in pediatric patients have not been established.
9. Drug Abuse and Dependence
9.1 Controlled Substance
HYSINGLA ER contains hydrocodone bitartrate, a Schedule II controlled substance.
9.2 Abuse
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal.
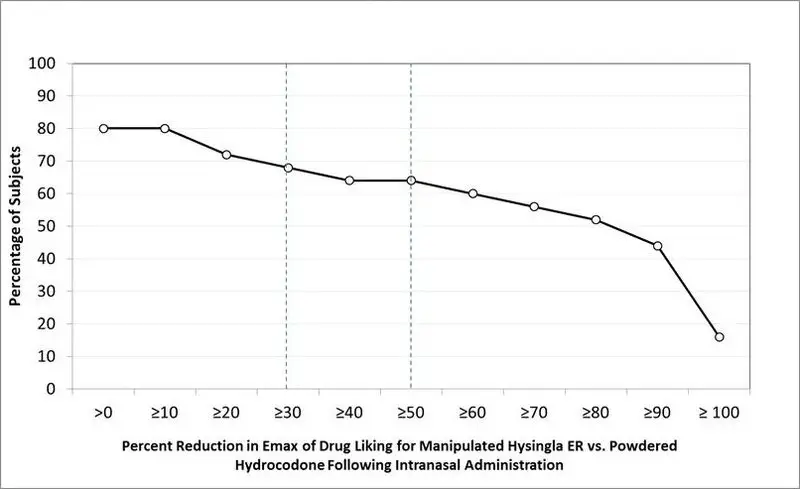
| *Bipolar scale (0=maximum negative response, 50=neutral response, 100=maximum positive response) ** Unipolar scale (0=maximum negative response, 100=maximum positive response) |
||
| VAS Scale (100 point) | HYSINGLA ER Manipulated | Hydrocodone Powder |
| Intranasal (n=25) | ||
| Drug Liking* | ||
| Mean (SE) | 65.4 (3.7) | 90.4 (2.6) |
| Median (Range) | 56 (50–100) | 100 (51–100) |
| Take Drug Again** | ||
| Mean (SE) | 36.4 (8.2) | 85.2 (5.0) |
| Median (Range) | 14 (0-100) | 100 (1-100) |
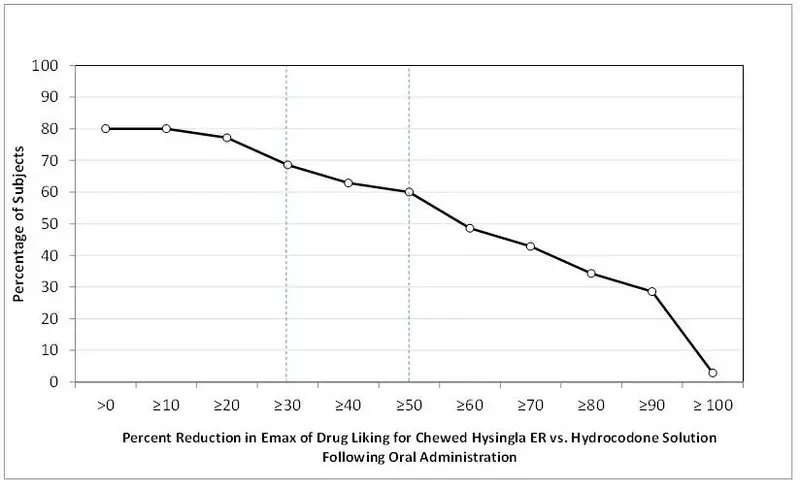
| *Bipolar scale (0=maximum negative response, 50=neutral response, 100=maximum positive response) ** Unipolar scale (0=maximum negative response, 100=maximum positive response) |
|||
| VAS Scale (100 point) | HYSINGLA ER | Hydrocodone Solution |
|
| Oral (n=35) | Intact | Chewed | |
| Drug Liking* | |||
| Mean (SE) | 63.3 (2.7) | 69.0 (3.0) | 94.0 (1.7) |
| Median (Range) | 58 (50–100) | 66 (50–100) | 100 (51–100) |
| Take Drug Again** | |||
| Mean (SE) | 34.3 (6.1) | 44.3 (6.9) | 89.7 (3.6) |
| Median (Range) | 24 (0-100) | 55 (0-100) | 100 (1-100) |
11. Hysingla ER Description
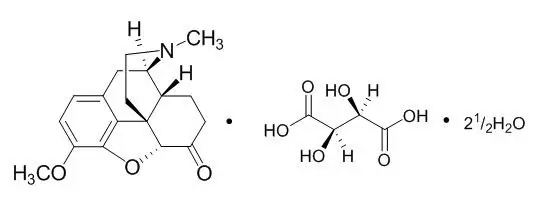
Empirical formula: C18H21NO3•C4H6O6•2½H2O; Molecular weight: 494.49.
The 30 mg tablets also contain Iron Oxide Yellow.
The 40 mg tablets also contain Iron Oxide Yellow, Iron Oxide Red, and Iron Oxide Black.
The 60 mg tablets also contain Iron Oxide Yellow and Iron Oxide Red.
The 80 mg tablets also contain Iron Oxide Red.
12. Hysingla ER - Clinical Pharmacology
12.3 Pharmacokinetics
| * median (minimum, maximum) | |||
| Dose Strength (mg) | AUCinf (ng•h/mL) | Cmax (ng/mL) | Tmax* (h) |
| 20 | 284 (128) | 14.6 (5.5) | 16 (6, 24) |
| 40 | 622 (252) | 33.9 (11.8) | 16 (6, 24) |
| 60 | 1009 (294) | 53.6 (15.4) | 14 (10, 30) |
| 80 | 1304 (375) | 69.1 (17.2) | 16 (10, 24) |
| 120 | 1787 (679) | 110 (44.1) | 14 (6, 30) |
Figure 3. Mean Steady-State Plasma Hydrocodone Concentration Profile
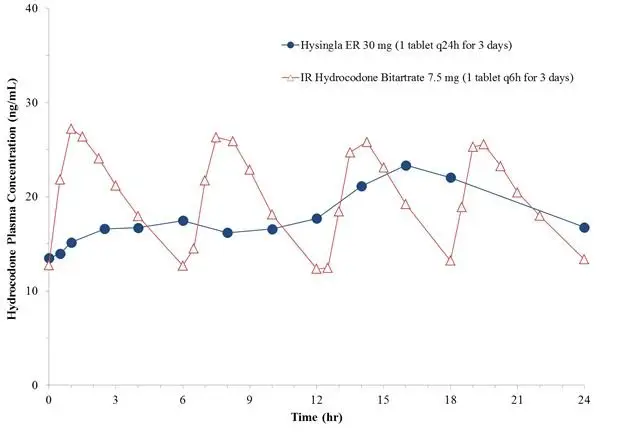
| * Mean (minimum, maximum); Percentage fluctuation in plasma concentration is derived as (Cmax,ss – Cmin, ss)*100/Cavg,ss. | ||||
| Regimen | AUC24,ss (ng•h/mL) | Cmax,ss (ng/mL) | Cmin,ss (ng/mL) | %Fluctuation* |
| HYSINGLA ER | ||||
| 30 mg q24h | 443 (128) | 26.4 (7.4) | 16.7 (5.2) | 61 (6.4,113) |
| 80 mg q24h | 1252 (352) | 82.6 (25.7) | 28.2 (12) | 105 (36,214) |
| 120 mg q24h | 1938 (729) | 135 (50) | 63.6 (29) | 97.9 (32, 250) |
Sex
Systemic exposure of hydrocodone (Cmax and AUC) was similar between males and females.
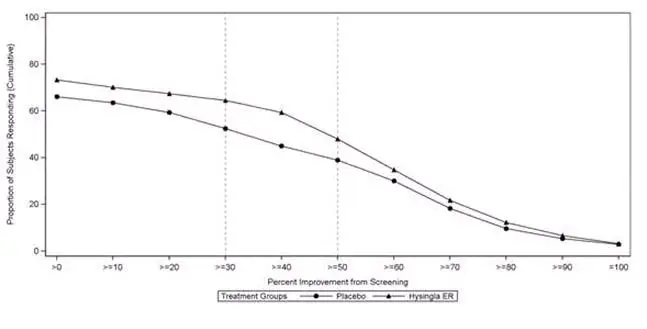
14. Clinical Studies
16. How is Hysingla ER supplied
Dispense in tight, light-resistant container, as defined by the USP.
Store HYSINGLA ER securely and dispose of properly [see Patient Counseling Information (17)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss with the patient and caregiver the availability of naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with HYSINGLA ER. Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program) [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
Educate patients and caregivers on how to recognize the signs and symptoms of an overdose.
If naloxone is prescribed, also advise patients and caregivers:
- How to treat with naloxone in the event of an opioid overdose
- To tell family and friends about their naloxone and to keep it in a place where family and friends can access it in an emergency
- To read the Patient Information (or other educational material) that will come with their naloxone. Emphasize the importance of doing this before an opioid emergency happens, so the patient and caregiver will know what to do.
- Use HYSINGLA ER exactly as prescribed to reduce the risk of life-threatening adverse reactions (e.g., respiratory depression) [see Warnings and Precautions (5.3)].
- Swallow tablets whole, one tablet at a time, with enough water to ensure swallowing immediately after placing in the mouth [see Dosage and Administration (2.1)].
- Do not pre-soak, lick, or otherwise wet the tablet prior to placing in the mouth [see Dosage and Administration (2.1)].
- Do not chew, crush, or dissolve the tablets [see Dosage and Administration (2.1)].
Important Discontinuation Instructions
- In order to avoid developing withdrawal symptoms, instruct patients not to discontinue HYSINGLA ER without first discussing a tapering plan with the prescriber [see Dosage and Administration (2.7)]
| Medication Guide HYSINGLA® ER (hye-SING-luh) (hydrocodone bitartrate) extended-release tablets, CII |
HYSINGLA ER is:
|
Important information about HYSINGLA ER:
|
Do not take HYSINGLA ER if you have:
|
Before taking HYSINGLA ER, tell your healthcare provider if you have a history of:
|
When taking HYSINGLA ER:
|
While taking HYSINGLA ER, DO NOT:
|
The possible side effects of HYSINGLA ER are:
These are not all the possible side effects of HYSINGLA ER. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov. Manufactured by: Purdue Pharma L.P., Stamford, CT 06901-3431, www.purduepharma.com or call 1-888-726-7535 |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued: 03/2021
| HYSINGLA ER
hydrocodone bitartrate tablet, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| HYSINGLA ER
hydrocodone bitartrate tablet, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| HYSINGLA ER
hydrocodone bitartrate tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| HYSINGLA ER
hydrocodone bitartrate tablet, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| HYSINGLA ER
hydrocodone bitartrate tablet, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| HYSINGLA ER
hydrocodone bitartrate tablet, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| HYSINGLA ER
hydrocodone bitartrate tablet, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Purdue Pharma LP (932323652) |
| Registrant - Purdue Pharma LP (932323652) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Purdue Pharmaceuticals L.P. | 132080875 | MANUFACTURE(59011-276, 59011-273, 59011-274, 59011-272, 59011-275, 59011-277, 59011-271) | |