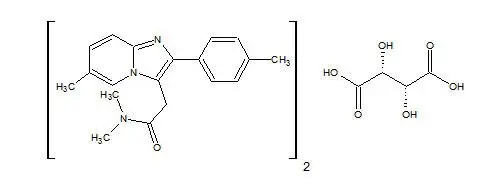
Drug Detail:Intermezzo (Zolpidem sublingual [ zole-pi-dem ])
Drug Class: Miscellaneous anxiolytics, sedatives and hypnotics
Highlights of Prescribing Information
INTERMEZZO® (zolpidem tartrate) sublingual tablets, CIV
Initial U.S. Approval: 1992
WARNING:
COMPLEX SLEEP BEHAVIORS
See full prescribing
information for complete box warning.
Complex sleep behaviors including sleep-walking, sleep- driving, and engaging in other activities while not fully awake may occur following use of INTERMEZZO. Some of these events may result in serious injuries, including death. Discontinue INTERMEZZO immediately if a patient experiences a complex sleep behavior (4, 5.1).
Indications and Usage for Intermezzo
INTERMEZZO is a GABAA agonist indicated for use as needed for the treatment of insomnia when a middle-of-the-night awakening is followed by difficulty returning to sleep (1)
Limitation of Use: Not indicated for the treatment of middle-of-the night awakening when the patient has fewer than 4 hours of bedtime remaining before the planned time of waking (1)
Intermezzo Dosage and Administration
- Take only if 4 hours of bedtime remain before the planned time of waking (2.1, 5.1)
- INTERMEZZO should be placed under the tongue and allowed to disintegrate completely before swallowing. The tablet should not be swallowed whole. (2.1)
- The effect of INTERMEZZO may be slowed if taken with or immediately after a meal (2.1)
- Recommended dose is 1.75 mg for women and 3.5 mg for men, taken only once per night if needed (2.2)
- Lower doses of CNS depressants may be necessary when taken concomitantly with INTERMEZZO (2.3)
- Co-administration with CNS depressants: Recommended dose is 1.75 mg for men and women (2.3)
- Geriatric patients and patients with hepatic impairment: Recommended dose is 1.75 mg for men and women (2.4, 2.5)
Dosage Forms and Strengths
1.75 mg and 3.5 mg sublingual tablets (3)
Contraindications
Patients who have experienced complex sleep behaviors after taking INTERMEZZO (4, 5.1)
Known hypersensitivity to zolpidem (4)
Warnings and Precautions
- CNS depressant effects: Impairs alertness and motor coordination, including risk of morning impairment. Risk increases with dose and use with other CNS depressants and alcohol. Instruct patients on correct use (5.2)
- Evaluate for co-morbid diagnoses: Re-evaluate if insomnia persists after 7 to 10 days of use (5.2)
- Severe anaphylactic/anaphylactoid reactions: Angioedema and anaphylaxis have been reported. Do not re-challenge if such reactions occur (5.3)
- Abnormal Thinking and Behavioral Changes: Changes including decreased inhibition, bizarre behavior, agitation and depersonalization have been reported. Immediately evaluate any new onset behavioral changes (5.5)
- Depression: Worsening of depression or suicidal thinking may occur. Prescribe the least number of tablets feasible to avoid intentional overdose (5.6)
- Respiratory Depression: Consider this risk before prescribing in patients with compromised respiratory function (5.7)
Adverse Reactions/Side Effects
Most commonly observed adverse reactions
(> 1% in adult patients) are headache, nausea, and fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Purdue Pharma at 1-888-726-7535 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CNS depressants, including alcohol: Possible adverse additive CNS depressant effects (5.1, 7.1)
- Imipramine: Decreased alertness observed (7.1)
- Chlorpromazine: Impaired alertness and psychomotor performance observed (7.1)
- Rifampin: Combination use may decrease effects (7.2)
- Ketoconazole: Combination use may increase effects (7.2)
Use In Specific Populations
- Pregnancy: Based on animal data, zolpidem may cause fetal harm. (8.1)
- Pediatric use: Safety and effectiveness of INTERMEZZO not established. With bedtime dosing of zolpidem, hallucinations observed (incidence 7%) (8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
Full Prescribing Information
Intermezzo Dosage and Administration
Dosage Forms and Strengths
INTERMEZZO is available as 1.75 mg and 3.5 mg tablets for sublingual administration.
INTERMEZZO 1.75 mg tablets are yellow, round, uncoated, biconvex, debossed with ZZ on one side.
INTERMEZZO 3.5 mg tablets are beige, round, uncoated, biconvex, debossed with ZZ on one side.
Warnings and Precautions
Adverse Reactions/Side Effects
- Complex Sleep Behaviors [See Warnings and Precautions (5.1)]
- CNS-depressant effects and next-day impairment [see Warnings and Precautions (5.2)]
- Serious anaphylactic and anaphylactoid reactions [see Warnings and Precautions (5.2)]
- Abnormal Thinking and Behavioral Changes [see Warnings and Precautions (5.5)]
- Withdrawal effects [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
| MedDRA System Organ Class Preferred Term | 3.5 mg INTERMEZZO
(n=150) | Placebo
(n=145) |
|---|---|---|
| Gastrointestinal Disorders | 4% | 2% |
| Nausea | 1% | 1% |
| General Disorders and Administration Site Conditions | 3% | 0% |
| Fatigue | 1% | 0% |
| Nervous System Disorders | 5% | 3% |
| Headache | 3% | 1% |
Use In Specific Populations
8.3 Nursing Mothers
Zolpidem is excreted in human milk. The effect of zolpidem on the nursing infant is not known.
8.5 Geriatric Use
| Adverse Reaction | 5 to 10 mg Oral Zolpidem tartrate | Placebo |
| Dizziness | 3% | 0% |
| Drowsiness | 5% | 2% |
| Diarrhea | 3% | 1% |
Drug Abuse and Dependence
9.1 Controlled Substance
Zolpidem tartrate is classified as a Schedule IV controlled substance by federal regulation.
How is Intermezzo supplied
Each sublingual tablet is individually packaged in a unit-dose pouch.
NDC 59011-256-30: Carton of 30 unit-dose pouches
Patient Counseling Information
See FDA-approved patient labeling (Medication Guide).
Suicide
Tell patients to immediately
report any suicidal thoughts.
Tell patients that the effect of INTERMEZZO may be slowed if taken with or immediately after a meal.
Instruct patients to remove the tablet from the unit-dose pouch just prior to dosing.
Advise patients NOT to take INTERMEZZO if they drank alcohol that day or before bed.
U.S. Patent Numbers 7,658,945; 7,682,628; 8,242,131; 8,252,809
Medication Guide
(zolpidem tartrate) sublingual tablet CIV
What is the most important information I should know about INTERMEZZO?
- Only take one tablet a night, if needed.
- Only take INTERMEZZO if you have at least 4 hours of bedtime left.
- Complex sleep behaviors that have caused serious injury and death. After taking INTERMEZZO, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing (complex sleep behaviors). The next morning, you may not remember that you did anything during the night. These activities may happen whether or not you drink alcohol or take other medicines that make you sleepy with INTERMEZZO.
INTERMEZZO may cause serious side effects, including:
- driving a car ("sleep-driving")
- making and eating food
- talking on the phone
- having sex
- sleep-walking
- Take INTERMEZZO exactly as prescribed
-
Do not take INTERMEZZO if you:
- drank alcohol that day or before bed.
- took another medicine to help you sleep.
- do not have at least 4 hours of bedtime remaining.
It is not known if INTERMEZZO is safe and effective in children.
Who should not take INTERMEZZO?
- Do not take INTERMEZZO if you have ever had a complex sleep behavior happen after taking INTERMEZZO.
- Do not take INTERMEZZO if you are allergic to zolpidem or any other ingredients in INTERMEZZO. See the end of this Medication Guide for a complete list of ingredients in INTERMEZZO.
- Do not take INTERMEZZO if you have had an allergic reaction to drugs containing zolpidem, such as Ambien, Ambien CR, Edluar, or Zolpimist.
Symptoms of a serious allergic reaction to INTERMEZZO can include:
- swelling of your face, lips, and throat that may cause difficulty breathing or swallowing
- nausea and vomiting
- have a history of depression, mental illness, or suicidal thoughts
- have a history of drug or alcohol abuse or addiction
- have kidney or liver disease
- have a lung disease or breathing problems
- are pregnant, planning to become pregnant, or breastfeeding
- See “What is the most important information I should know about INTERMEZZO”
- Read the "Instructions for Use" at the end of this Medication Guide for detailed instructions on how to take INTERMEZZO.
- Take INTERMEZZO exactly as prescribed. Only take one INTERMEZZO tablet per night if needed.
- Do not take INTERMEZZO if you drank alcohol that evening or before bed.
- While in bed, place the tablet under your tongue and allow it to break apart completely. Do not swallow it whole.
- You should not take INTERMEZZO with or right after a meal. INTERMEZZO may help you fall asleep faster when you take it on an empty stomach.
- Call your health care provider if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- If you take too much INTERMEZZO or overdose, get emergency treatment.
What are the possible side effects of INTERMEZZO?
INTERMEZZO may cause serious side effects, including:
- getting out of bed while not being fully awake and doing an activity that you do not know you are doing. (See “What is the most important information I should know about INTERMEZZO?”)
- abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, hallucinations, worsening of depression, and suicidal thoughts or actions.
- memory loss
- anxiety
- severe allergic reactions. Symptoms include swelling of the tongue or throat, trouble breathing, and nausea and vomiting. Get emergency medical help if you get these symptoms after taking INTERMEZZO.
The most common side effects of INTERMEZZO are:
- Headache
- Nausea
- Fatigue
You may report side effects to FDA at 1-800-FDA-1088.
How should I store INTERMEZZO?
- Store INTERMEZZO at room temperature, 68° to 77°F (20° to 25°C). Protect from moisture.
- Only open the pouch when you are ready to use INTERMEZZO.
Keep INTERMEZZO and all medicines out of reach of children.
General Information about INTERMEZZO
What are the ingredients in INTERMEZZO?
Active Ingredient: Zolpidem tartrate
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Instructions
for Use
INTERMEZZO® (in ter
mét zoh)
(zolpidem tartrate) sublingual tablet CIV
What is the most important Information I should know about INTERMEZZO?
- Only take 1 tablet a night if needed
- Only take INTERMEZZO if you have at least 4 hours of bedtime left
Using INTERMEZZO the wrong way can make you drowsy in the morning.
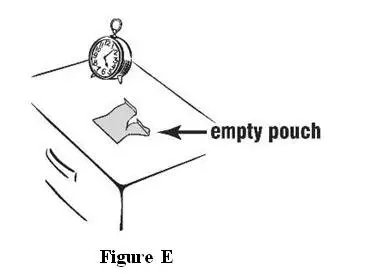
- Place only 1 INTERMEZZO pouch by your bed, and have a clock
or watch nearby (see Figure A).
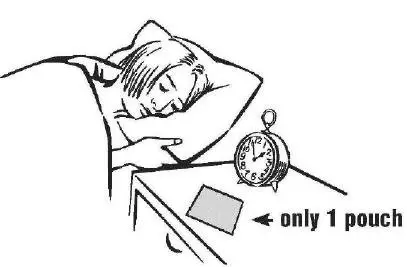
Figure A - Store all other unopened INTERMEZZO pouches with your other medicines away from your bedside.
- Only open the INTERMEZZO pouch when you are ready to use it.
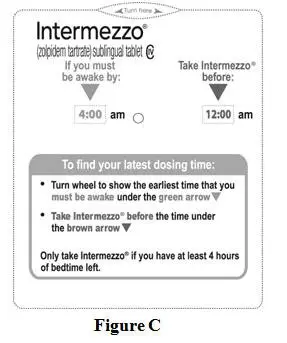
- You can either use the INTERMEZZO Dosing Time Chart (see Figure B) or the Dosing Time Tool (see Figure C) that comes with INTERMEZZO to find the latest time during the night you can take INTERMEZZO.
INTERMEZZO Dosing Time Chart (see Figure B):
- You can take INTERMEZZO if you have at least 4 hours of bedtime left before you must be awake.
- Find the earliest time you have to be up and awake in the column on the left.
- Find the latest time you can take INTERMEZZO on the same line in the column on the right.
| Figure B | |
| If you must be awake by: | Take INTERMEZZO before: |
| 4 am | 12 midnight |
| 5 am | 1 am |
| 6 am | 2 am |
| 7 am | 3 am |
| 8 am | 4 am |
| 9 am | 5 am |
INTERMEZZO Dosing Time Tool (see Figure C):
- Turn the INTERMEZZO Dosing Time Tool wheel to show the earliest time that you must be awake under the green arrow.
- Take INTERMEZZO before the time under the brown arrow.
During the night when you take INTERMEZZO:
- Only take INTERMEZZO if you have at least 4 hours of bedtime left before you have to be awake (see Figure B).
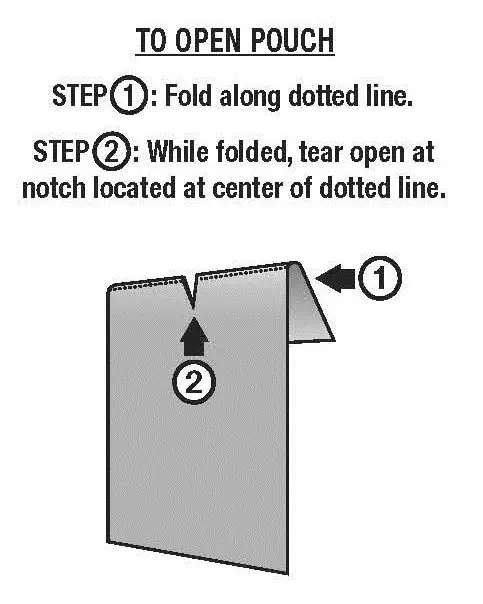
Step 2. Open the INTERMEZZO pouch you placed by your bed.
- Fold the INTERMEZZO pouch along the dotted line. While the INTERMEZZO pouch is folded, tear the pouch open at the notch at the center of the dotted line (see Figure D).
Step 3. Remove the tablet from the INTERMEZZO pouch.
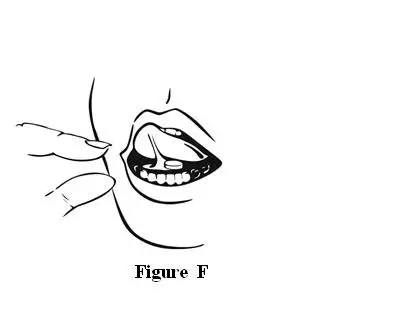
Step 6. Throw the empty INTERMEZZO pouch away in the morning.
| INTERMEZZO
zolpidem tartrate tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| INTERMEZZO
zolpidem tartrate tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Purdue Pharma LP (932323652) |
| Registrant - Purdue Pharma LP (932323652) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Pharmaceuticals, Inc. | 005286822 | MANUFACTURE(59011-255, 59011-256) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Corporation | 002346625 | PACK(59011-255, 59011-256) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Corporation | 143696495 | PACK(59011-255, 59011-256) | |