Drug Detail:Hyzaar (Hydrochlorothiazide and losartan [ hye-droe-klor-oh-thye-a-zide-and-loe-sar-tan ])
Drug Class: Angiotensin II inhibitors with thiazides
Highlights of Prescribing Information
HYZAAR® (losartan potassium and hydrochlorothiazide) tablets, for oral use
Initial U.S. Approval: 1995
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
When pregnancy is detected, discontinue HYZAAR as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1)
Indications and Usage for Hyzaar
HYZAAR is a combination of losartan, an angiotensin II receptor blocker (ARB) and hydrochlorothiazide, a diuretic indicated for:
- Treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1.1)
- Reduction of the risk of stroke in patients with hypertension and left ventricular hypertrophy. There is evidence that this benefit does not apply to Black patients. (1.2)
Hyzaar Dosage and Administration
Hypertension
- Usual starting dose: 50/12.5 mg once daily. (2.1)
- Titrate as needed to a maximum dose of 100/25 mg. (2.1)
Hypertensive Patients with Left Ventricular Hypertrophy
- Not controlled on monotherapy: Initiate with 50/12.5 mg. Titrate as needed to a maximum of 100/25 mg. (2.2)
Dosage Forms and Strengths
Tablets (losartan potassium/hydrochlorothiazide content): 50/12.5 mg; 100/12.5 mg; and 100/25 mg. (3)
Contraindications
- Hypersensitivity to any component of HYZAAR. (4)
- Anuria. (4)
- Coadministration with aliskiren in patients with diabetes. (4)
Warnings and Precautions
- Hypotension: Correct volume or salt depletion prior to administration of HYZAAR. (5.2)
- Monitor renal function and potassium in susceptible patients. (5.3)
- Observe for clinical signs of fluid or electrolyte imbalance. (5.5)
- Acute angle-closure glaucoma. (5.6)
- Exacerbation of systemic lupus erythematosus. (5.7)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥2% and greater than placebo) are dizziness, upper respiratory infection, cough, and back pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Organon LLC, a subsidiary of Organon & Co., at 1-844-674-3200 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Agents increasing serum potassium: Risk of hyperkalemia. (7.1)
- Lithium: Risk of lithium toxicity. (7.2)
- Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): increased risk of renal impairment and reduced diuretic, natriuretic, and antihypertensive effects. (7.3)
- Dual inhibition of the renin-angiotensin system: increased risk of renal impairment, hypotension, syncope, and hyperkalemia. (7.4)
- Antidiabetic drugs: dosage adjustment of antidiabetic may be required. (7.5)
- Cholestyramine and colestipol: Reduced absorption of thiazides. (7.5)
Use In Specific Populations
- Hepatic Impairment: HYZAAR is not recommended for initial therapy, because the recommended starting dose is not available. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Full Prescribing Information
WARNING: FETAL TOXICITY
When pregnancy is detected, discontinue HYZAAR as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)].
1. Indications and Usage for Hyzaar
1.1 Hypertension
HYZAAR® is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular (CV) events, primarily strokes and myocardial infarction. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including losartan and hydrochlorothiazide.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in Black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
This fixed dose combination is not indicated for initial therapy of hypertension, except when the hypertension is severe enough that the value of achieving prompt blood pressure control exceeds the risk of initiating combination therapy in these patients [see Clinical Studies (14) and Dosage and Administration (2.1)].
HYZAAR may be administered with other antihypertensive agents.
1.2 Hypertensive Patients with Left Ventricular Hypertrophy
HYZAAR is indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy, but there is evidence that this benefit does not apply to Black patients. [See Use in Specific Populations (8.6), Clinical Pharmacology (12.3), and Dosage and Administration (2.2).]
2. Hyzaar Dosage and Administration
2.1 Hypertension
The usual starting dose of HYZAAR is 50/12.5 (losartan 50 mg/hydrochlorothiazide 12.5 mg) once daily. The dosage can be increased after 3 weeks of therapy to a maximum of 100/25 (losartan 100 mg/hydrochlorothiazide 25 mg) once daily as needed to control blood pressure [see Clinical Studies (14.2)].
Initiate a patient whose blood pressure is not adequately controlled with losartan 50 mg monotherapy with HYZAAR 50/12.5 once daily. If blood pressure remains uncontrolled after about 3 weeks of therapy, the dosage may be increased to two tablets of HYZAAR 50/12.5 once daily or one tablet of HYZAAR 100/25 once daily.
Initiate a patient whose blood pressure is not adequately controlled with losartan 100 mg monotherapy with HYZAAR 100/12.5 (losartan 100 mg/hydrochlorothiazide 12.5 mg) once daily. If blood pressure remains uncontrolled after about 3 weeks of therapy, increase the dose to two tablets of HYZAAR 50/12.5 once daily or one tablet of HYZAAR 100/25 once daily.
Initiate a patient whose blood pressure is inadequately controlled with hydrochlorothiazide 25 mg once daily, or is controlled but who experiences hypokalemia with this regimen, on HYZAAR 50/12.5 once daily, reducing the dose of hydrochlorothiazide without reducing the overall expected antihypertensive response. Evaluate the clinical response to HYZAAR 50/12.5 and, if blood pressure remains uncontrolled after about 3 weeks of therapy, increase the dose to two tablets of HYZAAR 50/12.5 once daily or one tablet of HYZAAR 100/25 once daily.
2.2 Hypertensive Patients with Left Ventricular Hypertrophy
In patients whose blood pressure is not adequately controlled on 50 mg losartan potassium, initiate treatment with HYZAAR 50/12.5. If additional blood pressure reduction is needed, increase the dose to HYZAAR 100/12.5, followed by HYZAAR 100/25. For further blood pressure reduction add other antihypertensives [see Clinical Studies (14)].
3. Dosage Forms and Strengths
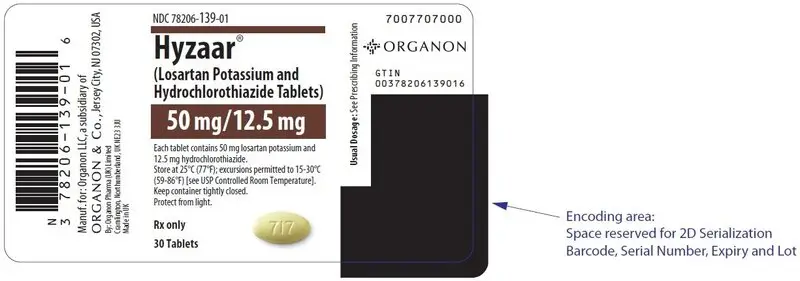
- HYZAAR 50/12.5 are yellow, oval, film-coated tablets, with code 717 on one side.
- HYZAAR 100/12.5 are white, oval, film-coated tablets, with code 745 on one side.
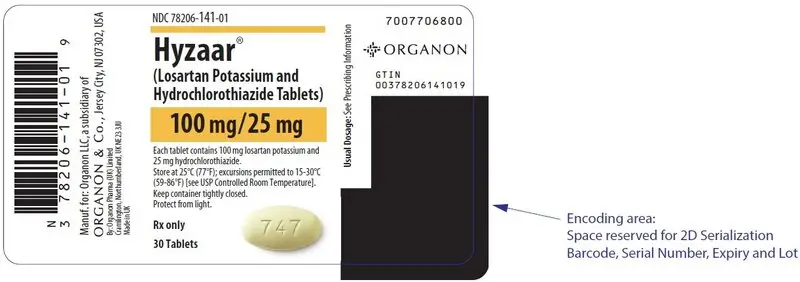
- HYZAAR 100/25 are light yellow, oval, film-coated tablets, with code 747 on one side.
4. Contraindications
HYZAAR is contraindicated:
- In patients who are hypersensitive to any component of this product.
- In patients with anuria
- For coadministration with aliskiren in patients with diabetes
5. Warnings and Precautions
5.1 Fetal Toxicity
HYZAAR can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue HYZAAR as soon as possible.
Thiazides cross the placental barrier and appear in cord blood. Adverse reactions include fetal or neonatal jaundice, thrombocytopenia [see Use in Specific Populations (8.1)].
5.2 Hypotension in Volume- or Salt-Depleted Patients
In patients with an activated renin-angiotensin system, such as volume- or salt-depleted patients (e.g., those being treated with high doses of diuretics), symptomatic hypotension may occur after initiation of treatment with HYZAAR. Correct volume or salt depletion prior to administration of HYZAAR. Do not use HYZAAR as initial therapy in patients with intravascular volume depletion.
5.3 Impaired Renal Function
Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on HYZAAR. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on HYZAAR [see Drug Interactions (7.3) and Use in Specific Populations (8.8)].
5.4 Hypersensitivity
Hypersensitivity reactions to hydrochlorothiazide may occur in patients with or without a history of allergy or bronchial asthma but are more likely in patients with such a history [see Adverse Reactions (6.2)].
5.5 Electrolyte and Metabolic Effects
In double-blind clinical trials of various doses of losartan potassium and hydrochlorothiazide, the incidence of hypertensive patients who developed hypokalemia (serum potassium <3.5 mEq/L) was 6.7% versus 3.5% for placebo; the incidence of hyperkalemia (serum potassium >5.7 mEq/L) was 0.4% versus 0% for placebo.
HYZAAR contains hydrochlorothiazide which can cause hypokalemia, hyponatremia and hypomagnesemia. Hypomagnesemia can result in hypokalemia which may be difficult to treat despite potassium repletion. HYZAAR also contains losartan which can cause hyperkalemia. Monitor serum electrolytes periodically [see Drug Interactions (7.1)].
Concomitant use of other drugs that may increase serum potassium may lead to hyperkalemia [see Drug Interactions (7.1)].
Hydrochlorothiazide may alter glucose tolerance and raise serum levels of cholesterol and triglycerides.
Hyperuricemia may occur or frank gout may be precipitated in patients receiving thiazide therapy. Because losartan decreases uric acid, losartan in combination with hydrochlorothiazide attenuates the diuretic-induced hyperuricemia.
Hydrochlorothiazide decreases urinary calcium excretion and may cause elevations of serum calcium. Monitor calcium levels.
5.6 Acute Myopia and Secondary Angle-Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Losartan potassium-hydrochlorothiazide has been evaluated for safety in 858 patients treated for essential hypertension and 3889 patients treated for hypertension and left ventricular hypertrophy. Most adverse reactions have been mild and transient in nature and have not required discontinuation of therapy. In controlled clinical trials, discontinuation of therapy due to clinical adverse events was required in only 2.8% and 2.3% of patients treated with the combination and placebo, respectively.
In these double-blind controlled clinical trials, adverse reactions occurring in greater than 2% of subjects treated with losartan-hydrochlorothiazide and at a greater rate than placebo were: back pain (2.1% vs 0.6%), dizziness (5.7% vs 2.9%), and upper respiratory infection (6.1% vs 4.6%).
The following additional adverse reactions have been reported in clinical trials with HYZAAR and/or the individual components:
Blood and the lymphatic system disorders: Anemia, aplastic anemia, hemolytic anemia, leukopenia, agranulocytosis.
Metabolism and nutrition disorders: Anorexia, hyperglycemia, hyperuricemia, electrolyte imbalance including hyponatremia and hypokalemia.
Psychiatric disorders: Insomnia, restlessness.
Nervous system disorders: Dysgeusia, headache, migraine, paraesthesias.
Eye disorders: Xanthopsia, transient blurred vision.
Cardiac disorders: Palpitation, tachycardia.
Vascular disorders: Dose-related orthostatic effects, necrotizing angiitis (vasculitis, cutaneous vasculitis).
Respiratory, thoracic and mediastinal disorders: Nasal congestion.
Gastrointestinal disorders: Dyspepsia, abdominal pain, gastric irritation, cramping, nausea, vomiting, pancreatitis, sialoadenitis.
Hepato-biliary disorders: Jaundice (intrahepatic cholestatic jaundice).
Skin and subcutaneous tissue disorders: Rash, pruritus, purpura, toxic epidermal necrolysis, urticaria, photosensitivity, cutaneous lupus erythematosus.
Musculoskeletal and connective tissue disorders: Muscle cramps, muscle spasm.
Renal and urinary disorders: Glycosuria, renal dysfunction, interstitial nephritis, renal failure.
Reproductive system and breast disorders: Erectile dysfunction/impotence.
General disorders and administration site conditions: Chest pain, malaise, weakness.
Investigations: Liver function abnormalities.
Cough
Persistent dry cough has been associated with ACE-inhibitor use and in practice can be a cause of discontinuation of ACE-inhibitor therapy. Two prospective, parallel-group, double-blind, randomized, controlled trials were conducted to assess the effects of losartan on the incidence of cough in hypertensive patients who had experienced cough while receiving ACE-inhibitor therapy. Patients who had typical ACE-inhibitor cough when challenged with lisinopril, whose cough disappeared on placebo, were randomized to losartan 50 mg, lisinopril 20 mg, or either placebo (one study, n=97) or 25 mg hydrochlorothiazide (n=135). The double-blind treatment period lasted up to 8 weeks. The incidence of cough is shown in Table 1 below.
|
|||
| Study 1* | HCTZ | Losartan | Lisinopril |
| Cough | 25% | 17% | 69% |
| Study 2† | Placebo | Losartan | Lisinopril |
| Cough | 35% | 29% | 62% |
These studies demonstrate that the incidence of cough associated with losartan therapy, in a population that all had cough associated with ACE-inhibitor therapy, is similar to that associated with hydrochlorothiazide or placebo therapy.
Cases of cough, including positive re-challenges, have been reported with the use of losartan in postmarketing experience.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of HYZAAR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Digestive: Hepatitis has been reported rarely in patients treated with losartan.
Hematologic: Thrombocytopenia.
Hypersensitivity: Angioedema, including swelling of the larynx and glottis, causing airway obstruction and/or swelling of the face, lips, pharynx, and/or tongue. Vasculitis, including Henoch-Schönlein purpura; anaphylactic reactions which can present as respiratory distress (including pneumonitis and pulmonary edema).
Musculoskeletal: Rhabdomyolysis.
Skin: Erythroderma.
Non-melanoma Skin Cancer: Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
7. Drug Interactions
7.1 Agents Increasing Serum Potassium
Coadministration of losartan with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients.
7.2 Lithium
Increases in serum lithium concentrations and lithium toxicity have been reported with concomitant use of angiotensin II receptor antagonists or thiazide diuretics. Monitor lithium levels in patients receiving HYZAAR and lithium.
7.4 Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, syncope, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy.
The Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial enrolled 1448 patients with type 2 diabetes, elevated urinary-albumin-to-creatinine ratio, and decreased estimated glomerular filtration rate (GFR 30 to 89.9 mL/min), randomized them to lisinopril or placebo on a background of losartan therapy and followed them for a median of 2.2 years. Patients receiving the combination of losartan and lisinopril did not obtain any additional benefit compared to monotherapy for the combined endpoint of decline in GFR, end-stage renal disease, or death, but experienced an increased incidence of hyperkalemia and acute kidney injury compared with the monotherapy group.
Closely monitor blood pressure, renal function, and electrolytes in patients on HYZAAR and other agents that affect the RAS.
Do not coadminister aliskiren with HYZAAR in patients with diabetes. Avoid use of aliskiren with HYZAAR in patients with renal impairment (GFR <60 mL/min).
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of HYZAAR in pediatric patients have not been established.
Neonates with a history of in utero exposure to HYZAAR: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusion or dialysis may be required as means of reversing hypotension and/or substituting for disordered renal function.
8.5 Geriatric Use
In a controlled clinical study for the reduction in the combined risk of cardiovascular death, stroke and myocardial infarction in hypertensive patients with left ventricular hypertrophy, 2857 patients (62%) were 65 years and over, while 808 patients (18%) were 75 years and over. In an effort to control blood pressure in this study, patients were coadministered losartan and hydrochlorothiazide 74% of the total time they were on study drug. No overall differences in effectiveness were observed between these patients and younger patients. Adverse events were somewhat more frequent in the elderly compared to non-elderly patients for both the losartan-hydrochlorothiazide and the control groups [see Clinical Pharmacology (12.3)].
8.6 Race
In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, Black patients with hypertension and left ventricular hypertrophy treated with atenolol had a lower risk of stroke, the primary composite endpoint, as compared with Black patients treated with losartan (both cotreated with hydrochlorothiazide in the majority of patients). In the subgroup of Black patients (n=533, 6% of the LIFE study patients), there were 29 primary endpoints among 263 patients on atenolol (11%, 26 per 1000 patient-years) and 46 primary endpoints among 270 patients (17%, 42 per 1000 patient-years) on losartan. This finding could not be explained on the basis of differences in the populations other than race or on any imbalances between treatment groups. In addition, blood pressure reductions in both treatment groups were consistent between Black and non-Black patients. Given the difficulty in interpreting subset differences in large trials, it cannot be known whether the observed difference is the result of chance. However, the LIFE study provides no evidence that the benefits of losartan on reducing the risk of cardiovascular events in hypertensive patients with left ventricular hypertrophy apply to Black patients [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Initiation of HYZAAR is not recommended for patients with hepatic impairment because the appropriate starting dose of losartan, 25 mg, is not available.
8.8 Renal Impairment
Changes in renal function have been reported in susceptible individuals [see Dosage and Administration (2.1), Warnings and Precautions (5.4), and Clinical Pharmacology (12.3)]. Safety and effectiveness of HYZAAR in patients with severe renal impairment (creatinine clearance <30 mL/min) have not been established.
11. Hyzaar Description
HYZAAR 50/12.5 (losartan potassium-hydrochlorothiazide), HYZAAR 100/12.5 (losartan potassium-hydrochlorothiazide) and HYZAAR 100/25 (losartan potassium-hydrochlorothiazide) tablets combine an angiotensin II receptor blocker acting on the AT1 receptor subtype and a diuretic, hydrochlorothiazide.
Losartan potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O, and its structural formula is:
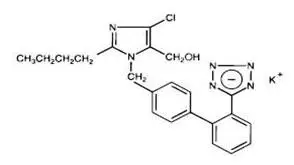 |
Losartan potassium is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone.
Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
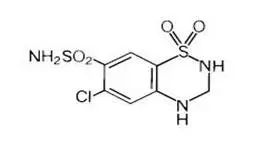 |
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.74, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
HYZAAR is available for oral administration in three tablet combinations of losartan and hydrochlorothiazide. HYZAAR 50/12.5 contains 50 mg of losartan potassium and 12.5 mg of hydrochlorothiazide. HYZAAR 100/12.5 contains 100 mg of losartan potassium and 12.5 mg of hydrochlorothiazide. HYZAAR 100/25 contains 100 mg of losartan potassium and 25 mg of hydrochlorothiazide. Inactive ingredients are microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, and titanium dioxide. HYZAAR 50/12.5 and HYZAAR 100/25 also contain D&C yellow No. 10 aluminum lake. HYZAAR 50/12.5, HYZAAR 100/12.5, and HYZAAR 100/25 may also contain carnauba wax.
HYZAAR 50/12.5 contains 4.24 mg (0.108 mEq) of potassium, HYZAAR 100/12.5 contains 8.48 mg (0.216 mEq) of potassium, and HYZAAR 100/25 contains 8.48 mg (0.216 mEq) of potassium.
12. Hyzaar - Clinical Pharmacology
14. Clinical Studies
14.2 Losartan Potassium-Hydrochlorothiazide
The 3 controlled studies of losartan and hydrochlorothiazide included over 1300 patients assessing the antihypertensive efficacy of various doses of losartan (25, 50 and 100 mg) and concomitant hydrochlorothiazide (6.25, 12.5 and 25 mg). A factorial study compared the combination of losartan/hydrochlorothiazide 50/12.5 mg with its components and placebo. The combination of losartan/hydrochlorothiazide 50/12.5 mg resulted in an approximately additive placebo-adjusted systolic/diastolic response (15.5/9.0 mmHg for the combination compared to 8.5/5.0 mmHg for losartan alone and 7.0/3.0 mmHg for hydrochlorothiazide alone). Another study investigated the dose-response relationship of various doses of hydrochlorothiazide (6.25, 12.5 and 25 mg) or placebo on a background of losartan (50 mg) in patients not adequately controlled (Sitting Diastolic Blood Pressure [SiDBP] 93-120 mmHg) on losartan (50 mg) alone. The third study investigated the dose-response relationship of various doses of losartan (25, 50 and 100 mg) or placebo on a background of hydrochlorothiazide (25 mg) in patients not adequately controlled (SiDBP 93-120 mmHg) on hydrochlorothiazide (25 mg) alone. These studies showed an added antihypertensive response at trough (24 hours post-dosing) of hydrochlorothiazide 12.5 or 25 mg added to losartan 50 mg of 5.5/3.5 and 10.0/6.0 mmHg, respectively. Similarly, there was an added antihypertensive response at trough when losartan 50 or 100 mg was added to hydrochlorothiazide 25 mg of 9.0/5.5 and 12.5/6.5 mmHg, respectively. There was no significant effect on heart rate.
There was no difference in response for men and women or in patients over or under 65 years of age.
Black patients had a larger response to hydrochlorothiazide than non-Black patients and a smaller response to losartan. The overall response to the combination was similar for Black and non-Black patients.
16. How is Hyzaar supplied
HYZAAR is supplied as a film-coated tablet.
| Losartan/ Hydrochlorothiazide | Color | Shape | Engraving | NDC 78206-xxx-xx | |
|---|---|---|---|---|---|
| Bottle/ 30 | Bottle/ 90 | ||||
| 50/12.5 mg | yellow | oval | 717 | 139-01 | 139-02 |
| 100/12.5 mg | white | oval | 745 | 140-01 | 140-02 |
| 100/25 mg | light yellow | oval | 747 | 141-01 | 141-02 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patient Information
HYZAAR®
(HY-zar)
(losartan potassium and hydrochlorothiazide tablets)
50/12.5 mg, 100/12.5 mg, 100/25 mg
Rx only
Read the Patient Information that comes with HYZAAR® before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition and treatment.
What is the most important information I should know about HYZAAR?
- HYZAAR can cause harm or death to an unborn baby.
- Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant.
- If you get pregnant while taking HYZAAR tell your doctor right away.
What is HYZAAR?
HYZAAR contains 2 prescription medicines, an angiotensin receptor blocker (ARB) and a diuretic (water pill). It is used to:
- lower high blood pressure (hypertension). HYZAAR is not usually the first medicine used to treat high blood pressure.
- lower the chance of stroke in patients with high blood pressure and a heart problem called left ventricular hypertrophy (LVH). HYZAAR may not help Black patients with this problem.
HYZAAR has not been studied in children less than 18 years old.
High Blood Pressure (hypertension). Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. The losartan ingredient in HYZAAR can help your blood vessels relax so your blood pressure is lower. The hydrochlorothiazide ingredient in HYZAAR works by making your kidneys pass more water and salt.
Left Ventricular Hypertrophy (LVH) is an enlargement of the walls of the left chamber of the heart (the heart's main pumping chamber). LVH can happen from several things. High blood pressure is the most common cause of LVH.
Who should not take HYZAAR?
Do not take HYZAAR if you:
- are allergic to any ingredients in HYZAAR. See a complete list of ingredients in HYZAAR at the end of this leaflet.
- are not passing urine.
- have diabetes and are taking a medicine called aliskiren to reduce blood pressure.
What should I tell my doctor before taking HYZAAR?
Tell your doctor about all your medical conditions including if you:
- are pregnant or planning to become pregnant. See "What is the most important information I should know about HYZAAR?"
- are breastfeeding or plan to breastfeed. HYZAAR can pass into your milk and may harm your baby. You and your doctor should decide if you will take HYZAAR or breastfeed. You should not do both.
- have been vomiting (throwing up), having diarrhea, sweating a lot, or not drinking enough fluids. These could cause you to have low blood pressure.
- have liver problems
- have kidney problems
- have systemic lupus erythematosus (Lupus; SLE)
- have diabetes
- have gout
- have any allergies
- have had skin cancer or if you develop a new skin lesion during the treatment. Treatment with hydrochlorothiazide may increase the risk of some types of skin cancer (non-melanoma skin cancer). Discuss with your doctor how to protect your skin from sun exposure and how often you should undergo skin cancer screening.
Tell your doctor about all of the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
HYZAAR and certain other medicines may interact with each other. Especially tell your doctor if you are taking:
- potassium supplements
- salt substitutes containing potassium
- other medicines that may increase serum potassium
- water pills (diuretics)
- lithium (a medicine used to treat a certain kind of depression)
- medicines used to treat pain and arthritis, called non-steroidal anti-inflammatory drugs (NSAIDs), including COX-2 inhibitors
- other medicines to reduce blood pressure.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I take HYZAAR?
- Take HYZAAR exactly as prescribed by your doctor. Your doctor may change your dose if needed.
- HYZAAR can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
- If you take too much HYZAAR, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
- Your doctor may do blood tests from time to time while you are taking HYZAAR.
What are the possible side effects of HYZAAR?
HYZAAR may cause the following side effects that may be serious:
- Injury or death of unborn babies. See "What is the most important information I should know about HYZAAR?"
- Allergic reaction. Symptoms of an allergic reaction are swelling of the face, lips, throat, or tongue and may include severe shortness of breath and/or difficulty breathing. Get emergency medical help right away and stop taking HYZAAR.
- Low blood pressure (hypotension). Low blood pressure may cause you to feel faint or dizzy. Lie down if you feel faint or dizzy. Call your doctor right away.
- If you have kidney problems, you may see a worsening in how well your kidneys work. Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.
- A new or worsening condition called systemic lupus erythematosus (Lupus; SLE)
-
Eye problems. One of the medicines in HYZAAR can cause eye problems that, if left untreated, may lead to vision loss. Symptoms of eye problems can happen within hours to weeks of starting HYZAAR. Tell your doctor right away if you have:
- decrease in vision
- eye pain
- Sensitivity of the skin to the sun and risk of skin cancer.
The most common side effects of HYZAAR in people with high blood pressure are:
- "colds" (upper respiratory infection)
- dizziness
- stuffy nose
- back pain
Tell your doctor if you get any side effect that bothers you or that won't go away. This is not a complete list of side effects. For a complete list, ask your doctor or pharmacist.
How should I store HYZAAR?
- Store HYZAAR at room temperature at 59°F to 86°F (15°C to 30°C).
- Keep HYZAAR in a tightly closed container, and keep HYZAAR out of the light.
- Keep HYZAAR and all medicines out of the reach of children.
General information about HYZAAR
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use HYZAAR for a condition for which it was not prescribed. Do not give HYZAAR to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about HYZAAR. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information that is written for health professionals.
What are the ingredients in HYZAAR?
Active ingredients: losartan potassium, hydrochlorothiazide
Inactive ingredients:
microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, titanium dioxide. HYZAAR 50/12.5 and HYZAAR 100/25 also contain D&C yellow No. 10 aluminum lake.
HYZAAR 50/12.5, HYZAAR 100/12.5, and HYZAAR 100/25 may also contain carnauba wax.
| HYZAAR
losartan potassium and hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| HYZAAR
losartan potassium and hydrochlorothiazide tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYZAAR
losartan potassium and hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Organon LLC (117494753) |