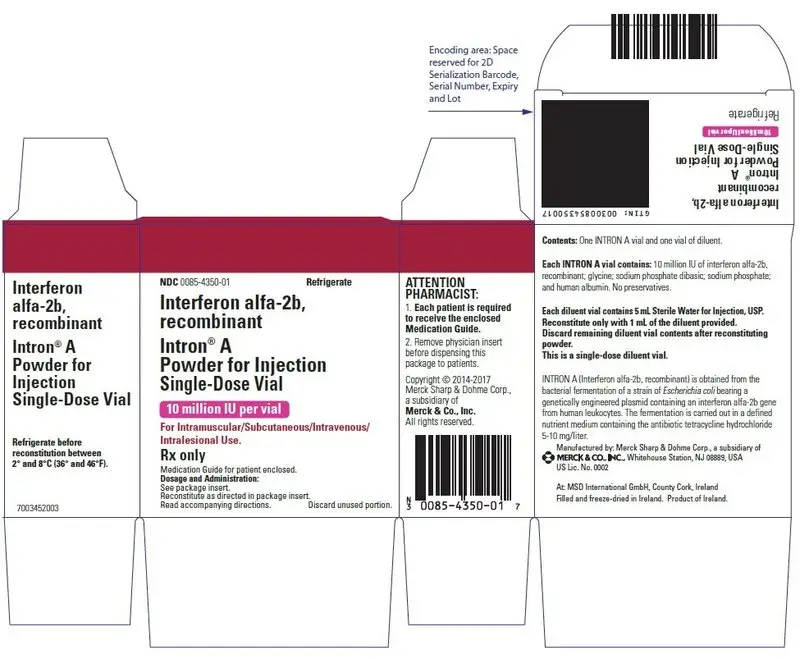
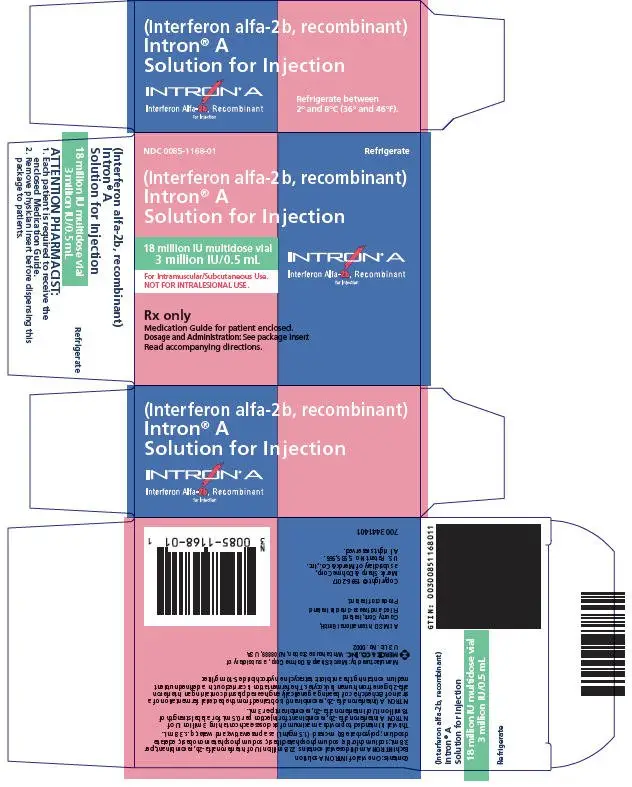
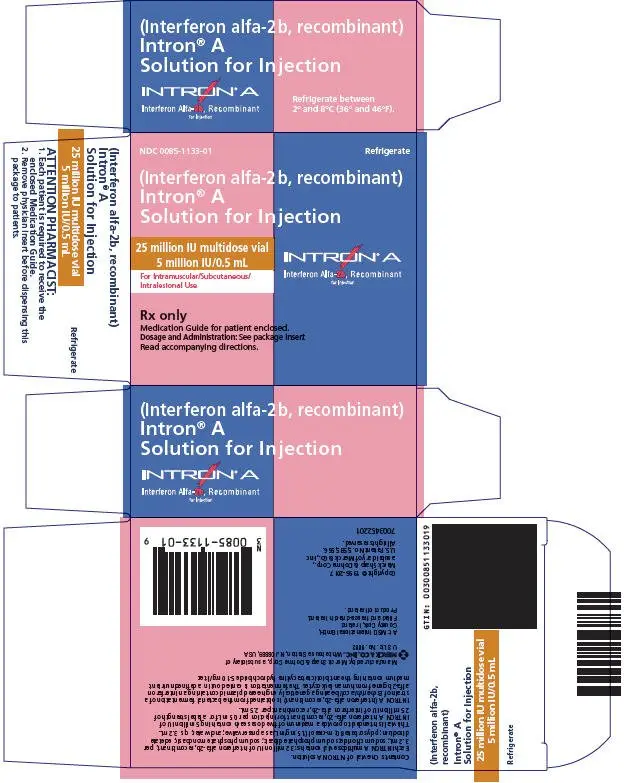
Drug Detail:Intron a (Interferon alfa-2b [ in-ter-fear-on-al-fa-2b ])
Drug Class: Antineoplastic interferons
WARNING
Alpha interferons, including INTRON® A, cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Patients with persistently severe or worsening signs or symptoms of these conditions should be withdrawn from therapy. In many but not all cases these disorders resolve after stopping INTRON A therapy. See WARNINGS and ADVERSE REACTIONS.
Intron A - Clinical Pharmacology
Chronic Hepatitis C
The safety and efficacy of INTRON A in the treatment of chronic hepatitis C was evaluated in 5 randomized clinical studies in which an INTRON A dose of 3 million IU three times a week (TIW) was assessed. The initial three studies were placebo-controlled trials that evaluated a 6-month (24-week) course of therapy. In each of the three studies, INTRON A therapy resulted in a reduction in serum alanine aminotransferase (ALT) in a greater proportion of patients versus control patients at the end of 6 months of dosing. During the 6 months of follow-up, approximately 50% of the patients who responded maintained their ALT response. A combined analysis comparing pretreatment and post-treatment liver biopsies revealed histological improvement in a statistically significantly greater proportion of INTRON A-treated patients compared to controls.
Two additional studies have investigated longer treatment durations (up to 24 months).5,6 Patients in the two studies to evaluate longer duration of treatment had hepatitis with or without cirrhosis in the absence of decompensated liver disease. Complete response to treatment was defined as normalization of the final two serum ALT levels during the treatment period. A sustained response was defined as a complete response at the end of the treatment period, with sustained normal ALT values lasting at least 6 months following discontinuation of therapy.
In Study 1, all patients were initially treated with INTRON A 3 million IU TIW subcutaneously for 24 weeks (run-in-period). Patients who completed the initial 24-week treatment period were then randomly assigned to receive no further treatment, or to receive 3 million IU TIW for an additional 48 weeks. In Study 2, patients who met the entry criteria were randomly assigned to receive INTRON A 3 million IU TIW subcutaneously for 24 weeks or to receive INTRON A 3 million IU TIW subcutaneously for 96 weeks. In both studies, patient follow-up was variable and some data collection was retrospective.
Results show that longer durations of INTRON A therapy improved the sustained response rate (see TABLE 2). In patients with complete responses (CR) to INTRON A therapy after 6 months of treatment (149/352 [42%]), responses were less often sustained if drug was discontinued (21/70 [30%]) than if it was continued for 18 to 24 months (44/79 [56%]). Of all patients randomized, the sustained response rate in the patients receiving 18 or 24 months of therapy was 22% and 26%, respectively, in the two trials. In patients who did not have a CR by 6 months, additional therapy did not result in significantly more responses, since almost all patients who responded to therapy did so within the first 16 weeks of treatment.
A subset (less than 50%) of patients from the combined extended dosing studies had liver biopsies performed both before and after INTRON A treatment. Improvement in necroinflammatory activity as assessed retrospectively by the Knodell (Study 1) and Scheuer (Study 2) Histology Activity Indices was observed in both studies. A higher number of patients (58%, 45/78) improved with extended therapy than with shorter (6 months) therapy (38%, 34/89) in this subset.
Combination treatment with INTRON A and REBETOL® (ribavirin USP) provided a significant reduction in virologic load and improved histologic response in adult patients with compensated liver disease who were treatment-naïve or had relapsed following therapy with alpha interferon alone; pediatric patients previously untreated with alpha interferon experienced a sustained virologic response. See REBETOL prescribing information for additional information.
Chronic Hepatitis B
Pediatrics
The safety and efficacy of INTRON A in the treatment of chronic hepatitis B was evaluated in one randomized controlled trial of 149 patients ranging from 1 year to 17 years of age. Seventy-two patients were treated with 3 million IU/m2 of INTRON A therapy administered subcutaneously three times a week (TIW) for 1 week; the dose was then escalated to 6 million IU/m2 TIW for a minimum of 16 weeks up to 24 weeks. The maximum weekly dosage was 10 million IU TIW. Seventy-seven patients were untreated controls. Study entry and response criteria were identical to those described in the adult patient population.
Patients treated with INTRON A therapy had a better response (loss of HBV DNA and HBeAg at 24 weeks of follow-up) compared to the untreated controls (24% [17/72] versus 10% [8/77] P=0.05). Sixteen of the 17 responders treated with INTRON A therapy remained HBV DNA and HBeAg negative and had a normal serum ALT 12 to 24 months after completion of treatment. Serum HBsAg became negative in 7 out of 17 patients who responded to INTRON A therapy. None of the control patients who had an HBV DNA and HBeAg response became HBsAg negative. At 24 weeks of follow-up, normalization of serum ALT was similar in patients treated with INTRON A therapy (17%, 12/72) and in untreated control patients (16%, 12/77). Patients with a baseline HBV DNA less than 100 pg/mL were more likely to respond to INTRON A therapy than were patients with a baseline HBV DNA greater than 100 pg/mL (35% versus 9%, respectively). Patients who contracted hepatitis B through maternal vertical transmission had lower response rates than those who contracted the disease by other means (5% versus 31%, respectively). There was no evidence that the effects on HBV DNA and HBeAg were limited to specific subpopulations based on age, gender, or race.
| 30 million IU/m2 TIW, SC and 35 million IU QD, SC | |||||
|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | ||||
|
|||||
| CD4<200 | 4/14 | (29%) | 0/19 | (0%) | |
| 200≤CD4≤400 | 6/12 | (50%) | 0/5 | (0%) | |
| } 58% | |||||
| CD4>400 | 5/7 | (71%) | 0/0 | (0%) | |
| Treatment Group* - Number of Patients (%) | |||
|---|---|---|---|
| Study Number | INTRON A 3 million IU 24 weeks of treatment | INTRON A 3 million IU 72 or 96 weeks of treatment† | Difference (Extended — 24 weeks) (95% CI)‡ |
|
|||
| ALT response at the end of follow-up | |||
| 1 | 12/101 (12%) | 23/104 (22%) | 10% (-3, 24) |
| 2 | 9/67 (13%) | 21/80 (26%) | 13% (-4, 30) |
| Combined Studies | 21/168 (12.5%) | 44/184 (24%) | 11.4% (2, 21) |
| ALT response at the end of treatment | |||
| 1 | 40/101 (40%) | 51/104 (49%) | -- |
| 2 | 32/67 (48%) | 35/80 (44%) | -- |
| Treatment Group† - Number of Patients (%) | |||||||
|---|---|---|---|---|---|---|---|
| Study Number | INTRON A 5 million IU QD | INTRON A 10 million IU TIW | Untreated Controls | P‡ Value | |||
|
|||||||
| 17 | 15/38 | (39%) | -- | -- | 3/42 | (7%) | 0.0009 |
| 2 | -- | -- | 10/24 | (42%) | 1/22 | (5%) | 0.005 |
| 38 | -- | -- | 13/24§ | (54%) | 2/27 | (7%)§ | NA§ |
| All Studies | 15/38 | (39%) | 23/48 | (48%) | 6/91 | (7%) | -- |
| Treatment Group - Number of Patients (%) | |||||||
|---|---|---|---|---|---|---|---|
| Study Number | INTRON A 5 million IU QD | INTRON A 10 million IU TIW | Untreated Controls | P† Value | |||
|
|||||||
| 1 | 16/38 | (42%) | -- | -- | 8/42 | (19%) | 0.03 |
| 2 | -- | -- | 10/24 | (42%) | 1/22 | (5%) | 0.0034 |
| 3 | -- | -- | 12/24‡ | (50%) | 2/27 | (7%)‡ | NA‡ |
| All Studies | 16/38 | (42%) | 22/48 | (46%) | 11/91 | (12%) | -- |
Contraindications
INTRON® A is contraindicated in patients with:
- Hypersensitivity to interferon alpha or any component of the product
- Autoimmune hepatitis
- Decompensated liver disease
INTRON A and REBETOL® combination therapy is additionally contraindicated in:
- Patients with hypersensitivity to ribavirin or any other component of the product
- Women who are pregnant
- Men whose female partners are pregnant
- Patients with hemoglobinopathies (e.g., thalassemia major, sickle cell anemia)
- Patients with creatinine clearance less than 50 mL/min.
See REBETOL prescribing information for additional information.
Warnings
Neuropsychiatric Disorders
DEPRESSION AND SUICIDAL BEHAVIOR INCLUDING SUICIDAL IDEATION, SUICIDAL ATTEMPTS, AND COMPLETED SUICIDES, HOMICIDAL IDEATION, AND AGGRESSIVE BEHAVIOR SOMETIMES DIRECTED TOWARDS OTHERS, HAVE BEEN REPORTED IN ASSOCIATION WITH TREATMENT WITH ALPHA INTERFERONS, INCLUDING INTRON A THERAPY. If patients develop psychiatric problems, including clinical depression, it is recommended that the patients be carefully monitored during treatment and in the 6-month follow-up period.
INTRON A should be used with caution in patients with a history of psychiatric disorders. INTRON A therapy should be discontinued for any patient developing severe psychiatric disorder during treatment. Obtundation and coma have also been observed in some patients, usually elderly, treated at higher doses. While these effects are usually rapidly reversible upon discontinuation of therapy, full resolution of symptoms has taken up to 3 weeks in a few severe episodes. If psychiatric symptoms persist or worsen, or suicidal or homicidal ideation or aggressive behavior towards others is identified, discontinue treatment with INTRON A and follow the patient closely, with psychiatric intervention as appropriate. Narcotics, hypnotics, or sedatives may be used concurrently with caution and patients should be closely monitored until the adverse effects have resolved. Suicidal ideation or attempts occurred more frequently among pediatric patients, primarily adolescents, compared to adult patients (2.4% versus 1%) during treatment and off-therapy follow-up. Cases of encephalopathy have also been observed in some patients, usually elderly, treated with higher doses of INTRON A.
Treatment with interferons may be associated with exacerbated symptoms of psychiatric disorders in patients with co-occurring psychiatric and substance use disorders. If treatment with interferons is initiated in patients with prior history or existence of psychiatric condition or with a history of substance use disorders, treatment considerations should include the need for drug screening and periodic health evaluation, including psychiatric symptom monitoring. Early intervention for re-emergence or development of neuropsychiatric symptoms and substance use is recommended.
Precautions
Drug Interactions
Interactions between INTRON A and other drugs have not been fully evaluated. Caution should be exercised when administering INTRON A therapy in combination with other potentially myelosuppressive agents such as zidovudine. Concomitant use of alpha interferon and theophylline decreases theophylline clearance, resulting in a 100% increase in serum theophylline levels.
Information for Patients
Patients receiving INTRON A alone or in combination with REBETOL® should be informed of the risks and benefits associated with treatment and should be instructed on proper use of the product. To supplement your discussion with a patient, you may wish to provide patients with a copy of the MEDICATION GUIDE.
Patients should be informed of, and advised to seek medical attention for, symptoms indicative of serious adverse reactions associated with this product. Such adverse reactions may include depression (suicidal ideation), cardiovascular (chest pain), ophthalmologic toxicity (decrease in/or loss of vision), pancreatitis or colitis (severe abdominal pain), and cytopenias (high persistent fevers, bruising, dyspnea). Patients should be advised that some side effects such as fatigue and decreased concentration might interfere with the ability to perform certain tasks. Patients who are taking INTRON A in combination with REBETOL must be thoroughly informed of the risks to a fetus. Female patients and female partners of male patients must be told to use two forms of birth control during treatment and for six months after therapy is discontinued (see MEDICATION GUIDE).
Patients should be advised to remain well hydrated during the initial stages of treatment and that use of an antipyretic may ameliorate some of the flu-like symptoms.
If a decision is made to allow a patient to self-administer INTRON A, they should be instructed, based on their treatment, if they should inject a dose of INTRON® A subcutaneously or intramuscularly. If it is too difficult for them to inject themselves, they should be instructed to ask someone who has been trained to give the injection to them. Patients should be instructed on the importance of site selection for self-administering the injection, as well as the importance on rotating the injection sites. A puncture resistant container for the disposal of needles and syringes should be supplied. Patients self-administering INTRON A should be instructed on the proper disposal of needles and syringes and cautioned against reuse.
Patients should be instructed that the Sterile Water for Injection vial supplied with Intron A Powder for Injection contains an excess amount of diluent (5 mL) and only 1 mL should be withdrawn to reconstitute Intron A Powder for Injection. The vial of Sterile Water for Injection is intended for single-dose only. Discard the unused portion of sterile water. Do not save or reuse.
Laboratory Tests
In addition to those tests normally required for monitoring patients, the following laboratory tests are recommended for all patients on INTRON A therapy, prior to beginning treatment and then periodically thereafter.
- Standard hematologic tests — including hemoglobin, complete and differential white blood cell counts, and platelet count.
- Blood chemistries — electrolytes, liver function tests, and TSH.
- Monitor hepatic function with serum bilirubin, ALT (alanine transaminase), AST (aspartate aminotransferase), alkaline phosphatase, and LDH (lactate dehydrogenase) at 2, 8 and 12 weeks following initiation of INTRON A, then every 6 months while receiving INTRON A. Permanently discontinue INTRON A for evidence of severe (Grade 3) hepatic injury or hepatic decompensation (Child-Pugh score >6 [class B and C]).
Those patients who have preexisting cardiac abnormalities and/or are in advanced stages of cancer should have electrocardiograms taken prior to and during the course of treatment.
Mild-to-moderate leukopenia and elevated serum liver enzyme (SGOT) levels have been reported with intralesional administration of INTRON A (see ADVERSE REACTIONS); therefore, the monitoring of these laboratory parameters should be considered.
Baseline chest X-rays are suggested and should be repeated if clinically indicated.
For malignant melanoma patients, differential WBC count and liver function tests should be monitored weekly during the induction phase of therapy and monthly during the maintenance phase of therapy.
For specific recommendations in chronic hepatitis C and chronic hepatitis B, see INDICATIONS AND USAGE.
Adverse Reactions/Side Effects
Overdosage
There is limited experience with overdosage. Postmarketing surveillance includes reports of patients receiving a single dose as great as 10 times the recommended dose. In general, the primary effects of an overdose are consistent with the effects seen with therapeutic doses of interferon alfa-2b. Hepatic enzyme abnormalities, renal failure, hemorrhage, and myocardial infarction have been reported with single administration overdoses and/or with longer durations of treatment than prescribed (see ADVERSE REACTIONS). Toxic effects after ingestion of interferon alfa-2b are not expected because interferons are poorly absorbed orally. Consultation with a poison center is recommended.
Intron A Dosage and Administration
General
IMPORTANT: INTRON® A is supplied as 1) Powder for Injection/Reconstitution; 2) Solution for Injection in Vials. Not all dosage forms and strengths are appropriate for some indications. It is important that you carefully read the instructions below for the indication you are treating to ensure you are using an appropriate dosage form and strength.
To enhance the tolerability of INTRON A, injections should be administered in the evening when possible.
To reduce the incidence of certain adverse reactions, acetaminophen may be administered at the time of injection.
The solution should be allowed to come to room temperature before using.
Malignant Melanoma
(see DOSAGE AND ADMINISTRATION, General)
INTRON A adjuvant treatment of malignant melanoma is given in two phases, induction and maintenance.
Dose Adjustment
NOTE: Regular laboratory testing should be performed to monitor laboratory abnormalities for the purpose of dose modifications (see PRECAUTIONS, Laboratory Tests).
- INTRON A should be withheld for severe adverse reactions, including granulocyte counts greater than 250/mm3 but less than 500/mm3 or SGPT/SGOT greater than 5-10× upper limit of normal, until adverse reactions abate. INTRON A treatment should be restarted at 50% of the previous dose.
- INTRON A should be permanently discontinued for:
- Toxicity that does not abate after withholding INTRON A
- Severe adverse reactions which recur in patients receiving reduced doses of INTRON A
- Granulocyte count less than 250/mm3 or SGPT/SGOT of greater than 10× upper limit of normal
Dose Adjustment
NOTE: Regular laboratory testing should be performed to monitor laboratory abnormalities for the purpose of dose modifications (see PRECAUTIONS, Laboratory Tests).
- INTRON A should be withheld for severe adverse reactions, including granulocyte counts greater than 250/mm3 but less than 500/mm3 or SGPT/SGOT greater than 5-10× upper limit of normal, until adverse reactions abate. INTRON A treatment should be restarted at 50% of the previous dose.
- INTRON A should be permanently discontinued for:
- Toxicity that does not abate after withholding INTRON A
- Severe adverse reactions which recur in patients receiving reduced doses of INTRON A
- Granulocyte count less than 250/mm3 or SGPT/SGOT of greater than 10× upper limit of normal
AIDS-Related Kaposi's Sarcoma
(see DOSAGE AND ADMINISTRATION, General)
Dose Adjustment
- INTRON A dose should be reduced by 50% or withheld for severe adverse reactions.
- INTRON A may be resumed at a reduced dose if severe adverse reactions abate with interruption of dosing.
- INTRON A should be permanently discontinued if severe adverse reactions persist or if they recur in patients receiving a reduced dose.
Chronic Hepatitis B Pediatrics
(see DOSAGE AND ADMINISTRATION, General)
Dose Adjustment
If severe adverse reactions or laboratory abnormalities develop during INTRON A therapy, the dose should be modified (50% reduction) or discontinued if appropriate, until the adverse reactions abate. If intolerance persists after dose adjustment, INTRON A therapy should be discontinued.
For patients with decreases in white blood cell, granulocyte or platelet counts, the following guidelines for dose modification should be followed:
| INTRON A Dose | White Blood Cell Count | Granulocyte Count | Platelet Count |
|---|---|---|---|
| Reduce 50% | <1.5 × 109/L | <0.75 × 109/L | <50 × 109/L |
| Permanently Discontinue | <1.0 × 109/L | <0.5 × 109/L | <25 × 109/L |
INTRON A therapy was resumed at up to 100% of the initial dose when white blood cell, granulocyte, and/or platelet counts returned to normal or baseline values.
PREPARATION AND ADMINISTRATION
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: 03/2023 | ||
| MEDICATION GUIDE
INTRON® A (In-tron-aye) (Interferon alfa-2b, recombinant) |
|||
| If you are taking INTRON A with REBETOL, also read the Medication Guide for REBETOL® (ribavirin) Capsules and Oral Solution.
INTRON A alone is a treatment for certain types of cancers and hepatitis B virus. INTRON A by itself or with REBETOL is a treatment for some people infected with hepatitis C virus. |
|||
| What is the most important information I should know about INTRON A? INTRON A can cause serious side effects that may cause death or worsen certain serious conditions that you may already have. Tell your healthcare provider right away if you have any of the symptoms listed below while taking INTRON A. If symptoms get worse, or become severe and continue, your healthcare provider may tell you to stop taking INTRON A permanently. In many, but not all people, these symptoms go away after they stop taking INTRON A. Heart problems. Some people who take INTRON A may develop heart problems, including: |
|||
|
| ||
| Stroke or symptoms of a stroke. Symptoms may include weakness, loss of coordination, and numbness. Stroke or symptoms of a stroke may happen in people who have some risk factors or no known risk factors for a stroke. Mental health problems, including suicide. INTRON A may cause you to develop mood or behavior problems that may get worse during treatment with INTRON A or after your last dose, including: |
|||
|
| ||
| If you have these symptoms, your healthcare provider should carefully monitor you during treatment with INTRON A and for 6 months after your last dose. New or worsening autoimmune disease. Some people taking INTRON A develop autoimmune diseases (a condition where the body's immune cells attack other cells or organs in the body), including rheumatoid arthritis, systemic lupus erythematosus, sarcoidosis, and psoriasis. In some people who already have an autoimmune disease, the disease may get worse while on INTRON A. Infections. Some people who take INTRON A may get an infection. Symptoms may include: |
|||
|
| ||
| During treatment with INTRON A, you should see a healthcare provider regularly for check-ups and blood tests to make sure that your treatment is working, and to check for side effects. | |||
| What is INTRON A?
INTRON A is a prescription medicine that is used:
|
|||
| Who should not take INTRON A?
Do not take INTRON A if you:
|
|||
| What should I tell my healthcare provider before taking INTRON A? Before you take INTRON A, tell your healthcare provider about all of your health problems, including if you:
Especially tell your healthcare provider if you take:
|
|||
How should I take INTRON A?
|
|||
| What are the possible side effects of INTRON A? INTRON A may cause serious side effects including:
|
|||
|
| ||
|
|||
|
| ||
|
|||
|
| ||
You may need to have a chest X-ray or other tests if you develop fever, cough, shortness of breath, or other symptoms of a lung problem during treatment with INTRON A.
|
|||
|
| ||
|
|||
|
| ||
|
|||
|
| ||
The most common side effects of INTRON A include:
These are not all the side effects of INTRON A. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1–800–FDA–1088. |
|||
| How should I store INTRON A? INTRON A Solution for Injection:
Before mixing, store in the refrigerator between 36°F to 46°F (2°C to 8°C).
|
|||
| General Information about the safe and effective use of INTRON A
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use INTRON A for a condition for which it was not prescribed. Do not give INTRON A to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about INTRON A. If you would like more information, ask your healthcare provider. You can ask your healthcare provider or pharmacist for information about INTRON A that was written for health care professionals. |
|||
| What are the ingredients in INTRON A? Active ingredient: interferon alfa-2b Inactive ingredients: |
|||
|
|||
| Manufactured by: Merck Sharp & Dohme LLC, Rahway, NJ 07065, USA U.S. License Number 0002 Copyright © 1996-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates. All rights reserved. usmg-mk2958-mf-2303r015 For more information, go to www.IntronA.com or call 1-800-622-4477. |
|||
Instructions for Use
INTRON® A (In-tron-aye)
(Interferon alfa-2b, recombinant)
Solution for Injection
Be sure that you read, understand and follow these instructions before injecting INTRON A. Your healthcare provider should show you how to prepare, measure and inject INTRON A properly before you use it for the first time. Ask your healthcare provider if you have any questions.
Before starting, collect all of the supplies that you will need to use for preparing and injecting INTRON A. For each injection, you will need the following supplies:
- 1 vial of INTRON A solution
- 1 single-dose disposable syringe and needle
- 1 cotton ball or gauze
- 2 alcohol swabs
- 1 sharps disposal container for throwing away (dispose of) your used syringes, needles, and vials. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
Important:
- Never re-use disposable syringes and needles.
- Make sure you have the right syringe and needle to use with INTRON A. Your healthcare provider should tell you what syringes and needles to use to inject INTRON A.
How should I prepare a dose of INTRON® A?
- 1
- Find a well lit, clean, flat working surface.
- 2.
- Before removing INTRON A from the carton, look at the expiration date printed on the carton. Make sure that the expiration date has not passed. Do not use if the expiration date has passed.
- 3.
- Wash your hands well with soap and warm water (See Figure A). Keep your work area, your hands and injection site clean to decrease the risk of infection.
- 4.
- Remove 1 vial of INTRON A solution from the carton (See Figure B).
- 5.
- Look at the vial of INTRON A. The solution should be clear and colorless, without particles. Do not use the vial of INTRON A if the medicine is cloudy, has particles or is not clear and colorless.
- 6.
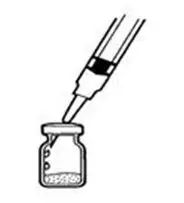
- Remove the protective plastic cap from the top of the INTRON A vial. Clean the rubber stopper on the top of the INTRON A vial with an alcohol swab (See Figure C).
- 7.
- Gently warm the INTRON A solution by slowly rolling the vial in the palms of your hands for about one minute (See Figure D). Do not shake the vial.
- 8.
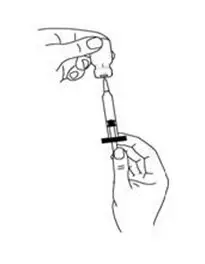
- Open the package of the syringe you are using (See Figure E) and if it does not have a needle attached, then attach a new needle to the syringe.
- 9.
- Remove the protective cap from the needle of the syringe. Fill the syringe with air by pulling back on the plunger to the mark on the syringe that matches the dose prescribed by your healthcare provider (See Figure F).
- 10.
- Hold the vial of INTRON A Solution for Injection on your flat working surface (See Figure G). Do not touch the cleaned rubber stopper.
- 11.
- Push the needle straight down through the middle of the rubber stopper of the vial containing the INTRON A solution (See Figure H). Slowly inject all the air from the syringe into the air space above the solution.
- 12.
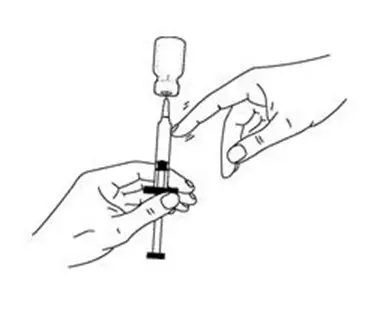
- Keep the needle in the vial. Turn the vial upside down (See Figure I).
- Make sure the tip of the needle is in the INTRON A solution.
- Slowly pull the plunger back to fill the syringe with INTRON A solution to the dose (mL or cc) prescribed by your healthcare provider.
- 13.
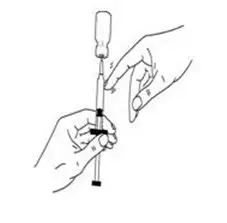
- With the needle still in the vial, check the syringe for air bubbles (See Figure J).
- If there are any air bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe.
- Slowly push the plunger up to remove the air bubbles.
- If you push solution back into the vial, slowly pull back on the plunger to draw the dose (mL or cc) prescribed by your healthcare provider.
- 14.
- Do not remove the needle from the vial. Lay the vial and syringe on its side on your flat work surface until you are ready to inject the INTRON A solution.
How should I choose a site for injection?
Based on your treatment, your healthcare provider will tell you if you should inject a dose of INTRON® A under the skin (subcutaneous injection) or into the muscle (intramuscular injection). If it is too difficult for you to inject, ask someone who has been trained to give injections to help you.
For Subcutaneous Injection
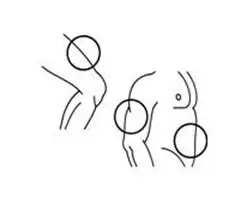
The best sites for injection are areas on your body with a layer of fat between skin and muscle such as (See Figure K):
- the front of your middle thighs
- the outer area of your upper arms
- the abdomen, except around your belly button (navel)
For Intramuscular Injection
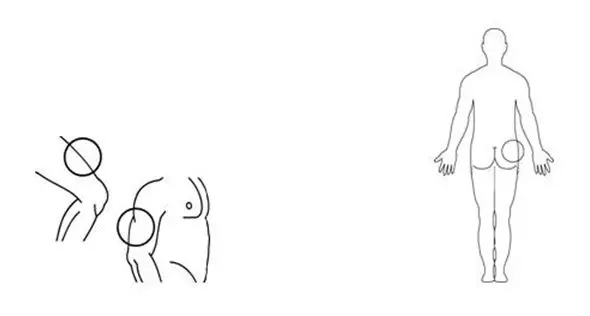
The best sites for injection into your muscle are (See Figure L):
- the front of your middle thighs
- your upper arms
- the upper outer areas of your buttocks
You should use a different site each time you inject INTRON® A to avoid soreness at any one site. Do not inject INTRON A into an area where the skin is irritated, red, bruised, infected or has scars, stretch marks or lumps.
How should I inject a dose of INTRON® A?
- 15.
- Clean the injection site with a new alcohol swab. Wait for the area to dry.
- 16.
- Pick up the vial and syringe from your flat work surface. Remove the syringe and needle from the vial.
- Hold the syringe in the hand that you will use to inject INTRON A.
- Do not touch the needle or allow it to touch the work surface.
- 17.
- With your other hand, pinch a fold of the skin at the cleaned injection site.
For subcutaneous injection (under the skin):
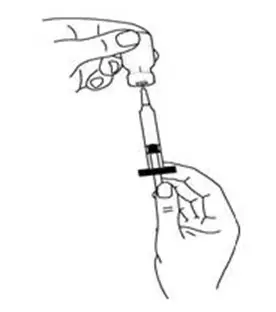
- Hold the syringe (like a pencil) at a 45-degree angle to the skin. With a quick "dart-like" motion, push the needle into the skin (See Figure M).
For intramuscular injection (into the muscle):
- Hold the syringe (like a pencil) at a 90-degree angle to the skin. With a quick "dart-like" motion, push the needle into the muscle (See Figure N).
- 18.
- After the needle is inserted, remove the hand used to pinch the skin. Use it to hold the syringe barrel.
- Pull the plunger back slightly.
- If no blood is present in the syringe, inject the medicine by gently pressing the plunger all the way down the syringe barrel, until the syringe is empty.
-
If blood comes into the syringe, the needle has entered a blood vessel. Do not inject INTRON® A.
- Withdraw the needle and dispose of the syringe and needle in the sharps disposal container. See "How should I dispose of used syringes, needles and vials?" at the end of this Instructions for Use
- If there is bleeding, cover the injection site with a bandage.
- Then, repeat steps 1 through 18 with a new dose of INTRON A and inject the medicine at a new injection site.
- 19.
- When the syringe is empty, pull the needle out of the skin.
- Place a cotton ball or gauze over the injection site and press for several seconds. Do not massage the injection site.
- If there is bleeding, cover the injection site with a bandage.
- 20.
- Dispose of used syringes, needles and vials in the sharps disposal container. See "How should I dispose of used syringes, needles, and vials?" below.
How should I dispose of used syringes, needles, and vials?
- Put your used needles, syringes and vials in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles, syringes and vials in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Always keep the sharps disposal container out of the reach of children.
How should I store INTRON® A?
- Store in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze Intron A.
- Allow the INTRON A Solution for Injection to come to room temperature before using. INTRON A Solution in Multidose vials for Injection may be used to give more than 1 injection of medicine.
- Throw away any unused INTRON A Solution for Injection remaining in the vial after 1 month
- Keep away from heat.
- Keep INTRON A and all medicines out of the reach of children.
Instructions for Use
INTRON® A (In-tron-aye)
(Interferon alfa-2b, recombinant)
Powder for Solution
Be sure that you read, understand, and follow these instructions before injecting INTRON A. Your healthcare provider should show you how to prepare, measure, and inject INTRON A properly before you use it for the first time. Ask your healthcare provider if you have any questions.
Before starting, collect all of the supplies that you will need to use for preparing and injecting INTRON A. For each injection you will need the following supplies:
- 1 vial of INTRON A powder for solution
- 1 vial of sterile water for injection (diluent). The vial contains an excess amount of sterile water (5 mL). You will only need to withdraw 1 mL to prepare your single dose.
- 1 single-dose disposable syringe and needle
- 1 cotton ball or gauze
- 2 alcohol swabs
- 1 sharps disposal container for throwing away (dispose of) your used syringes, needles, and vials. See "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
Important:
- Never re-use disposable syringes and needles.
- The vial of mixed INTRON A should be used right away. Do not mix more than 1 vial of INTRON A at a time. If you do not use the vial of prepared solution right away, store it in a refrigerator and use within 24 hours. See the end of this Instructions for Use for information about "How should I store INTRON A?"
- After mixing, throw away (discard) the vial of INTRON A after you withdraw one dose of medicine.
- Make sure you have the right syringe and needle to use with INTRON A. Your healthcare provider should tell you what syringes and needles to use to inject INTRON A.
How should I prepare a dose of INTRON A?
Before you inject INTRON A, the powder must be mixed with 1 mL (cc) of the sterile water for injection (diluent) from the INTRON A vial package.
- 1.
- Find a clean, well-lit, flat work surface.
- 2.
- Get one of your INTRON A vial packages. Check the date printed on the carton. Make sure that the expiration date has not passed.
- 3.
- Wash your hands well with soap and water. Keep your work area, your hands, and injection site clean to decrease the risk of infection (See Figure A).
- 4.
- Gently warm the vial of diluent by slowly rolling the vial in the palms of your hands for one minute (See Figure B).
- 5.
- Remove the protective plastic cap from the tops of both vials (INTRON A powder and the diluent). Clean the rubber stopper on the top of both vials with an alcohol swab (See Figure C).
- 6.
- Open the package for the syringe (See Figure D) you are using and if it does not have a needle attached, then attach a new needle to the syringe.
- 7.
- Remove the needle cover from the syringe. Fill the syringe with air by pulling the plunger back to 1 mL (See Figure E).
- 8.
- Hold the diluent vial on your flat work surface. Do not touch the cleaned rubber stopper (See Figure F).
- 9.
- Push the needle straight down through the middle of the rubber stopper of the diluent vial and slowly inject all the air from the syringe into the air space above the diluent (See Figure G).
- 10.
- Keep the needle in the vial. Turn the vial upside down and make sure the tip of the needle is in the diluent.
- Important: The sterile water for injection vial contains an excess amount of sterile water (5 mL). You will only need to withdraw 1 mL to prepare your single dose.
- Slowly pull the plunger back to fill the syringe with diluent to the 1 mL mark on the side of the syringe (See Figure H).
- 11.
- With the needle still inserted in the vial, check the syringe for air bubbles (See Figure I).
- If there are any air bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe.
- Slowly push the plunger up to remove the air bubbles.
- If you push diluent back into the vial, slowly pull back on the plunger to again draw 1 mL of diluent back into the syringe.
- 12.
- Remove the needle from the vial. Do not let the syringe touch anything.
- 13.
- Throw away the diluent that is left over in the vial. Do not save any leftover diluent or use it again. See "How should I dispose of used syringes, needles, and vials" at the end of this Instructions for Use.
- 14.
- Insert the needle through the center of the rubber stopper of the INTRON A powder vial. Do not touch the cleaned rubber stopper.
- Place the needle tip, at an angle, against the side of the INTRON A powder vial (See Figure J).
- Slowly push the plunger down to inject the diluent into the vial. The stream of liquid should run down the sides of the glass vial.
- To prevent bubbles from forming, do not aim the stream of diluent directly on the medicine in the bottom of the vial.
- Do not remove the needle from the vial.
- 15.
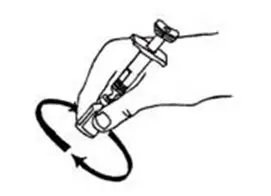
- Gently swirl the INTRON A vial in a circular motion until the powder is completely dissolved (See Figure K).
- Do not shake the vial. If any powder remains undissolved in the vial, gently turn the vial upside down until all of the powder is dissolved.
- The solution may look cloudy or bubbly for a few minutes. If air bubbles do form, wait until the solution settles and all bubbles rise to the top. Then withdraw your dose from the vial.
- 16.
- After the INTRON A completely dissolves, the solution should be clear and colorless to light yellow, without particles. Do not use the mixed solution if you see particles in it, or it is not clear and colorless to light yellow.
- 17.
- With the needle in the vial, turn the vial upside down (See Figure L).
- Hold the vial with one hand. Be sure the tip of the needle is in the INTRON A solution. Slowly pull the plunger back to fill the syringe with the exact amount of INTRON A into the syringe that your healthcare provider told you to use.
- 18.
- With the needle still inserted in the vial, check the syringe for air bubbles. If you see any air bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe (See Figure M).
- 19.
- Slowly push the plunger up to remove the air bubbles. If you push solution back into the vial, slowly pull back on the plunger again to draw the correct amount of INTRON A solution back into the syringe.
- 20.
- Do not remove the needle from the vial. Lay the vial and syringe on its side on your flat work surface until you are ready to inject the INTRON A solution.
How should I choose a site for injection?
Based on your treatment, your healthcare provider will tell you if you should inject a dose of INTRON® A under the skin (subcutaneous injection) or into the muscle (intramuscular injection). If it is too difficult for you to inject, ask someone who has been trained to give injections to help you.
For Subcutaneous Injection
The best sites for injection are areas on your body with a layer of fat between skin and muscle, such as (See Figure N):
- the front of your middle thighs
- the outer area of your upper arms
- the abdomen, except around your belly-button (navel)
For Intramuscular Injection
The best sites for injection into your muscle are (See Figure O):
- the front of your middle thighs
- your upper arms
- the upper outer areas of your buttocks
You should use a different site each time you inject INTRON® A to avoid soreness at any one site. Do not inject INTRON A into an area where the skin is irritated, red, bruised, infected or has scars, stretch marks, or lumps.
How should I inject a dose of INTRON® A?
- 21.
- Clean the injection site with a new alcohol swab. Wait for the skin to dry.
- 22.
- Pick up the vial and syringe from your flat work surface. Remove the syringe and needle from the vial.
- Hold the syringe in the hand that you will use to inject INTRON A.
- Do not touch the needle or allow it to touch the work surface.
- 23.
- With one hand, pinch a fold of the skin at the cleaned injection site.
- 24.
-
For subcutaneous injection (under the skin):
- With the other hand, hold the syringe (like a pencil) at a 45-degree angle to the skin. With a quick "dart-like" motion, push the needle into the skin (See Figure P).
- 25.
-
For intramuscular injection (into the muscle):
- Hold the syringe (like a pencil) at a 90-degree angle to the skin.
- With a quick "dart-like" motion, push the needle into the muscle (See Figure Q).
- 26.
- After the needle is inserted, remove the hand used to pinch the skin and use it to hold the syringe barrel.
- Pull the plunger back slightly.
- If no blood is present in the syringe, inject the medicine by gently pushing the plunger all the way down the syringe barrel, until the syringe is empty.
-
If blood comes into the syringe, the needle has entered a blood vessel. Do not inject INTRON® A.
- Withdraw the needle and dispose of the syringe and needle in the sharps disposal container. See section "How should I dispose of used syringes, needles, and vials?" at the end of this Instructions for Use.
- If there is bleeding, cover the injection site with a bandage.
- Then, repeat steps 1 through 25 with a new dose of INTRON A and inject the medicine at a new injection site.
- 27.
- When the syringe is empty, pull the needle out of the skin.
- Place a cotton ball or gauze over the injection site and press for several seconds. Do not massage the injection site.
- If there is bleeding, cover the injection site with a bandage.
- 28.
- Dispose of used syringes, needles, and vials in the sharps disposal container. (See "How should I dispose of used syringes, needles, and vials?" below.)
How should I dispose of used syringes, needles, and vials?
- Put your used needles, syringes and vials in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles, syringes and vials in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Always keep the sharps disposal container out of the reach of children.
- Before mixing, store in the refrigerator between 36°F to 46°F (2°C to 8°C).
- After mixing the INTRON A Powder for Injection, use the solution right away or store the solution in the refrigerator for up to 24 hours between 36°F to 46°F (2°C to 8°C). Allow the solution to come to room temperature before using.
- Do not freeze Intron A.
- Keep away from heat.
- Throw away any medicine left in the vial after you withdraw 1 dose.
Keep INTRON A and all medicines out of the reach of children.
| INTRON A
interferon alfa-2b kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| INTRON A
interferon alfa-2b kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| INTRON A
interferon alfa-2b kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| INTRON A
interferon alfa-2b injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| INTRON A
interferon alfa-2b injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |