Drug Detail:Imovax rabies (human diploid cell) (Rabies vaccine (human diploid cell) [ ray-beez-vax-een ])
Drug Class: Viral vaccines
Imovax Rabies Description
The Imovax® Rabies Vaccine produced by Sanofi Pasteur SA is a sterile, stable, freeze-dried suspension of rabies virus prepared from strain PM-1503-3M obtained from the Wistar Institute, Philadelphia, PA.
The virus is harvested from infected human diploid cells, MRC-5 strain, concentrated by ultrafiltration and is inactivated by beta-propiolactone. One dose of reconstituted vaccine contains less than 100 mg human albumin, less than 150 mcg neomycin sulfate and 20 mcg of phenol red indicator. Beta-propiolactone, a residual component of the manufacturing process, is present in less than 50 parts per million.
The finished, freeze-dried vaccine is provided for intramuscular administration in a single dose vial containing no preservative. After reconstitution, immediately administer the full 1.0 mL amount of vaccine. If it cannot be administered promptly, discard.
The potency of one dose (1.0 mL) of Imovax Rabies vaccine is equal to or greater than 2.5 international units of rabies antigen.
Related/similar drugs
RabAvert, rabies vaccine, purified chick embryo cell, Imogam Rabies-HT, HyperRAB S/DIndications and Usage for Imovax Rabies
Imovax Rabies is a vaccine indicated for pre-exposure and post-exposure prophylaxis against rabies. Imovax Rabies vaccine is approved for use in all age groups.
1. Rationale of treatment
Physicians must evaluate each possible rabies exposure. Local or state public health officials should be consulted if questions arise about the need for prophylaxis. (11)
The following factors should be considered before antirabies prophylaxis is initiated.
2. Pre- and post-exposure prophylaxis of rabies
A. Pre-exposure
Pre-exposure immunization should be offered to rabies researchers, certain laboratory workers and other persons in high-risk groups, such as veterinarians and their staff, and animal handlers. Pre-exposure vaccination also should be considered for persons whose activities bring them into frequent contact with rabies virus or potentially rabid bats, raccoons, skunks, cats, dogs, or other species at risk for having rabies. In addition, some international travelers might be candidates for pre-exposure vaccination if they are likely to come in contact with animals in areas where dog or other animal rabies is enzootic and immediate access to appropriate medical care, including rabies vaccine and immune globulin, might be limited. (11)
Vaccination is recommended for children living in or visiting countries where exposure to rabid animals is a constant threat. Worldwide statistics indicate children are more at risk than adults.
Pre-exposure prophylaxis is administered for several reasons. First, although pre-exposure vaccination does not eliminate the need for additional medical evaluation after a rabies exposure, it simplifies management by eliminating the need for Rabies Immune Globulin (RIG) and decreases the number of doses of vaccine needed. This is particularly important for persons at high risk for being exposed to rabies in areas where modern immunizing products might not be available or where cruder, less safe biologics might be used, placing the exposed person at increased risk for adverse events. Second, pre-exposure prophylaxis might offer partial immunity to persons whose post-exposure prophylaxis is delayed. Finally, pre-exposure prophylaxis might provide some protection to persons at risk for unrecognized exposures to rabies. (11)
PRE-EXPOSURE RABIES PROPHYLAXIS GUIDE
Pre-exposure prophylaxis consists of three 1.0 mL doses of Imovax Rabies vaccine administered intramuscularly, using a sterile needle and syringe, one injection per day on Days 0, 7, and 21 or 28. In adults and older children, the vaccine should be administered in the deltoid muscle. In infants and small children, the anterolateral aspect of the thigh may be preferable, depending on age and body mass.
Administration of booster doses of vaccine depends on exposure risk category and serologic testing as noted in Table 1.
Immunosuppressed persons should postpone pre-exposure vaccinations and consider avoiding activities for which rabies pre-exposure prophylaxis is indicated. When this course is not possible, immunosuppressed persons who are at risk for rabies should have their viral neutralizing antibody titers checked after completing the pre-exposure series. If no acceptable antibody response is detected, the patient should be managed in consultation with their physician and appropriate public health officials. (11)
| Risk category | Nature of risk | Typical populations | Pre-exposure recommendations |
|---|---|---|---|
|
|||
| Continuous | Virus present continuously and often in high concentrations. Specific exposures likely to go unrecognized. Bite, nonbite, or aerosol exposure. | Rabies research laboratory workers; rabies biologics production workers. | Primary course. Serologic testing every 6 months; booster vaccination if antibody titer is below acceptable level.* |
| Frequent | Exposure usually episodic, with source recognized, but exposure also might be unrecognized. Bite, nonbite, or aerosol exposure. | Rabies diagnostic laboratory workers, cavers, veterinarians and staff, and animal-control and wildlife workers in areas where rabies is enzootic. All persons who frequently handle bats. | Primary course. Serologic testing every 2 years; booster vaccination if antibody titer is below acceptable level.* |
| Infrequent (greater than population at large) | Exposure nearly always episodic with source recognized. Bite or nonbite exposure. | Veterinarians and animal-control staff working with terrestrial animals in areas where rabies is uncommon to rare. Veterinary students. Travelers visiting areas where rabies is enzootic and immediate access to appropriate medical care including biologics is limited. | Primary course. No serologic testing or booster vaccination. |
| Rare (population at large) | Exposure always episodic with source recognized. Bite or nonbite exposure. | US population at large, including persons in areas where rabies is epizootic. | No vaccination necessary. |
B. Post-exposure
The essential components of rabies post-exposure prophylaxis are wound treatment and, for previously unvaccinated persons, the administration of both human rabies immune globulin (RIG) and vaccine. (11)
POST-EXPOSURE RABIES PROPHYLAXIS GUIDE
The following recommendations are only a guide. In applying them, take into account the animal species involved, the circumstances of the bite or other exposure, the vaccination status of the animal, the availability of the exposing animal for observation or rabies testing, and the presence of rabies in the region (see Table 2). Local or state public health officials should be consulted if questions arise about the need for rabies prophylaxis. (11)
| Animal type | Evaluation and disposition of animal | Post-exposure prophylaxis recommendations |
|---|---|---|
|
||
| Dogs, cats, and ferrets | Healthy and available for 10 days observation. | Persons should not begin prophylaxis unless animal develops clinical signs of rabies.* |
| Rabid or suspected rabid. | Immediately begin prophylaxis. | |
| Unknown (eg, escaped). | Consult public health officials. | |
| Skunks, raccoons, foxes, and most other carnivores; bats† | Regard as rabid unless animal proven negative by laboratory tests.‡ | Consider immediate prophylaxis. |
| Livestock, small rodents (rabbits and hares), large rodents (woodchucks and beavers), and other mammals | Consider individually. | Consult public health officials. Bites from squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, mice, other small rodents, rabbits, and hares almost never require anti-rabies post-exposure prophylaxis. |
Warnings
- Do not inject the vaccine into the gluteal area as administration in this area may result in lower neutralizing antibody titers. (11)
- The product is provided in a single dose vial. Because the single dose vial contains no preservative, it is not to be used as a multidose vial for intradermal injection.
- In both pre-exposure and post-exposure immunization, the full 1.0 mL dose should be given intramuscularly.
- Serum sickness type reactions have been reported in persons receiving booster doses of rabies vaccine for pre-exposure prophylaxis. The reaction is characterized by onset approximately 2 to 21 days post-booster, presents with a generalized urticaria, and may also include arthralgia, arthritis, angioedema, nausea, vomiting, fever, and malaise. None of the reported reactions were life-threatening. This has been reported in up to 7% of persons receiving booster vaccination. (13)
- Rare cases of neurologic illness resembling Guillain-Barré syndrome, (14) (15) a transient neuroparalytic illness, that resolved without sequelae in 12 weeks and a focal subacute central nervous system disorder temporally associated with HDCV, have been reported. (16)
- This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD, or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
All serious systemic neuroparalytic or anaphylactic reactions to a rabies vaccine should be immediately reported to VAERS at 1-800-822-7967 (http://vaers.hhs.gov) or Sanofi Pasteur Inc., 1-800-VACCINE (1-800-822-2463).
- Vaccination with Imovax Rabies vaccine may not protect 100% of vaccinated individuals.
Precautions
- IN ADULTS AND CHILDREN THE VACCINE SHOULD BE INJECTED INTO THE DELTOID MUSCLE. IN INFANTS AND SMALL CHILDREN, THE ANTEROLATERAL ASPECT OF THE THIGH MAY BE PREFERABLE.
- When a person with a history of hypersensitivity must be given rabies vaccine, antihistamines may be given. Epinephrine (1:1000) and other appropriate agents should be readily available to counteract anaphylactic reactions, and the person should be carefully observed after immunization.
- While the concentration of antibiotics in each dose of vaccine is extremely small, persons with known hypersensitivity to any of these agents, or any other component of the vaccine, could manifest an allergic reaction. While the risk is small, it should be weighed in light of the potential risk of contracting rabies.
Imovax Rabies Dosage and Administration
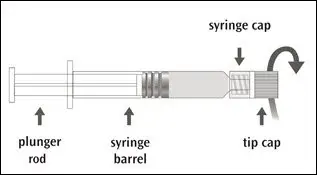
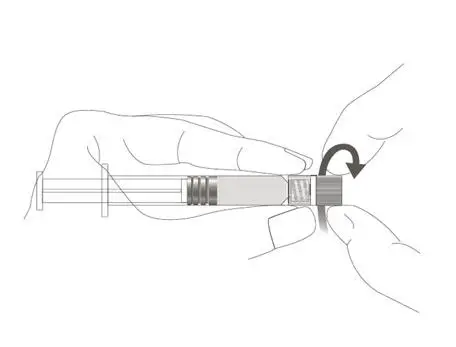
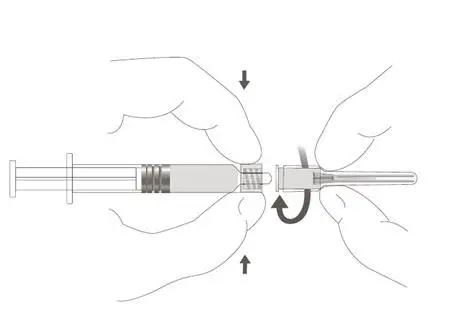
The package contains a vial of freeze-dried vaccine, a Luer-Lok™ syringe containing 1.0 mL of diluent with a plunger rod either inserted into the syringe or provided separately, and a sterile reconstitution needle. The syringe and its package should be inspected prior to use for evidence of leakage, premature activation of the plunger rod, or a faulty tip seal. If evidence of such defects is observed, the product should not be used.
| IMOVAX RABIES
rabies virus strain pm-1503-3m antigen (propiolactone inactivated) and water kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| IMOVAX RABIES
rabies virus strain pm-1503-3m antigen (propiolactone inactivated) and water kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur | 578763542 | MANUFACTURE(49281-250, 49281-252) | |