Drug Detail:Ibsrela (Tenapanor)
Drug Class: NHE3 inhibitors
Highlights of Prescribing Information
IBSRELA (tenapanor) tablets, for oral use
Initial U.S. Approval: 2019
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
See full prescribing information for complete boxed warning.
- IBSRELA is contraindicated in patients less than 6 years of age; in young juvenile rats, tenapanor caused death presumed to be due to dehydration. (4, 8.4)
- Avoid use of IBSRELA in patients 6 years to less than 12 years of age. (5.1, 8.4)
- The safety and effectiveness of IBSRELA have not been established in pediatric patients less than 18 years of age. (8.4)
Indications and Usage for Ibsrela
IBSRELA is a sodium/hydrogen exchanger 3 (NHE3) inhibitor indicated for treatment of irritable bowel syndrome with constipation (IBS-C) in adults. (1)
Ibsrela Dosage and Administration
- The recommended dosage in adults is 50 mg, orally twice daily. (2)
- Take immediately prior to breakfast or the first meal of the day and immediately prior to dinner. (2)
Dosage Forms and Strengths
Tablets: 50 mg tenapanor. (3)
Contraindications
- Pediatric patients less than 6 years of age. (4, 5.1, 8.4)
- Patients with known or suspected mechanical gastrointestinal obstruction. (4)
Warnings and Precautions
Diarrhea: Patients may experience severe diarrhea. If severe diarrhea occurs, suspend dosing and rehydrate patient. (5.2)
Adverse Reactions/Side Effects
Most common adverse reactions (≥2%) are diarrhea, abdominal distension, flatulence and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ardelyx at 1-844-427-7352 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
OATP2B1 Substrates: Potential for reduced exposure of the concomitant drug (e.g., enalapril). Monitor for signs related to loss of efficacy and adjust the dosage of the concomitantly administered drug as needed. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2022
Related/similar drugs
Linzess, Amitiza, lubiprostone, Trulance, linaclotide, plecanatideFull Prescribing Information
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
- IBSRELA is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile rats administration of tenapanor caused deaths presumed to be due to dehydration [see Contraindications (4), Use in Specific Populations (8.4)].
- Avoid use of IBSRELA in patients 6 years to less than 12 years of age [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
- The safety and effectiveness of IBSRELA have not been established in patients less than 18 years of age [see Use in Specific Populations (8.4)].
1. Indications and Usage for Ibsrela
IBSRELA is indicated for treatment of irritable bowel syndrome with constipation (IBS-C) in adults.
2. Ibsrela Dosage and Administration
The recommended dosage of IBSRELA in adults is 50 mg orally twice daily.
3. Dosage Forms and Strengths
Tablets: 50 mg tenapanor supplied as an oval, white to off-white tablet debossed with "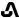 50" on one side and "5791" on the other side.
50" on one side and "5791" on the other side.
4. Contraindications
IBSRELA is contraindicated in:
- Patients less than 6 years of age due to the risk of serious dehydration [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
- Patients with known or suspected mechanical gastrointestinal obstruction.
5. Warnings and Precautions
5.1 Risk of Serious Dehydration in Pediatric Patients
IBSRELA is contraindicated in patients below 6 years of age. The safety and effectiveness of IBSRELA in patients less than 18 years of age have not been established. In young juvenile rats (less than 1 week old; approximate human age equivalent of less than 2 years of age), decreased body weight and deaths occurred, presumed to be due to dehydration, following oral administration of tenapanor. There are no data available in older juvenile rats (human age equivalent 2 years to less than 12 years).
Avoid the use of IBSRELA in patients 6 years to less than 12 years of age. Although there are no data in older juvenile rats, given the deaths in younger rats and the lack of clinical safety and efficacy data in pediatric patients, avoid the use of IBSRELA in patients 6 years to less than 12 years of age [see Contraindications (4), Warnings and Precautions (5.2), Use in Specific Populations (8.4)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect data from 1203 adult patients with IBS-C in two randomized, double-blind, placebo-controlled clinical trials (Trial 1 and Trial 2). Patients were randomized to receive placebo or IBSRELA 50 mg twice daily for up to 52 weeks. Demographic characteristics were comparable between treatment groups in the two trials [see Clinical Studies (14)].
7. Drug Interactions
7.1 OATP2B1 Substrates
Tenapanor is an inhibitor of intestinal uptake transporter, OATP2B1 [see Clinical Pharmacology (12.3)]. Drugs which are substrates of OATP2B1 may have reduced exposures when concomitantly taken with IBSRELA. Monitor for signs related to loss of efficacy and adjust the dosage of concomitantly administered drug as needed.
Enalapril is a substrate of OATP2B1. When enalapril was coadministered with tenapanor (30 mg twice daily for five days, a dosage 0.6 times the recommended dosage), the peak exposure (Cmax) of enalapril and its active metabolite, enalaprilat, decreased by approximately 70% and total systemic exposures (AUC) decreased by approximately 50% to 65% compared to when enalapril was administered alone [see Clinical Pharmacology (12.3)].
Monitor blood pressure and increase the dosage of enalapril, if needed, when IBSRELA is coadministered with enalapril.
8. Use In Specific Populations
8.4 Pediatric Use
IBSRELA is contraindicated in patients less than 6 years of age. Avoid IBSRELA in patients 6 years to less than 12 years of age [see Contraindications (4), Warnings and Precautions (5.1)].
The safety and effectiveness of IBSRELA in patients less than 18 years of age have not been established.
In nonclinical studies, deaths occurred in young juvenile rats (less than 1 week-old-rats approximate human age equivalent of less than 2 years of age) following oral administration of tenapanor, as described below in Juvenile Animal Toxicity Data.
8.5 Geriatric Use
Of the 1203 patients in placebo-controlled clinical trials of IBSRELA, 100 (8%) were 65 years of age and older. No overall differences in safety or effectiveness were observed between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
10. Overdosage
Based on nonclinical data, overdose of IBSRELA may result in gastrointestinal adverse effects such as diarrhea as a result of exaggerated pharmacology with a risk for dehydration if diarrhea is severe or prolonged [see Warnings and Precautions (5.1)].
11. Ibsrela Description
IBSRELA (tenapanor) tablets contain tenapanor hydrochloride as an active ingredient. Tenapanor hydrochloride is a sodium/hydrogen exchanger 3 (NHE3) inhibitor for oral use. The chemical name for tenapanor hydrochloride is 12,15-Dioxa-2,7,9-triazaheptadecanamide, 17-[[[3-[(4S)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl]phenyl]sulphonyl]amino]-N-[2-[2-[2-[[[3-[(4S)-6,8-dichloro-1,2,3,4-tetrahydro-2-methyl-4-isoquinolinyl]phenyl]sulphonyl]amino]ethoxy]ethoxy]ethyl]-8-oxo-, hydrochloride (1:2). Tenapanor hydrochloride has the molecular formula of C50H68Cl6N8O10S2, the molecular weight of 1218 Daltons, and the chemical structure below:
Tenapanor hydrochloride is a white to off-white to light brown hygroscopic amorphous solid. It is practically insoluble in water.
IBSRELA tablets contain 50 mg of tenapanor (equivalent to 53.2 mg of tenapanor hydrochloride). Inactive ingredients in the tablet are colloidal silicon dioxide, hypromellose, low-substituted hydroxypropyl cellulose, microcrystalline cellulose, propyl gallate, stearic acid, tartaric acid powder, titanium dioxide and triacetin.
12. Ibsrela - Clinical Pharmacology
12.1 Mechanism of Action
Tenapanor is a locally acting inhibitor of the sodium/hydrogen exchanger 3 (NHE3), an antiporter expressed on the apical surface of the small intestine and colon primarily responsible for the absorption of dietary sodium. In vitro and animal studies indicate its major metabolite, M1, is not active against NHE3. By inhibiting NHE3 on the apical surface of the enterocytes, tenapanor reduces absorption of sodium from the small intestine and colon, resulting in an increase in water secretion into the intestinal lumen, which accelerates intestinal transit time and results in a softer stool consistency.
Tenapanor has also been shown to reduce abdominal pain by decreasing visceral hypersensitivity and by decreasing intestinal permeability in animal models. In rat model of colonic hypersensitivity, tenapanor reduced visceral hyperalgesia and normalized colonic sensory neuronal excitability.
14. Clinical Studies
The efficacy of IBSRELA for the treatment of IBS-C was established in two double-blind, placebo-controlled, randomized, multicenter trials in adult patients: Trial 1 (TEN-01-302; NCT02686138) and Trial 2 (TEN-01-301; NCT02621892). The intent-to-treat (ITT) analysis population included 620 patients in Trial 1 and 606 patients in Trial 2 with mean age of 46 years (range 18 to 75 years), 80% females, 64% White and 31% Black/African American. In these clinical trials, IBSRELA was administered immediately prior to breakfast or the first meal of the day and immediately prior to dinner.
To enter the trials, all patients met Rome III criteria for IBS-C and were required to meet the following clinical criteria during the 2-week baseline run-in period:
- a mean abdominal pain score of at least 3 on a 0-to-10-point numeric rating scale where a score of 0 indicates no pain and 10 indicates very severe pain
- less than 3 complete spontaneous bowel movements (CSBMs) per week, where a CSBM is defined as a spontaneous bowel movement (SBM) that is associated with a sense of complete evacuation (an SBM is a bowel movement occurring in the absence of laxative use)
- less than or equal to 5 SBMs per week
The trial designs were identical through the first 12 weeks of treatment, and thereafter differed in that Trial 1 continued for an additional 14 weeks of treatment (26 weeks double-blind treatment), whereas Trial 2 included a 4-week randomized withdrawal (RW) period.
Efficacy of IBSRELA was assessed using responder analyses based on daily diary entries.
In both trials, the primary endpoint was the proportion of responders, where a responder was defined as a patient achieving both the stool frequency and abdominal pain intensity responder criteria in the same week for at least 6 of the first 12 weeks of treatment. The stool frequency (CSBM) and abdominal pain responder criteria assessed each week were defined as:
- CSBM responder: a patient who experienced an increase of at least 1 CSBM in weekly average from baseline.
- Abdominal pain responder: a patient who experienced at least a 30% reduction in the weekly average of abdominal pain score compared with baseline.
The responder rates for the primary endpoint and components of the primary endpoint (CSBM and abdominal pain), which were pre-specified key secondary endpoints, are shown in Table 2.
|
|||
| Trial 1 | |||
| IBSRELA N=293 | Placebo N=300 | Treatment Difference [95% CI*] |
|
| Responder† | 37% | 24% | 13% [6%, 20%] |
| Components of Responder Endpoint: | |||
| CSBM Responder‡ | 47% | 33% | |
| Abdominal Pain Responder§ | 50% | 38% | |
| Trial 2 | |||
| Responder Rates | IBSRELA N=307 | Placebo N=299 | Treatment Difference [95% CI*] |
| Responder† | 27% | 19% | 8% [2%, 15%] |
| Components of Responder Endpoint: | |||
| CSBM Responder‡ | 34% | 29% | |
| Abdominal Pain Responder§ | 44% | 33% | |
In Trials 1 and 2, the proportion of responders for 9 out of the first 12 weeks, including at least 3 of the last 4 weeks, was greater in IBSRELA-treated patients compared to placebo-treated patients. In addition, in Trial 1, the proportion of responders for 13 out of 26 weeks was greater in IBSRELA-treated patients compared to placebo-treated patients.
In both trials, improvements from baseline in average weekly CSBMs and abdominal pain were observed by Week 1, with improvement maintained through the end of treatment.
In IBSRELA-treated patients re-randomized to placebo in Trial 2, CSBM frequency and abdominal pain severity worsened on average over the 4-week period but remained improved compared to baseline. Patients who continued on IBSRELA maintained their response to therapy on average over the additional 4 weeks. Patients on placebo who were re-randomized to IBSRELA had an average increase in CSBM frequency and a decrease in abdominal pain.
16. How is Ibsrela supplied
IBSRELA tablets contain 50 mg tenapanor and are oval, white to off-white, debossed with " 50" on one side and "5791" on the other side.
50" on one side and "5791" on the other side.
IBSRELA is supplied in a white, opaque, high-density polyethylene bottle containing 60 tablets with a silica gel canister (as the desiccant) and screw-top polypropylene child-resistant cap lined and induction-activated aluminum foil liner (NDC 73154-050-60).
17. Patient Counseling Information
Advise the patients to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: May 2021 |
| Medication Guide IBSRELA® (ibs rel`a) (tenapanor) tablets, for oral use |
|
What is the most important information I should know about IBSRELA?
See "What are the possible side effects of IBSRELA?" for more information about side effects. |
|
| What is IBSRELA?
IBSRELA is a prescription medicine used in adults to treat:
|
|
Who should not take IBSRELA?
|
|
Before you take IBSRELA, tell your doctor about all your medical conditions, including if you:
|
|
How should I take IBSRELA?
|
|
| What are the possible side effects of IBSRELA? IBSRELA can cause serious side effects, including:
|
|
How should I store IBSRELA?
|
|
| General information about the safe and effective use of IBSRELA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use IBSRELA for a condition for which it was not prescribed. Do not give IBSRELA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about IBSRELA that is written for health professionals. |
|
| What are the ingredients in IBSRELA? Active ingredient: tenapanor hydrochloride Inactive ingredients: colloidal silicon dioxide, hypromellose, low-substituted hydroxypropyl cellulose, microcrystalline cellulose, propyl gallate, stearic acid, tartaric acid, titanium dioxide, and triacetin. Manufactured for and distributed by Ardelyx, Inc. Waltham, MA 02451 USA IBSRELA® is a registered trademark of Ardelyx, Inc. Patent: www.IBSRELA-patents.com For more information, go to www.ardelyx.com or call 1-844-427-7352 |
|
| IBSRELA
tenapanor hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ardelyx, Inc. (827436556) |






