Drug Detail:Invokamet (Canagliflozin and metformin [ kan-a-gli-floe-zin-and-met-for-min ])
Drug Class: Antidiabetic combinations
Highlights of Prescribing Information
INVOKAMET ®(canagliflozin and metformin hydrochloride (HCl) tablets), for oral use
INVOKAMET ®XR (canagliflozin and metformin HCl extended-release tablets), for oral use
Initial U.S. Approval: 2014
WARNING: LACTIC ACIDOSIS
See full prescribing information for complete boxed warning.
- Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. Symptoms included malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Laboratory abnormalities included elevated blood lactate levels, anion gap acidosis, increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL. ( 5.1)
- Risk factors include renal impairment, concomitant use of certain drugs, age >65 years old, radiological studies with contrast, surgery and other procedures, hypoxic states, excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the Full Prescribing Information. ( 5.1)
- If lactic acidosis is suspected, discontinue INVOKAMET or INVOKAMET XR and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended. ( 5.1)
Recent Major Changes
| Indications and Usage ( 1) | 07/2023 |
| Dosage and Administration ( 2.2, 2.3, 2.4, 2.5, 2.7) | 07/2023 |
| Contraindications ( 4) | 07/2023 |
| Warnings and Precautions ( 5.2) | 07/2023 |
Indications and Usage for Invokamet
INVOKAMET and INVOKAMET XR are a combination of canagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, and metformin HCl, a biguanide, indicated:
- As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus ( 1)
- Canagliflozin is indicated to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease ( 1)
- Canagliflozin is indicated to reduce the risk of end-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria ( 1).
Limitations of Use:
Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus ( 1)
Invokamet Dosage and Administration
- Individualize starting dose based on the patient's current regimen and renal function ( 2.2, 2.3, 2.4)
- Initiation of INVOKAMET or INVOKAMET XR is not recommended in patients with an eGFR less than 45 mL/min/1.73 m 2, due to the metformin component ( 2.4)
- INVOKAMET: one tablet, twice daily with meals, recommended starting dose of canagliflozin is 50 mg twice daily and metformin HCl 500 mg twice daily ( 2.2)
- INVOKAMET XR: two tablets, once daily with the morning meal. Swallow whole. Never crush, cut, or chew ( 2.2)
- Canagliflozin dose can be increased to a total daily dose of 300 mg in patients tolerating 100 mg who have an eGFR of 60 mL/min/1.73 m 2or greater and require additional glycemic control. Do not exceed a total daily canagliflozin dose of 300 mg ( 2.2)
- Gradually escalate metformin HCl dose to reduce the gastrointestinal side effects while not exceeding a total daily dose of 2,000 mg ( 2.3)
- Assess renal function before initiating and as clinically indicated ( 2.1, 2.3)
- Dose adjustment for patients with renal impairment may be required ( 2.4)
- See full prescribing information for INVOKAMET and INVOKAMET XR dosage modifications due to drug interactions. ( 2.5)
- May need to be discontinued at time of, or prior to, iodinated contrast imaging procedures ( 2.6)
- Withhold INVOKAMET or INVOKAMET XR at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting ( 2.7).
Dosage Forms and Strengths
INVOKAMET tablets:
- Canagliflozin 50 mg and metformin HCl 500 mg ( 3)
- Canagliflozin 50 mg and metformin HCl 1,000 mg ( 3)
- Canagliflozin 150 mg and metformin HCl 500 mg ( 3)
- Canagliflozin 150 mg and metformin HCl 1,000 mg ( 3)
INVOKAMET XR extended-release tablets:
- Canagliflozin 50 mg and metformin HCl 500 mg ( 3)
- Canagliflozin 50 mg and metformin HCl 1,000 mg ( 3)
- Canagliflozin 150 mg and metformin HCl 500 mg ( 3)
- Canagliflozin 150 mg and metformin HCl 1,000 mg ( 3)
Contraindications
- Severe renal impairment (eGFR less than 30 mL/min/1.73 m 2) ( 4)
- Metabolic acidosis, including diabetic ketoacidosis ( 4, 5.1)
- Serious hypersensitivity reaction to canagliflozin or metformin HCl ( 4, 5.9)
Warnings and Precautions
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis: Consider ketone monitoring in patients at risk for ketoacidosis, as indicated. Assess for ketoacidosis regardless of presenting blood glucose levels and discontinue INVOKAMET or INVOKAMET XR if ketoacidosis is suspected. Monitor patients for resolution of ketoacidosis before restarting ( 5.2)
- Lower Limb Amputation: Consider factors that may increase the risk of amputation before initiating INVOKAMET or INVOKAMET XR. Monitor patients for infection or ulcers of lower limb and discontinue if these occur ( 5.3)
- Volume Depletion: May result in acute kidney injury. Before initiating, assess and correct volume status in patients with renal impairment, elderly patients, or patients on loop diuretics. Monitor for signs and symptoms during therapy ( 5.4)
- Urosepsis and pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated ( 5.5)
- Hypoglycemia: Consider a lower dose of insulin or insulin secretagogue to reduce the risk of hypoglycemia when used in combination ( 5.6)
- Necrotizing fasciitis of the perineum (Fournier's gangrene): Serious, life-threatening cases have occurred in both females and males. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment ( 5.7)
- Genital mycotic infections: Monitor and treat if indicated ( 5.8)
- Hypersensitivity reactions: Discontinue and monitor until signs and symptoms resolve ( 5.9)
- Bone fracture: Consider factors that contribute to fracture risk before initiating INVOKAMET or INVOKAMET XR ( 5.10)
- Vitamin B 12deficiency : Metformin HCl may lower vitamin B 12levels. Measure hematological parameters annually and vitamin B 12at 2- to 3-year intervals and manage any abnormalities ( 5.11)
Adverse Reactions/Side Effects
- Most common adverse reactions associated with canagliflozin (5% or greater incidence): female genital mycotic infections, urinary tract infection, and increased urination ( 6.1)
- Most common adverse reactions associated with metformin HCl (5% or greater incidence) are diarrhea, nausea, vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Pharmaceuticals, Inc. at 1-800-526-7736 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Carbonic Anhydrase Inhibitors:May increase risk of lactic acidosis. Consider more frequent monitoring ( 7)
- Drugs that Reduce Metformin Clearance:May increase risk of lactic acidosis. Consider benefits and risks of concomitant use ( 7)
- See full prescribing information for additional drug interactions and information on interference of INVOKAMET and INVOKAMET XR with laboratory tests. ( 7)
Use In Specific Populations
- Pregnancy: Advise females of the potential risk to a fetus especially during the second and third trimesters ( 8.1)
- Lactation: Not recommended when breastfeeding ( 8.2)
- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy ( 8.3)
- Geriatrics: Higher incidence of adverse reactions related to reduced intravascular volume. Assess renal function more frequently ( 6.1, 8.5)
- Renal impairment: Higher incidence of adverse reactions related to hypotension and renal function ( 8.6)
- Hepatic impairment: Avoid use in patients with hepatic impairment ( 8.7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2023
Related/similar drugs
Repatha, Mounjaro, metformin, Ozempic, Xarelto, simvastatin, TrulicityFull Prescribing Information
WARNING: LACTIC ACIDOSIS
- Post-marketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [see Warnings and Precautions (5.1)] .
- Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment [see Warnings and Precautions (5.1)] .
- Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the full prescribing information [see Dosage and Administration (2.2, 2.3), Contraindications (4), Warnings and Precautions (5.1), Drug Interactions (7), and Use in Specific Populations (8.6, 8.7)] .
- If metformin-associated lactic acidosis is suspected, immediately discontinue INVOKAMET or INVOKAMET XR and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [see Warnings and Precautions (5.1)] .
1. Indications and Usage for Invokamet
INVOKAMET and INVOKAMET XR are a combination of canagliflozin and metformin HCl indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Canagliflozin is indicated to reduce the risk of major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction and nonfatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease (CVD).
Canagliflozin is indicated to reduce the risk of end-stage kidney disease (ESKD), doubling of serum creatinine, cardiovascular (CV) death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria greater than 300 mg/day.
2. Invokamet Dosage and Administration
2.1 Prior to Initiation of INVOKAMET or INVOKAMET XR
Assess renal function before initiating INVOKAMET or INVOKAMET XR and as clinically indicated [see Dosage and Administration (2.4)and Warnings and Precautions (5.1, 5.4), Contraindications (4)].
In patients with volume depletion, correct this condition before initiating INVOKAMET or INVOKAMET XR [see Warnings and Precautions (5.4)and Use in Specific Populations (8.5, 8.6)] .
2.3 Recommended Dosage and Administration
Individualize the starting dose of INVOKAMET or INVOKAMET XR based on the patient's current regimen and renal function [see Dosage and Administration (2.4)] . Table 1 presents the recommended starting dosage of INVOKAMET and INVOKAMET XR based on the patient's current regimen. For the available strengths of the canagliflozin and metformin components in INVOKAMET and INVOKAMET XR, see Dosage Forms and Strengths (3).
| Current Regimen | INVOKAMET
Recommended Dosage Administered as one tablet, orally, twice daily with meals | INVOKAMET XR
Recommended Dosage Administered as two tablets, orally, once daily with the morning meal |
|---|---|---|
|
||
| Not treated with either canagliflozin or metformin HCl | Total daily dosage is canagliflozin 100 mg and metformin HCl 1,000 mg | |
| Metformin HCl * | Total daily dosage is canagliflozin 100 mg and the nearest appropriate total daily dosage of metformin HCl | |
| Canagliflozin | The same total daily dosage of canagliflozin and a total daily dosage of metformin HCl 1,000 mg | |
| Canagliflozin and metformin HCl * | The same total daily dosage of canagliflozin and the nearest appropriate total daily dosage of metformin HCl | |
2.4 Recommended Dosage for Patients with Renal Impairment
- Initiation of INVOKAMET or INVOKAMET XR is not recommended in patients with an eGFR less than 45 mL/min/1.73 m 2, due to the metformin component.
- Table 2 provides dosage recommendations for patients with renal impairment, based on eGFR [see Use in Specific Populations (8.6)and Clinical Studies (14.3)].
| Estimated Glomerular Filtration Rate
[eGFR (mL/min/1.73 m 2)] | Recommended Dosage |
|---|---|
| eGFR 45 to less than 60 | The maximum recommended dosage of canagliflozin is 100 mg daily. |
| eGFR 30 to less than 45 | Assess the benefit risk of continuing INVOKAMET or INVOKAMET XR. The maximum recommended dosage of canagliflozin is 100 mg daily. |
| eGFR less than 30 | Contraindicated. If eGFR falls below 30 during treatment; discontinue INVOKAMET or INVOKAMET XR [see Contraindications (4)] . |
2.5 Concomitant Use with UDP-Glucuronosyltransferase (UGT) Enzyme Inducers
When co-administering INVOKAMET or INVOKAMET XR with an inducer of UGT (e.g., rifampin, phenytoin, phenobarbital, ritonavir), increase the total daily dosage of canagliflozin based on renal function [see Drug Interactions (7)] :
- In patients with eGFR 60 mL/min/1.73 m 2or greater, increase the total daily dosage of canagliflozin to 200 mg in patients currently tolerating a total daily dosage of canagliflozin 100 mg. The maximum recommended dosage of canagliflozin is 300 mg daily.
- In patients with eGFR less than 60 mL/min/1.73 m 2, increase the total daily dosage of canagliflozin to a maximum of 200 mg in patients currently tolerating a total daily dosage of canagliflozin 100 mg.
2.6 Discontinuation for Iodinated Contrast Imaging Procedures
Discontinue INVOKAMET or INVOKAMET XR at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR of less than 60 mL/min/1.73 m 2; in patients with a history of liver disease, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart INVOKAMET or INVOKAMET XR if renal function is stable [see Warnings and Precautions (5.1)] .
2.7 Temporary Interruption for Surgery
Withhold INVOKAMET or INVOKAMET XR at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting. Resume INVOKAMET or INVOKAMET XR when the patient is clinically stable and has resumed oral intake [see Warnings and Precautions (5.2)and Clinical Pharmacology (12.2)].
3. Dosage Forms and Strengths
INVOKAMET (canagliflozin and metformin HCl) tablets are available as follows:
| Canagliflozin Strength | Metformin HCl Strength | Color/Shape | Tablet Identifiers * |
|---|---|---|---|
|
|||
| 50 mg | 500 mg | white/capsule-shaped | CM 155 |
| 50 mg | 1,000 mg | beige/capsule-shaped | CM 551 |
| 150 mg | 500 mg | yellow/capsule-shaped | CM 215 |
| 150 mg | 1,000 mg | purple/capsule-shaped | CM 611 |
INVOKAMET XR (canagliflozin and metformin HCl) extended-release tablets are available as follows:
| Canagliflozin Strength | Metformin HCl Strength | Color/Shape | Tablet Identifiers * |
|---|---|---|---|
|
|||
| 50 mg | 500 mg | almost white to light orange/oblong, biconvex | CM1 |
| 50 mg | 1,000 mg | pink/oblong, biconvex | CM3 |
| 150 mg | 500 mg | orange/oblong, biconvex | CM2 |
| 150 mg | 1,000 mg | reddish brown/oblong, biconvex | CM4 |
4. Contraindications
INVOKAMET or INVOKAMET XR is contraindicated in patients:
- With severe renal impairment (eGFR less than 30 mL/min/1.73 m 2) [see Warnings and Precautions (5.1)and Use in Specific Populations (8.6)].
- With acute or chronic metabolic acidosis, including diabetic ketoacidosis [see Warnings and Precautions (5.2)] .
- With serious hypersensitivity reaction to canagliflozin or metformin HCl, such as anaphylaxis or angioedema [see Warnings and Precautions (5.9)and Adverse Reactions (6)] .
5. Warnings and Precautions
5.1 Lactic Acidosis
There have been post-marketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypothermia, hypotension and resistant bradyarrhythmias have occurred with severe acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate:pyruvate ratio; metformin plasma levels generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of INVOKAMET or INVOKAMET XR. In INVOKAMET or INVOKAMET XR-treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin is dialyzable, with a clearance of up to 170 mL/min under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and if these symptoms occur instruct them to discontinue INVOKAMET or INVOKAMET XR and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
5.2 Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
In patients with type 1 diabetes mellitus, INVOKAMET or INVOKAMET XR significantly increases the risk of diabetic ketoacidosis, a life-threatening event, beyond the background rate. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was markedly increased in patients who received sodium glucose transporter 2 (SGLT2) inhibitors compared to patients who received placebo; this risk may be greater with higher doses of INVOKAMET or INVOKAMET XR. INVOKAMET or INVOKAMET XR is not indicated for glycemic control in patients with type 1 diabetes mellitus.
Type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are also risk factors for ketoacidosis. There have been postmarketing reports of fatal events of ketoacidosis in patients with type 2 diabetes mellitus using SGLT2 inhibitors, including INVOKAMET or INVOKAMET XR.
Precipitating conditions for diabetic ketoacidosis or other ketoacidosis include acute febrile illness, reduced caloric intake, ketogenic diet, surgery, insulin dose reduction, volume depletion, and alcohol abuse.
Signs and symptoms are consistent with dehydration and severe metabolic acidosis and include nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. Blood glucose levels at presentation may be below those typically expected for diabetic ketoacidosis (e.g., less than 250 mg/dL). Ketoacidosis and glucosuria may persist longer than typically expected. Urinary glucose excretion persists for 3 days after discontinuing INVOKAMET or INVOKAMET XR [see Clinical Pharmacology (12.2)] ; however, there have been postmarketing reports of ketoacidosis and glucosuria lasting greater than 6 days and some up to 2 weeks after discontinuation of SGLT2 inhibitors.
Consider ketone monitoring in patients at risk for ketoacidosis if indicated by the clinical situation. Assess for ketoacidosis regardless of presenting blood glucose levels in patients who present with signs and symptoms consistent with severe metabolic acidosis. If ketoacidosis is suspected, discontinue INVOKAMET or INVOKAMET XR, promptly evaluate, and treat ketoacidosis, if confirmed. Monitor patients for resolution of ketoacidosis before restarting INVOKAMET or INVOKAMET XR.
Withhold INVOKAMET or INVOKAMET XR, if possible, in temporary clinical situations that could predispose patients to ketoacidosis. Resume INVOKAMET or INVOKAMET XR when the patient is clinically stable and has resumed oral intake [see Dosage and Administration (2.7)] .
Educate all patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue INVOKAMET or INVOKAMET XR and seek medical attention immediately if signs and symptoms occur.
5.3 Lower Limb Amputation
An increased risk of lower limb amputations associated with canagliflozin, a component of INVOKAMET or INVOKAMET XR, versus placebo was observed in CANVAS (5.9 vs 2.8 events per 1000 patient-years) and CANVAS-R (7.5 vs 4.2 events per 1000 patient-years), two randomized, placebo-controlled trials evaluating patients with type 2 diabetes who had either established cardiovascular disease or were at risk for cardiovascular disease. The risk of lower limb amputations was observed at both the 100 mg and 300 mg once daily dosage regimens. The amputation data for CANVAS and CANVAS-R are shown in Tables 4 and 5, respectively [see Adverse Reactions (6.1)] .
Amputations of the toe and midfoot (99 out of 140 patients with amputations receiving canagliflozin in the two trials) were the most frequent; however, amputations involving the leg, below and above the knee, were also observed (41 out of 140 patients with amputations receiving canagliflozin in the two trials). Some patients had multiple amputations, some involving both lower limbs.
Lower limb infections, gangrene, and diabetic foot ulcers were the most common precipitating medical events leading to the need for an amputation. The risk of amputation was highest in patients with a baseline history of prior amputation, peripheral vascular disease, and neuropathy.
Before initiating INVOKAMET or INVOKAMET XR, consider factors in the patient history that may predispose to the need for amputations, such as a history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers. Counsel patients about the importance of routine preventative foot care. Monitor patients receiving INVOKAMET or INVOKAMET XR for signs and symptoms of infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue INVOKAMET or INVOKAMET XR if these complications occur.
5.4 Volume Depletion
Canagliflozin can cause intravascular volume contraction which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions (6.1)] . There have been post-marketing reports of acute kidney injury which are likely related to volume depletion, some requiring hospitalizations and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including canagliflozin. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m 2), elderly patients, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating INVOKAMET or INVOKAMET XR in patients with one or more of these characteristics, assess and correct volume status. Monitor for signs and symptoms of volume depletion after initiating therapy.
5.5 Urosepsis and Pyelonephritis
There have been postmarketing reports of serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization in patients receiving canagliflozin. Treatment with INVOKAMET or INVOKAMET XR increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6)] .
5.6 Hypoglycemia with Concomitant Use of Sulfonylurea or Insulin
Insulin and insulin secretagogues are known to cause hypoglycemia. INVOKAMET or INVOKAMET XR may increase the risk of hypoglycemia when combined with insulin or an insulin secretagogue [see Adverse Reactions (6.1)] . Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with INVOKAMET or INVOKAMET XR.
5.7 Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier's gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in postmarketing surveillance in patients with diabetes mellitus receiving SGLT2 inhibitors, including canagliflozin. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with INVOKAMET or INVOKAMET XR presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue INVOKAMET or INVOKAMET XR, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
5.8 Genital Mycotic Infections
Canagliflozin increases the risk of genital mycotic infections. Patients with a history of genital mycotic infections and uncircumcised males were more likely to develop genital mycotic infections [see Adverse Reactions (6.1)] . Monitor and treat appropriately.
5.9 Hypersensitivity Reactions
Hypersensitivity reactions, including angioedema and anaphylaxis, have been reported with canagliflozin. These reactions generally occurred within hours to days after initiating canagliflozin. If hypersensitivity reactions occur, discontinue use of INVOKAMET or INVOKAMET XR; treat and monitor until signs and symptoms resolve [see Contraindications (4)and Adverse Reactions (6.1, 6.2)] .
5.10 Bone Fracture
An increased risk of bone fracture, occurring as early as 12 weeks after treatment initiation, was observed in patients using canagliflozin in the CANVAS trial [see Clinical Studies (14.2)] . Consider factors that contribute to fracture risk prior to initiating INVOKAMET or INVOKAMET XR [see Adverse Reactions (6.1)] .
5.11 Vitamin B 12Levels
In metformin HCl clinical trials of 29-week duration, a decrease to subnormal levels of previously normal serum vitamin B 12levels was observed in approximately 7% of patients. Such decrease, possibly due to interference with B 12absorption from the B 12-intrinsic factor complex, may be associated with anemia but appears to be rapidly reversible with discontinuation of metformin HCl or vitamin B 12supplementation. Certain individuals (those with inadequate vitamin B 12or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B 12levels. Measure hematologic parameters on an annual basis and vitamin B 12at 2- to 3-year intervals in patients on INVOKAMET or INVOKAMET XR and manage any abnormalities [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following important adverse reactions are also discussed elsewhere in the labeling:
- Lactic Acidosis [see Boxed Warningand Warnings and Precautions (5.1, 5.4)]
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes and Other Ketoacidosis [see Warnings and Precautions (5.2)]
- Lower Limb Amputation [see Warnings and Precautions (5.3)]
- Volume Depletion [see Warnings and Precautions (5.4)]
- Urosepsis and Pyelonephritis [see Warnings and Precautions (5.5)]
- Hypoglycemia with Concomitant Use of Sulfonylurea or Insulin [see Warnings and Precautions (5.6)]
- Necrotizing Fasciitis of the Perineum (Fournier's gangrene) [see Warnings and Precautions (5.7)]
- Genital Mycotic Infections [see Warnings and Precautions (5.8)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.9)]
- Bone Fracture [see Warnings and Precautions (5.10)]
- Vitamin B 12Deficiency [see Warnings and Precautions (5.11)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of canagliflozin. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7. Drug Interactions
| Carbonic Anhydrase Inhibitors | |
| Clinical Impact: | Carbonic anhydrase inhibitors frequently cause a decrease in serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs with INVOKAMET or INVOKAMET XR may increase the risk for lactic acidosis. |
| Intervention: | Consider more frequent monitoring of these patients. |
| Examples: | Topiramate or other carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) |
| Drugs That Reduce Metformin Clearance | |
| Clinical Impact: | Concomitant use of drugs that interfere with common renal tubular transport systems involved in the renal elimination of metformin (e.g., organic cationic transporter-2 [OCT2] / multidrug and toxin extrusion [MATE] inhibitors could increase systemic exposure to metformin and may increase the risk for lactic acidosis [see Clinical Pharmacology (12.3)]. |
| Intervention: | Consider the benefits and risks of concomitant use. |
| Examples: | Ranolazine, vandetanib, dolutegravir, and cimetidine |
| Alcohol | |
| Clinical Impact: | Alcohol is known to potentiate the effect of metformin HCl on lactate metabolism. |
| Intervention: | Warn patients against excessive alcohol intake while receiving INVOKAMET or INVOKAMET XR. |
| UGT Enzyme Inducers | |
| Clinical Impact: | UGT enzyme inducers decrease canagliflozin exposure which may reduce the effectiveness of INVOKAMET or NVOKAMET XR. |
| Intervention: | For patients with eGFR 60 mL/min/1.73 m
2or greater, if an inducer of UGTs is co-administered with INVOKAMET or INVOKAMET XR, increase the total daily dose of canagliflozin to 200 mg in patients currently tolerating INVOKAMET or INVOKAMET XR with a total daily dose of canagliflozin 100 mg. The total daily dose of canagliflozin may be increased to 300 mg in patients currently tolerating canagliflozin 200 mg and who require additional glycemic control.
For patients with eGFR less than 60 mL/min/1.73 m 2, if an inducer of UGTs is co-administered with INVOKAMET or INVOKAMET XR, increase the total daily dose of canagliflozin to 200 mg in patients currently tolerating canagliflozin 100 mg [see Dosage and Administration (2.5)and Clinical Pharmacology (12.3)] . |
| Examples: | Rifampin, phenytoin, phenobarbital, ritonavir |
| Insulin Secretagogues or Insulin | |
| Clinical Impact: | The risk of hypoglycemia is increased when INVOKAMET or INVOKAMET XR is used concomitantly with insulin secretagogues (e.g., sulfonylurea) or insulin. |
| Intervention: | Concomitant use may require a lower dosage of the insulin secretagogue or insulin to reduce the risk of hypoglycemia. |
| Drugs Affecting Glycemic Control | |
| Clinical Impact: | Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. |
| Intervention: | When such drugs are administered to a patient receiving INVOKAMET or INVOKAMET XR, monitor for loss of blood glucose control. When such drugs are withdrawn from a patient receiving INVOKAMET or INVOKAMET XR, monitor for hypoglycemia. |
| Examples: | Thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blockers, and isoniazid. |
| Digoxin | |
| Clinical Impact: | Canagliflozin increases digoxin exposure [see Clinical Pharmacology (12.3)] . |
| Intervention: | Monitor patients taking INVOKAMET or INVOKAMET XR with concomitant digoxin for a need to adjust dose of digoxin. |
| Lithium | |
| Clinical Impact: | Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. |
| Intervention: | Monitor serum lithium concentration more frequently during INVOKAMET or INVOKAMET XR initiation and dosage changes. |
| Drug/Laboratory Test Interference | |
| Positive Urine Glucose Test | |
| Clinical Impact: | SGLT2 inhibitors increase urinary glucose excretion which will lead to positive urine glucose tests. |
| Intervention: | Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
| Interference with 1,5-anhydroglucitol (1,5-AG) Assay | |
| Clinical Impact: | Measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. |
| Intervention: | Monitoring glycemic control with 1,5-AG assay is not recommended in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control. |
8. Use In Specific Populations
8.2 Lactation
Data
Published clinical lactation studies report that metformin is present in human milk which resulted in infant doses approximately 0.11% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.13 and 1. However, the studies were not designed to definitely establish the risk of use of metformin HCl during lactation because of small sample size and limited adverse event data collected in infants.
Radiolabeled canagliflozin administered to lactating rats on day 13 post-partum was present at a milk/plasma ratio of 1.40, indicating that canagliflozin and its metabolites are transferred into milk at a concentration comparable to that in plasma. Juvenile rats directly exposed to canagliflozin showed a risk to the developing kidney (renal pelvic and tubular dilatations) during maturation.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with metformin HCl may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Safety and effectiveness of INVOKAMET or INVOKAMET XR in pediatric patients under 18 years of age have not been established.
10. Overdosage
Overdose of metformin HCl has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin HCl use has been established. Lactic acidosis has been reported in approximately 32% of metformin HCl overdose cases [see Warnings and Precautions (5.1)] .
In the event of an overdose with INVOKAMET or INVOKAMET XR, contact the Poison Control Center. Employ the usual supportive measures (e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive treatment) as dictated by the patient's clinical status. Canagliflozin was negligibly removed during a 4-hour hemodialysis session. Canagliflozin is not expected to be dialyzable by peritoneal dialysis. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful partly for removal of accumulated metformin from patients in whom INVOKAMET or INVOKAMET XR overdosage is suspected.
11. Invokamet Description
INVOKAMET ®(canagliflozin and metformin HCl) and INVOKAMET ®XR (canagliflozin and metformin HCl extended-release tablets) contain canagliflozin and metformin HCl.
12. Invokamet - Clinical Pharmacology
12.3 Pharmacokinetics
Specific Populations
Trials characterizing the pharmacokinetics of canagliflozin and metformin after administration of INVOKAMET or INVOKAMET XR were not conducted in patients with renal and hepatic impairment. Descriptions of the individual components in this patient population are described below.
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
Canagliflozin has been studied in combination with metformin HCl alone, metformin HCl and sulfonylurea, metformin HCl and sitagliptin, metformin HCl and a thiazolidinedione (i.e., pioglitazone), and metformin HCl and insulin (with or without other anti-hyperglycemic agents). The efficacy of canagliflozin was compared to a dipeptidyl peptidase-4 (DPP-4) inhibitor (sitagliptin), both as add-on combination therapy with metformin HCl and sulfonylurea, and a sulfonylurea (glimepiride), both as add-on combination therapy with metformin HCl.
There have been no clinical efficacy trials conducted with INVOKAMET or INVOKAMET XR; however, bioequivalence of INVOKAMET or INVOKAMET XR to canagliflozin and metformin HCl co-administered as individual tablets was demonstrated in healthy subjects.
Canagliflozin as Add-on Combination Therapy with Metformin HCl
A total of 1,284 patients with type 2 diabetes inadequately controlled on metformin HCl monotherapy (greater than or equal to 2,000 mg/day or at least 1,500 mg/day if higher dose not tolerated) participated in a 26-week, double-blind, placebo- and active-controlled trial to evaluate the efficacy and safety of canagliflozin in combination with metformin HCl. The mean age was 55 years, 47% of patients were men, and the mean baseline eGFR was 89 mL/min/1.73 m 2. Patients already on the required metformin HCl dose (N=1009) were randomized after completing a 2-week, single-blind, placebo run-in period. Patients taking less than the required metformin HCl dose or patients on metformin HCl in combination with another antihyperglycemic agent (N=275) were switched to metformin HCl monotherapy (at doses described above) for at least 8 weeks before entering the 2-week, single-blind, placebo run-in. After the placebo run-in period, patients were randomized to canagliflozin 100 mg, canagliflozin 300 mg, sitagliptin 100 mg, or placebo, administered once daily as add-on therapy to metformin HCl.
At the end of treatment, canagliflozin 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to metformin HCl. Canagliflozin 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in significant reduction in fasting plasma glucose (FPG), in improved postprandial glucose (PPG), and in percent body weight reduction compared to placebo when added to metformin HCl (see Table 14). Statistically significant (p<0.001 for both doses) mean changes from baseline in systolic blood pressure relative to placebo were -5.4 mmHg and -6.6 mmHg with canagliflozin 100 mg and 300 mg, respectively.
| Efficacy Parameter | Placebo + Metformin HCl
(N=183) | Canagliflozin 100 mg + Metformin HCl
(N=368) | Canagliflozin 300 mg + Metformin HCl
(N=367) |
|---|---|---|---|
|
|||
| HbA 1C(%) | |||
| Baseline (mean) | 7.96 | 7.94 | 7.95 |
| Change from baseline (adjusted mean) | -0.17 | -0.79 | -0.94 |
| Difference from placebo (adjusted mean) (95% CI) † | -0.62
‡
(-0.76, -0.48) | -0.77
‡
(-0.91, -0.64) |
|
| Percent of patients achieving HbA 1C< 7% | 30 | 46 ‡ | 58 ‡ |
| Fasting Plasma Glucose (mg/dL) | |||
| Baseline (mean) | 164 | 169 | 173 |
| Change from baseline (adjusted mean) | 2 | -27 | -38 |
| Difference from placebo (adjusted mean) (95% CI) † | -30
‡
(-36, -24) | -40
‡
(-46, -34) |
|
| 2-hour Postprandial Glucose (mg/dL) | |||
| Baseline (mean) | 249 | 258 | 262 |
| Change from baseline (adjusted mean) | -10 | -48 | -57 |
| Difference from placebo (adjusted mean) (95% CI) † | -38
‡
(-49, -27) | -47
‡
(-58, -36) |
|
| Body Weight | |||
| Baseline (mean) in kg | 86.7 | 88.7 | 85.4 |
| % change from baseline (adjusted mean) | -1.2 | -3.7 | -4.2 |
| Difference from placebo (adjusted mean) (95% CI) † | -2.5
‡
(-3.1, -1.9) | -2.9
‡
(-3.5, -2.3) |
|
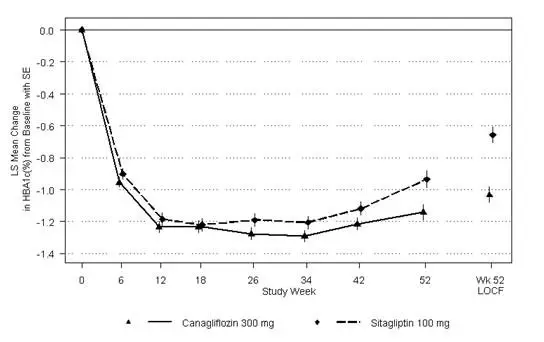
Canagliflozin as Add-on Combination Therapy with Metformin HCl and Sitagliptin
A total of 217 patients with type 2 diabetes inadequately controlled on the combination of metformin HCl (greater than or equal to 1,500 mg/day) and sitagliptin 100 mg/day (or equivalent fixed-dose combination) participated in a 26-week, double-blind, placebo-controlled trial to evaluate the efficacy and safety of canagliflozin in combination with metformin HCl and sitagliptin. The mean age was 57 years, 58% of patients were men, 73% of patients were Caucasian, 15% were Asian, and 12% were Black or African-American. The mean baseline eGFR was 90 mL/min/1.73 m 2and the mean baseline BMI was 32 kg/m 2. The mean duration of diabetes was 10 years. Eligible patients entered a 2-week, single-blind, placebo run-in period and were subsequently randomized to canagliflozin 100 mg or placebo, administered once daily as add-on to metformin HCl and sitagliptin. Patients with a baseline eGFR of 70 mL/min/1.73 m 2or greater who were tolerating canagliflozin 100 mg and who required additional glycemic control (fasting finger stick 100 mg/dL or greater at least twice within 2 weeks) were up-titrated to canagliflozin 300 mg. While up-titration occurred as early as Week 4, most (90%) patients randomized to canagliflozin were up-titrated to canagliflozin 300 mg by 6 to 8 weeks.
At the end of 26 weeks, canagliflozin once daily resulted in a statistically significant improvement in HbA 1C(p<0.001) compared to placebo when added to metformin HCl and sitagliptin (see Table 16).
| Efficacy Parameter | Placebo + Metformin HCl and Sitagliptin
(N=108 *) | Canagliflozin + Metformin HCl and Sitagliptin
(N=109 *) |
|---|---|---|
|
||
| HbA 1C(%) | ||
| Baseline (mean) | 8.40 | 8.50 |
| Change from baseline (adjusted mean) | -0.03 | -0.83 |
| Difference from placebo (adjusted mean) (95% CI) †‡ | -0.81
§
(-1.11; -0.51) |
|
| Percent of patients achieving HbA 1C< 7% ¶ | 9 | 28 |
| Fasting Plasma Glucose (mg/dL)# | ||
| Baseline (mean) | 180 | 185 |
| Change from baseline (adjusted mean) | -3 | -28 |
| Difference from placebo (adjusted mean) (95% CI) | -25
§
(-39; -11) |
|
Canagliflozin as Add-on Combination Therapy with Metformin HCl and Sulfonylurea
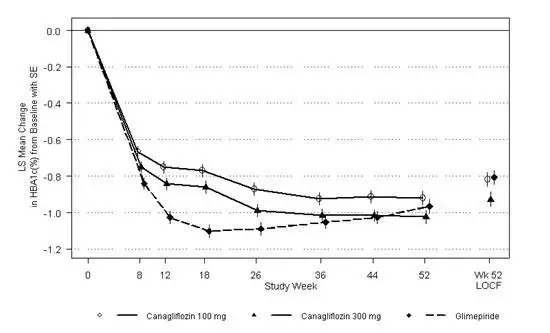
A total of 469 patients with type 2 diabetes inadequately controlled on the combination of metformin HCl (greater than or equal to 2,000 mg/day or at least 1,500 mg/day if higher dose not tolerated) and sulfonylurea (maximal or near-maximal effective dose) participated in a 26-week, double-blind, placebo-controlled trial to evaluate the efficacy and safety of canagliflozin in combination with metformin HCl and sulfonylurea. The mean age was 57 years, 51% of patients were men, and the mean baseline eGFR was 89 mL/min/1.73 m 2. Patients already on the protocol-specified doses of metformin HCl and sulfonylurea (N=372) entered a 2-week, single-blind, placebo run-in period. Other patients (N=97) were required to be on a stable protocol-specified dose of metformin HCl and sulfonylurea for at least 8 weeks before entering the 2-week run-in period. Following the run-in period, patients were randomized to canagliflozin 100 mg, canagliflozin 300 mg, or placebo administered once daily as add-on to metformin HCl and sulfonylurea.
At the end of treatment, canagliflozin 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to metformin HCl and sulfonylurea. Canagliflozin 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7.0%, in a significant reduction in fasting plasma glucose (FPG), and in percent body weight reduction compared to placebo when added to metformin HCl and sulfonylurea (see Table 17).
| Efficacy Parameter | Placebo + Metformin HCl and Sulfonylurea
(N=156) | Canagliflozin 100 mg + Metformin HCl
and Sulfonylurea (N=157) | Canagliflozin 300 mg + Metformin HCl
and Sulfonylurea (N=156) |
|---|---|---|---|
|
|||
| HbA 1C(%) | |||
| Baseline (mean) | 8.12 | 8.13 | 8.13 |
| Change from baseline (adjusted mean) | -0.13 | -0.85 | -1.06 |
| Difference from placebo (adjusted mean) (95% CI) † | -0.71
‡
(-0.90, -0.52) | -0.92
‡
(-1.11, -0.73) |
|
| Percent of patients achieving HbA 1C< 7% | 18 | 43 ‡ | 57 ‡ |
| Fasting Plasma Glucose (mg/dL) | |||
| Baseline (mean) | 170 | 173 | 168 |
| Change from baseline (adjusted mean) | 4 | -18 | -31 |
| Difference from placebo (adjusted mean) (95% CI) † | -22
‡
(-31, -13) | -35
‡
(-44, -25) |
|
| Body Weight | |||
| Baseline (mean) in kg | 90.8 | 93.5 | 93.5 |
| % change from baseline (adjusted mean) | -0.7 | -2.1 | -2.6 |
| Difference from placebo (adjusted mean) (95% CI) † | -1.4
‡
(-2.1, -0.7) | -2.0
‡
(-2.7, -1.3) |
|
Canagliflozin as Add-on Combination Therapy with Metformin HCl and Pioglitazone
A total of 342 patients with type 2 diabetes inadequately controlled on the combination of metformin HCl (greater than or equal to 2,000 mg/day or at least 1,500 mg/day if higher dose not tolerated) and pioglitazone (30 or 45 mg/day) participated in a 26-week, double--blind, placebo-controlled trial to evaluate the efficacy and safety of canagliflozin in combination with metformin HCl and pioglitazone. The mean age was 57 years, 63% of patients were men, and the mean baseline eGFR was 86 mL/min/1.73 m 2. Patients already on protocol-specified doses of metformin HCl and pioglitazone (N=163) entered a 2-week, single-blind, placebo run-in period. Other patients (N=181) were required to be on stable protocol-specified doses of metformin HCl and pioglitazone for at least 8 weeks before entering the 2-week run-in period. Following the run-in period, patients were randomized to canagliflozin 100 mg, canagliflozin 300 mg, or placebo, administered once daily as add-on to metformin HCl and pioglitazone.
At the end of treatment, canagliflozin 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to metformin HCl and pioglitazone. Canagliflozin 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in significant reduction in fasting plasma glucose (FPG), and in percent body weight reduction compared to placebo when added to metformin HCl and pioglitazone (see Table 19). Statistically significant (p<0.05 for both doses) mean changes from baseline in systolic blood pressure relative to placebo were -4.1 mmHg and -3.5 mmHg with canagliflozin 100 mg and 300 mg, respectively.
| Efficacy Parameter | Placebo + Metformin HCl and Pioglitazone
(N=115) | Canagliflozin 100 mg + Metformin HCl and Pioglitazone
(N=113) | Canagliflozin 300 mg + Metformin HCl and Pioglitazone
(N=114) |
|---|---|---|---|
|
|||
| HbA 1C(%) | |||
| Baseline (mean) | 8.00 | 7.99 | 7.84 |
| Change from baseline (adjusted mean) | -0.26 | -0.89 | -1.03 |
| Difference from placebo (adjusted mean) (95% CI) † | -0.62
‡
(-0.81, -0.44) | -0.76
‡
(-0.95, -0.58) |
|
| Percent of patients achieving HbA 1C< 7% | 33 | 47 ‡ | 64 ‡ |
| Fasting Plasma Glucose (mg/dL) | |||
| Baseline (mean) | 164 | 169 | 164 |
| Change from baseline (adjusted mean) | 3 | -27 | -33 |
| Difference from placebo (adjusted mean) (95% CI) † | -29
‡
(-37, -22) | -36
‡
(-43, -28) |
|
| Body Weight | |||
| Baseline (mean) in kg | 94.0 | 94.2 | 94.4 |
| % change from baseline (adjusted mean) | -0.1 | -2.8 | -3.8 |
| Difference from placebo (adjusted mean) (95% CI) † | -2.7
‡
(-3.6, -1.8) | -3.7
‡
(-4.6, -2.8) |
|
Canagliflozin as Add-on Combination Therapy with Insulin (With or Without Other Anti-Hyperglycemic Agents, Including Metformin HCl)
A total of 1,718 patients with type 2 diabetes inadequately controlled on insulin greater than or equal to 30 units/day or insulin in combination with other antihyperglycemic agents participated in an 18-week, double-blind, placebo-controlled substudy of a cardiovascular trial to evaluate the efficacy and safety of canagliflozin in combination with insulin. Of these patients, a subgroup of 432 patients with inadequate glycemic control received canagliflozin or placebo plus metformin HCl and ≥ 30 units/day of insulin over 18 weeks.
In this subgroup, the mean age was 61 years, 67% of patients were men, and the mean baseline eGFR was 81 mL/min/1.73 m 2. Patients on metformin HCl in combination with basal, bolus, or basal/bolus insulin for at least 10 weeks entered a 2-week, single-blind, placebo run-in period. Approximately 74% of these patients were on a background of metformin HCl and basal/bolus insulin regimen. After the run-in period, patients were randomized to canagliflozin 100 mg, canagliflozin 300 mg, or placebo, administered once daily as add-on to metformin HCl and insulin. The mean daily insulin dose at baseline was 93 units, which was similar across treatment groups.
At the end of treatment, canagliflozin 100 mg and 300 mg once daily resulted in a statistically significant improvement in HbA 1C(p<0.001 for both doses) compared to placebo when added to metformin HCl and insulin. Canagliflozin 100 mg and 300 mg once daily also resulted in a greater proportion of patients achieving an HbA 1Cless than 7%, in significant reductions in fasting plasma glucose (FPG), and in percent body weight reductions compared to placebo (see Table 20). Statistically significant (p=0.023 for the 100 mg and p<0.001 for the 300 mg dose) mean change from baseline in systolic blood pressure relative to placebo was –3.5 mmHg and -6 mmHg with canagliflozin 100 mg and 300 mg, respectively. Fewer patients on canagliflozin in combination with metformin HCl and insulin required glycemic rescue therapy: 3.6% of patients receiving canagliflozin 100 mg, 2.7% of patients receiving canagliflozin 300 mg, and 6.2% of patients receiving placebo. An increased incidence of hypoglycemia was observed in this trial, which is consistent with the expected increase of hypoglycemia when an agent not associated with hypoglycemia is added to insulin [see Warnings and Precautions (5.6)and Adverse Reactions (6.1)] .
| Efficacy Parameter | Placebo + Metformin HCl + Insulin
(N=145) | Canagliflozin 100 mg + Metformin HCl + Insulin
(N=139) | Canagliflozin 300 mg + Metformin HCl + Insulin
(N=148) |
|---|---|---|---|
|
|||
| HbA 1C(%) | |||
| Baseline (mean) | 8.15 | 8.20 | 8.22 |
| Change from baseline (adjusted mean) | 0.03 | -0.64 | -0.79 |
| Difference from placebo (adjusted mean) (95% CI) † | -0.66
‡
(-0.81, -0.51) | -0.82
‡
(-0.96, -0.67) |
|
| Percent of patients achieving HbA 1C< 7% | 9 | 19 § | 29 ‡ |
| Fasting Plasma Glucose (mg/dL) | |||
| Baseline | 163 | 168 | 167 |
| Change from baseline (adjusted mean) | 1 | -16 | -24 |
| Difference from placebo (adjusted mean) (97.5% CI) † | -16
‡
(-28, -5) | -25
‡
(-36, -14) |
|
| Body Weight | |||
| Baseline (mean) in kg | 102.3 | 99.7 | 101.1 |
| % change from baseline (adjusted mean) | 0.0 | -1.7 | -2.7 |
| Difference from placebo (adjusted mean) (97.5% CI) † | -1.7
‡
(-2.4, -1.0) | -2.7
‡
(-3.4, -2.0) |
|
14.2 Canagliflozin Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease
Canagliflozin is indicated to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease (CVD).
The CANVAS and CANVAS-R trials were multicenter, multi-national, randomized, double-blind parallel group, with similar inclusion and exclusion criteria. Patients eligible for enrollment in both CANVAS and CANVAS-R trials were: 30 years of age or older and had established, stable, cardiovascular, cerebrovascular, peripheral artery disease (66% of the enrolled population) or were 50 years of age or older and had two or more other specified risk factors for cardiovascular disease (34% of the enrolled population).
The integrated analysis of the CANVAS and CANVAS-R trials compared the risk of Major Adverse Cardiovascular Event (MACE) between canagliflozin and placebo when these were added to and used concomitantly with standard of care treatments for diabetes and atherosclerotic cardiovascular disease. The primary endpoint, MACE, was the time to first occurrence of a three-part composite outcome which included cardiovascular death, non-fatal myocardial infarction and non-fatal stroke.
In CANVAS, patients were randomly assigned 1:1:1 to canagliflozin 100 mg, canagliflozin 300 mg, or matching placebo. In CANVAS-R, patients were randomly assigned 1:1 to canagliflozin 100 mg or matching placebo, and titration to 300 mg was permitted at the investigator's discretion (based on tolerability and glycemic needs) after Week 13. Concomitant antidiabetic and atherosclerotic therapies could be adjusted, at the discretion of investigators, to ensure participants were treated according to the standard care for these diseases.
A total of 10,134 patients were treated (4,327 in CANVAS and 5,807 in CANVAS-R; total of 4,344 randomly assigned to placebo and 5,790 to canagliflozin) for a mean exposure duration of 149 weeks (223 weeks [4.3 years] in CANVAS and 94 weeks [1.8 years] in CANVAS-R) .Approximately 78% of the trial population was Caucasian, 13% was Asian, and 3% was Black. The mean age was 63 years and approximately 64% were male.
The mean HbA 1Cat baseline was 8.2% and mean duration of diabetes was 13.5 years with 70% of patients having had diabetes for 10 years or more. Approximately 31%, 21% and 17% reported a past history of neuropathy, retinopathy and nephropathy, respectively, and the mean eGFR 76 mL/min/1.73 m 2. At baseline, patients were treated with one (19%) or more (80%) antidiabetic medications including metformin (77%), insulin (50%), and sulfonylurea (43%).
At baseline, the mean systolic blood pressure was 137 mmHg, the mean diastolic blood pressure was 78 mmHg, the mean LDL was 89 mg/dL, the mean HDL was 46 mg/dL, and the mean urinary albumin to creatinine ratio (UACR) was 115 mg/g. At baseline, approximately 80% of patients were treated with renin angiotensin system inhibitors, 53% with beta-blockers, 13% with loop diuretics, 36% with non-loop diuretics, 75% with statins, and 74% with antiplatelet agents (mostly aspirin). During the trial, investigators could modify anti-diabetic and cardiovascular therapies to achieve local standard of care treatment targets with respect to blood glucose, lipid, and blood pressure. More patients receiving canagliflozin compared to placebo initiated anti-thrombotics (5.2% vs 4.2%) and statins (5.8% vs 4.8%) during the trial.
For the primary analysis, a stratified Cox proportional hazards model was used to test for non-inferiority against a pre-specified risk margin of 1.3 for the hazard ratio of MACE.
In the integrated analysis of CANVAS and CANVAS-R trials, canagliflozin reduced the risk of first occurrence of MACE. The estimated hazard ratio (95% CI) for time to first MACE was 0.86 (0.75, 0.97). Refer to Table 21. Vital status was obtained for 99.6% of patients across the trials. The Kaplan-Meier curve depicting time to first occurrence of MACE is shown in Figure 3.
| Placebo
N=4347 (%) | Canagliflozin
N=5795 (%) | Hazard ratio
(95% CI) † |
|
|---|---|---|---|
|
|||
| Composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke
(time to first occurrence) ‡,§,¶, | 426 (10.4) | 585 (9.2) | 0.86 (0.75, 0.97) |
| Non-fatal myocardial infarction §,¶ | 159 (3.9) | 215 (3.4) | 0.85 (0.69, 1.05) |
| Non-fatal Stroke §,¶ | 116 (2.8) | 158 (2.5) | 0.90 (0.71, 1.15) |
| Cardiovascular Death §,¶ | 185 (4.6) | 268 (4.1) | 0.87 (0.72, 1.06) |
Figure 3: Time to First Occurrence of MACE
14.3 Canagliflozin Renal and Cardiovascular Outcomes in Patients with Diabetic Nephropathy and Albuminuria
Canagliflozin is indicated to reduce the risk of end-stage kidney disease (ESKD), doubling of serum creatinine, cardiovascular (CV) death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria > 300 mg/day.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation Trial (CREDENCE) was a multinational, randomized, double-blind, placebo-controlled trial comparing canagliflozin with placebo in patients with type 2 diabetes mellitus, an eGFR ≥ 30 to < 90 mL/min/1.73 m 2and albuminuria (urine albumin/creatinine ˃ 300 to ≤ 5000 mg/g) who were receiving standard of care including a maximum-tolerated, labeled daily dose of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB).
The primary objective of CREDENCE was to assess the efficacy of canagliflozin relative to placebo in reducing the composite endpoint of end stage kidney disease (ESKD), doubling of serum creatinine, and renal or CV death.
Patients were randomized to receive canagliflozin 100 mg (N=2,202) or placebo (N=2,199) and treatment was continued until the initiation of dialysis or renal transplantation.
The median follow-up duration for the 4,401 randomized subjects was 137 weeks. Vital status was obtained for 99.9% of subjects.
The population was 67% White, 20% Asian, and 5% Black; 32% were of Hispanic or Latino ethnicity. The mean age was 63 years and 66% were male.
At randomization, the mean HbA 1cwas 8.3%, the median urine albumin/creatinine was 927 mg/g, the mean eGFR was 56.2 mL/min/1.73 m 2, 50% had prior CV disease, and 15% reported a history of heart failure. The most frequent antihyperglycemic agents (AHA) medications used at baseline were insulin (66%), biguanides (58%), and sulfonylureas (29%). Nearly all subjects (99.9%) were on ACEi or ARB at randomization, approximately 60% were taking an anti-thrombotic agent (including aspirin), and 69% were on a statin.
The primary composite endpoint in the CREDENCE study was the time to first occurrence of ESKD (defined as an eGFR < 15 mL/min/1.73 m 2, initiation of chronic dialysis or renal transplant), doubling of serum creatinine, and renal or CV death. Canagliflozin 100 mg significantly reduced the risk of the primary composite endpoint based on a time-to-event analysis [HR: 0.70; 95% CI: 0.59, 0.82; p<0.0001] (see Figure 4). The treatment effect reflected a reduction in progression to ESKD, doubling of serum creatinine and cardiovascular death as shown in Table 22 and Figure 4. There were few renal deaths during the trial. Canagliflozin 100 mg also significantly reduced the risk of hospitalization for heart failure [HR: 0.61; 95% CI: 0.47 to 0.80; p<0.001].
| Placebo | canagliflozin | ||||
|---|---|---|---|---|---|
| Endpoint | N=2,199
(%) | Event Rate * | N=2,202
(%) | Event Rate * | HR
†
(95% CI) |
| Intent-To-Treat Analysis Set (time to first occurrence)
The individual components do not represent a breakdown of the composite outcomes, but rather the total number of subjects experiencing an event during the course of the study. |
|||||
|
|||||
| Primary Composite Endpoint (ESKD, doubling of serum creatinine, renal death, or CV death) | 340 (15.5) | 6.1 | 245 (11.1) | 4.3 | 0.70
(0.59, 0.82) ‡ |
| ESKD | 165 (7.5) | 2.9 | 116 (5.3) | 2.0 | 0.68
(0.54, 0.86) |
| Doubling of serum creatinine | 188 (8.5) | 3.4 | 118 (5.4) | 2.1 | 0.60
(0.48, 0.76) |
| Renal death | 5 (0.2) | 0.1 | 2 (0.1) | 0.0 | |
| CV death | 140 (6.4) | 2.4 | 110 (5.0) | 1.9 | 0.78
(0.61, 1.00) |
| CV death or hospitalization for heart failure | 253 (11.5) | 4.5 | 179 (8.1) | 3.1 | 0.69
(0.57, 0.83) § |
| CV death, non-fatal myocardial infarction or non-fatal stroke | 269 (12.2) | 4.9 | 217 (9.9) | 3.9 | 0.80
(0.67, 0.95) ¶ |
| Non-fatal myocardial infarction | 87 (4.0) | 1.6 | 71 (3.2) | 1.3 | 0.81
(0.59, 1.10) |
| Non-fatal stroke | 66 (3.0) | 1.2 | 53 (2.4) | 0.9 | 0.80
(0.56, 1.15) |
| Hospitalization for heart failure | 141 (6.4) | 2.5 | 89 (4.0) | 1.6 | 0.61
(0.47, 0.80) § |
| ESKD, doubling of serum creatinine or renal death | 224 (10.2) | 4.0 | 153 (6.9) | 2.7 | 0.66
(0.53, 0.81) ‡ |
The Kaplan-Meier curve (Figure 4) shows time to first occurrence of the primary composite endpoint of ESKD, doubling of serum creatinine, renal death, or CV death. The curves begin to separate by Week 52 and continue to diverge thereafter.
Figure 4: CREDENCE: Time to First Occurrence of the Primary Composite Endpoint
16. How is Invokamet supplied
INVOKAMET ®tablets are available in bottles of 60 in the strengths listed below:
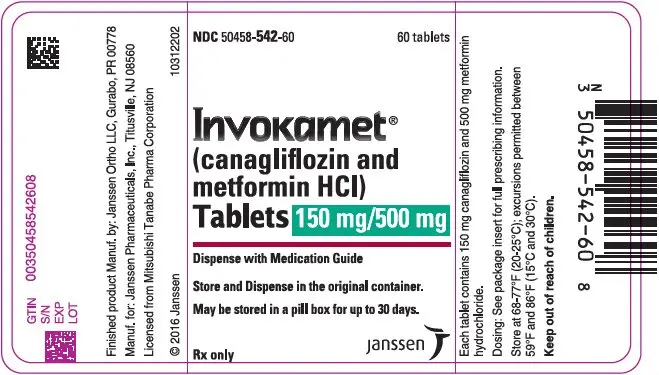
| INVOKAMET | TABLET STRENGTH | |||
|---|---|---|---|---|
| canagliflozin/metformin HCl tablets | 50 mg/500 mg | 50 mg/1000 mg | 150 mg/500 mg | 150 mg/1000 mg |
| Color | White | Beige | Yellow | Purple |
| Tablet Identification | CM | CM | CM | CM |
| 155 | 551 | 215 | 611 | |
| Capsule-shaped, film-coated tablets | ||||
| NDC | 50458-540-60 | 50458-541-60 | 50458-542-60 | 50458-543-60 |
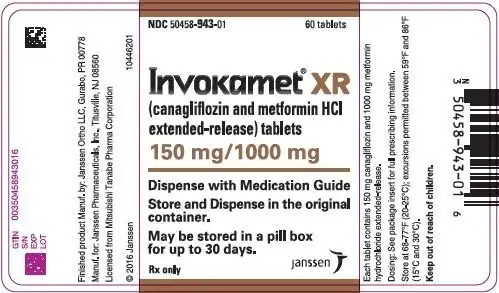
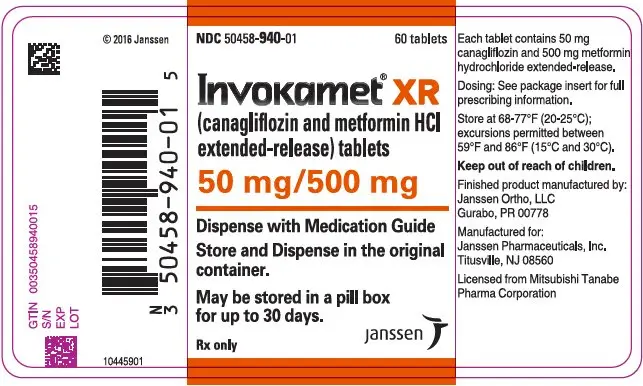
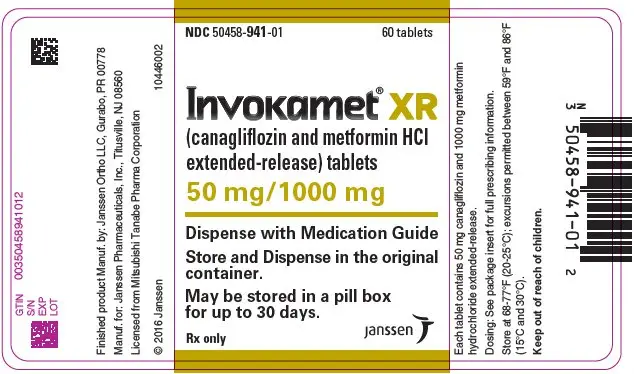
INVOKAMET ®XR tablets are available in bottles of 60 in the strengths listed below:
| INVOKAMET XR | TABLET STRENGTH | |||
|---|---|---|---|---|
| canagliflozin/metformin HCl extended-release tablets | 50 mg/500 mg | 50 mg/1000 mg | 150 mg/500 mg | 150 mg/1000 mg |
| Color | Almost White to Light Orange | Pink | Orange | Reddish Brown |
| Tablet Identification | CM1 | CM3 | CM2 | CM4 |
| Oblong, biconvex, film-coated tablets, a thin line on the tablet side may be visible. | ||||
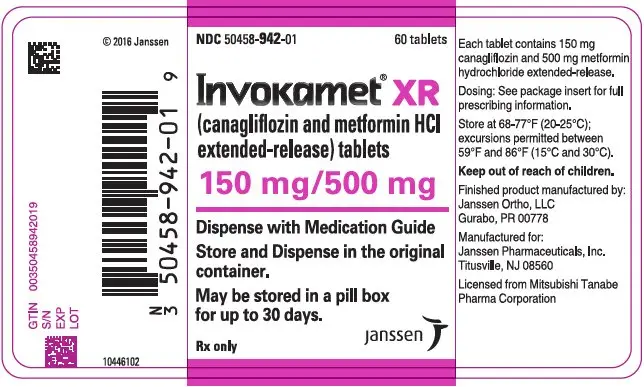
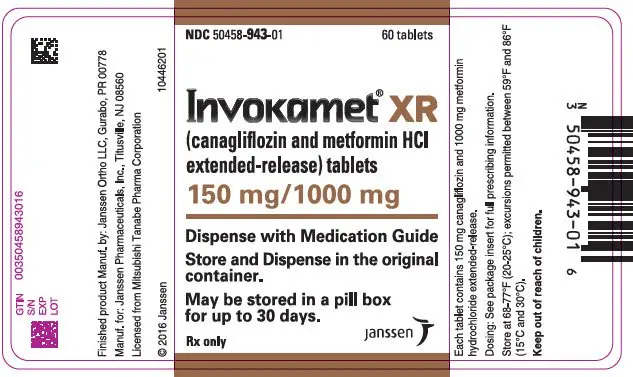
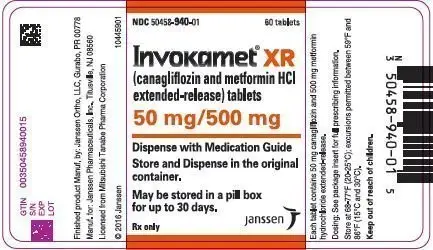
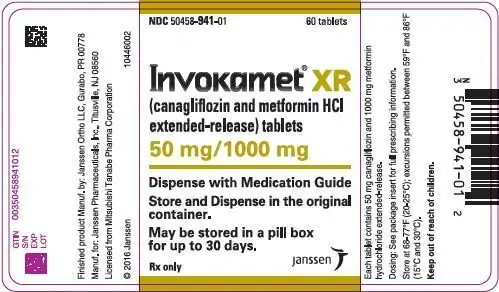
| NDC | 50458-940-01 | 50458-941-01 | 50458-942-01 | 50458-943-01 |
17. Patient Counseling Information
Advise the patient to read the FDA-Approved Patient Labeling (Medication Guide) .
| Medication Guide
INVOKAMET ®(in vok' a met) (canagliflozin and metformin hydrochloride (HCl)) tablets, for oral use and INVOKAMET ®(in vok' a met) XR (canagliflozin and metformin HCl) extended-release tablets, for oral use |
||||
|---|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised 07/2023 | |||
|
||||
|
|
|||
|
Most people who have had lactic acidosis had other conditions that, in combination with metformin use, led to the lactic acidosis. Tell your doctor if you have any of the following, because you have a higher chance for getting lactic acidosis with INVOKAMET or INVOKAMET XR if you: |
||||
|
||||
|
The best way to keep from having a problem with lactic acidosis from metformin is to tell your doctor if you have any of the problems in the list above. Your doctor will decide to stop your INVOKAMET or INVOKAMET XR for a while if you have any of these things.
|
||||
|
|
|||
Call your doctor right away if you have new pain or tenderness, any sores, ulcers, or infections in your leg or foot.Your doctor may decide to stop your INVOKAMET or INVOKAMET XR for a while if you have any of these signs or symptoms. Talk to your doctor about proper foot care.
Talk to your doctor about what you can do to prevent dehydration including how much fluid you should drink on a daily basis. Call your healthcare provider right away if you reduce the amount of food or liquid you drink, for example if you cannot eat or you start to lose liquids from your body, for example from vomiting, diarrhea, or being in the sun too long. |
||||
|
||||
|
||||
|
|
|||
| Talk to your doctor about what to do if you get symptoms of a yeast infection of the vagina or penis. Your doctor may suggest you use an over-the-counter antifungal medicine. Talk to your doctor right away if you use an over-the-counter antifungal medication and your symptoms do not go away. | ||||
| INVOKAMET or INVOKAMET XR can have other serious side effects.See " What are the possible side effects of INVOKAMET or INVOKAMET XR?" | ||||
|
What is INVOKAMET or INVOKAMET XR?
|
||||
|
Do not take INVOKAMET or INVOKAMET XR if you:
|
||||
|
Before taking INVOKAMET or INVOKAMET XR, tell your doctor about all of your medical conditions, including if you:
Tell your doctor about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. INVOKAMET or INVOKAMET XR may affect the way other medicines work and other medicines may affect how INVOKAMET or INVOKAMET XR works. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. |
||||
|
How should I take INVOKAMET or INVOKAMET XR?
|
||||
|
What should I avoid while taking INVOKAMET or INVOKAMET XR?
|
||||
|
What are the possible side effects of INVOKAMET or INVOKAMET XR? INVOKAMET or INVOKAMET XR may cause serious side effects including: |
||||
|
||||
|
|
|
||
|
||||
|
|
|
||
Other common side effects of INVOKAMET or INVOKAMET XR include: |
||||
|
|
|
||
|
|
|||
|
These are not all the possible side effects of INVOKAMET or INVOKAMET XR. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Janssen Pharmaceuticals, Inc. at 1-800-526-7736. |
||||
|
How should I store INVOKAMET or INVOKAMET XR?
Keep INVOKAMET and INVOKAMET XR and all medicines out of the reach of children. |
||||
|
General information about the safe and effective use of INVOKAMET or INVOKAMET XR. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use INVOKAMET or INVOKAMET XR for a condition for which it was not prescribed. Do not give INVOKAMET or INVOKAMET XR to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about INVOKAMET or INVOKAMET XR that is written for health professionals. |
||||
|
What are the ingredients in INVOKAMET? Active ingredients: canagliflozin and metformin HCl Inactive ingredients: The tablet core contains croscarmellose sodium, hypromellose, magnesium stearate, and microcrystalline cellulose. The magnesium stearate is vegetable-sourced. In addition, the tablet coating contains Macrogol/PEG3350, polyvinyl alcohol (partially hydrolyzed), talc, titanium dioxide, iron oxide yellow (50 mg/1,000 mg and 150 mg/500 mg tablets only), iron oxide red (50 mg/1,000 mg, 150 mg/500 mg and 150 mg/1,000 mg tablets only), and iron oxide black (150 mg/1,000 mg tablets only). What are the ingredients of INVOKAMET XR? Active ingredients: canagliflozin and metformin HCl Inactive ingredients: The tablet core contains croscarmellose sodium, hydroxypropyl cellulose, hypromellose, lactose anhydrous, magnesium stearate (vegetable-sourced), microcrystalline cellulose, polyethylene oxide, and silicified microcrystalline cellulose (50 mg/500 mg and 50 mg/1,000 mg tablets only). In addition, the tablet coating contains macrogol/PEG3350, polyvinyl alcohol (partially hydrolyzed), talc, titanium dioxide, iron oxide red, iron oxide yellow, and iron oxide black (50 mg/1,000 mg and 150 mg/1,000 mg tablets only). |
||||
|
Manufactured for: Janssen Pharmaceuticals, Inc., Titusville, NJ 08560. Licensed from Mitsubishi Tanabe Pharma Corporation. For patent information: www.janssenpatents.com © 2014 – 2020 Janssen Pharmaceutical Companies For more information about INVOKAMET or INVOKAMET XR, call 1-800-526-7736 or visit our websites at www.invokamet.com or www.invokametxr.com. |
||||
| INVOKAMET
canagliflozin and metformin hydrochloride tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| INVOKAMET
canagliflozin and metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| INVOKAMET
canagliflozin and metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| INVOKAMET
canagliflozin and metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| INVOKAMET XR
canagliflozin and metformin hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| INVOKAMET XR
canagliflozin and metformin hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| INVOKAMET XR
canagliflozin and metformin hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| INVOKAMET XR
canagliflozin and metformin hydrochloride tablet, film coated, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Janssen Pharmaceuticals, Inc. (063137772) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceuticals Inc | 063137772 | analysis(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Puerto Rico, Inc. (Manati) | 143814544 | manufacture(50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PHAST Gesellschaft für Pharmazeutische Qualitätsstandards mbH | 331156161 | analysis(50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceutica NV | 370005019 | analysis(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ajinomoto OmniChem | 400344443 | api manufacture(50458-540, 50458-541, 50458-542, 50458-543) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceutica NV | 400345889 | api manufacture(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) , analysis(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Farmhispania | 462038145 | api manufacture(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) , analysis(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Rolabo Outsourcing S.L. (Spain) | 513603225 | api manufacture(50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Cilag SpA | 542797928 | manufacture(50458-540, 50458-541, 50458-542, 50458-543) , pack(50458-540, 50458-541, 50458-542, 50458-543) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Johnson & Johnson Private Limited (Mumbai) | 677603030 | analysis(50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Ortho LLC | 805887986 | manufacture(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) , analysis(50458-540, 50458-940, 50458-941, 50458-942, 50458-943) , pack(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) , label(50458-540, 50458-541, 50458-542, 50458-543, 50458-940, 50458-941, 50458-942, 50458-943) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Granules India Limited | 918457644 | api manufacture(50458-540, 50458-541, 50458-542, 50458-543) , analysis(50458-540, 50458-541, 50458-542, 50458-543) | |