Drug Detail:Iodixanol (Iodixanol [ eye-oh-dix-an-ol ])
Drug Class: Non-ionic iodinated contrast media
Highlights of Prescribing Information
IODIXANOL injection, for intra-arterial or intra-venous use
Initial U.S. Approval: 1996
WARNING: NOT FOR INTRATHECAL USE
See full prescribing information for complete boxed warning
Inadvertent intrathecal administration may cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema. (4, 5.1)
Recent Major Changes
| Warnings and Precautions, Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age (5.8) | 04/2023 |
Indications and Usage for Iodixanol Injection
Iodixanol injection is a radiographic contrast agent indicated for the following:
Intra-arterial Procedures ( 1.1)
Adults and pediatric patients 12 years of age and over
- Intra-arterial digital subtraction angiography (270 mg Iodine/mL and 320 mg Iodine/mL).
- Angiocardiography (left ventriculography and selective coronary arteriography), peripheral arteriography, visceral arteriography, and cerebral arteriography (320 mg Iodine/mL).
Pediatric patients less than 12 years of age
- Angiocardiography, cerebral arteriography, and visceral arteriography (320 mg Iodine/mL).
Intravenous Procedures ( 1.2)
Adults and pediatric patients 12 years of age and over
- Computed tomography (CT) imaging head and body (270 mg Iodine/mL and 320 mg Iodine/mL).
- Excretory urography (270 mg Iodine/mL and 320 mg Iodine/mL).
- Peripheral venography (270 mg Iodine/mL).
- Coronary computed tomography angiography (CCTA) to assist diagnostic evaluation of patients with suspected coronary artery disease (320 mg Iodine/mL).
Pediatric patients less than 12 years of age
- CT imaging of the head and body (270 mg Iodine/mL).
- Excretory urography (270 mg Iodine/mL).
Iodixanol Injection Dosage and Administration
- Individualize the combination of volume and concentration of iodixanol injection considering age, body weight, size of the vessel, rate of blood flow within the vessel, and other applicable factors. ( 2.1, 2.2, 2.3, 2.4)
- For CT of the head and body, iodixanol injection may be used with an automated contrast injection system or contrast media management system cleared for use with iodixanol injection. (2.5)
- For the adult patients, the maximum recommended total dose of iodine is 80 grams. ( 2.1)
- Patients should be adequately hydrated prior to and following the intravascular administration of iodinated contrast agents. ( 2.1, 5.3)
- See full prescribing information for complete dosing and administration information. (2)
Dosage Forms and Strengths
Injection: In concentrations of 270 mg and 320 mg of organically bound iodine per mL (550 mg and 652 mg of Iodixanol per mL). ( 3)
Contraindications
- Not indicated for intrathecal use. ( 4)
Warnings and Precautions
- Hypersensitivity Reactions: Life-threatening or fatal reactions can occur. Always have emergency equipment and trained personnel available. (5.2)
- Contrast-Induced Acute Kidney Injury: Acute injury including renal failure can occur. Minimize dose and maintain adequate hydration to minimize risk. (5.3)
- Cardiovascular Adverse Reactions: Hemodynamic disturbances including shock and cardiac arrest may occur during or after administration. (5.4)
- Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age: Individualize thyroid function monitoring based on risk factors such as prematurity. (5.8)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence greater than 0.5%) in adult patients after iodixanol injection: Discomfort, warmth, pain; Cardiovascular: angina. Gastrointestinal: diarrhea, nausea, vomiting. Nervous System: agitation, anxiety, insomnia, nervousness, dizziness, headache, migraine, unusual skin sensations, sensory disturbance, fainting, sensation of spinning. Skin: itchy rash, severe itching, hives. Special Senses: Smell, taste, and vision alteration. ( 6.1) Pediatric patients experienced similar adverse reactions. ( 6.3)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Lactation: A lactating woman may pump and discard breast milk for 10 hours after iodixanol administration. ( 8.2)
- Geriatrics: Exercise caution in dose selection for elderly patients. ( 8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2023
Related/similar drugs
iohexol, barium sulfate, Omnipaque 350, Omnipaque 300, Volumen, DaTscan, iopromideFull Prescribing Information
WARNING: NOT FOR INTRATHECAL USE
Inadvertent intrathecal administration may cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema [see Contraindications (4) and Adverse Reactions (5.1)].
1. Indications and Usage for Iodixanol Injection
Iodixanol injection is indicated in for:
1.1 Intra-arterial Procedures
Adult and pediatric patients 12 years of age and older
- (270 mg Iodine/mL and 320 mg Iodine/mL) intra-arterial digital subtraction angiography (IA-DSA).
- (320 mg Iodine/mL) angiocardiography (left ventriculography and selective coronary arteriography), peripheral arteriography, visceral arteriography, and cerebral arteriography.
Pediatric patients less than 12 years of age
- (320 mg Iodine/mL) angiocardiography, cerebral arteriography, and visceral arteriography.
1.2 Intravenous Procedures
Adult and pediatric patients 12 years of age and older
- (270 mg Iodine/mL and 320 mg Iodine/mL) CT imaging of the head and body.
- (270 mg Iodine/mL and 320 mg Iodine/mL) excretory urography.
- (270 mg Iodine/mL) peripheral venography.
- (320 mg Iodine/mL) coronary computed tomography angiography (CCTA) to assist in the diagnostic evaluation of patients with suspected coronary artery disease.
Pediatric patients less than 12 years of age
- (270 mg Iodine/mL) CT imaging of the head and body.
- (270 mg Iodine/mL) excretory urography.
2. Iodixanol Injection Dosage and Administration
2.1 Important Dosage and Administration Instructions
- Iodixanol injection is for intravascular use only [see Boxed Warning, Contraindications (4), and Warnings and Precautions (5.1)]
- Use sterile technique for all handling and administration of iodixanol injection.
- Do not use if tamper-evident ring is broken or missing.
- Warm iodixanol injection and administer at body or room temperature.
- Inspect iodixanol injection for particulate matter or discoloration before administration, whenever solution and container permit. Do not administer if iodixanol injection contains particulate matter or is discolored.
- Do not mix iodixanol injection with, or inject in intravenous lines containing, other drugs or total nutritional admixtures.
- Use the lowest dose necessary to obtain adequate visualization.
- Individualize the volume, strength, and rate of administration of iodixanol injection. Consider factors such as age, bodyweight, vessel size, blood flow rate within the vessel, anticipated pathology, degree and extent of opacification required, structures or area to be examined, disease processes affecting the patient, and equipment and technique to be employed.
- The maximum recommended total dose of iodine for adults is 80 grams.
- Avoid extravasation when injecting iodixanol injection; especially in patients with severe arterial or venous disease [see Warnings and Precautions (5.6)].
- Hydrate patients before and after iodixanol injection administration [see Warnings and Precautions (5.3)] .
2.2 Intra-arterial Dosage and Administration
- Intra-arterial digital subtraction angiography (IA-DSA) (270 mg Iodine/mL and 320 mg Iodine/mL)
- Angiocardiography (left ventriculography and selective coronary arteriography), peripheral arteriography, visceral arteriography, and cerebral arteriography (320 mg Iodine/mL)
Use injection rates approximately equal to the flow rate in the vessel being injected. The usual single injection volumes or total dose per patient (mL/kg) for adults and adolescents over 12 years of age are listed in Table 1:
TABLE 1
ADULTS and PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER
IODIXANOL INJECTION SINGLE DOSE RECOMMENDATIONS FOR INJECTION INTO SELECTED ARTERIES
| ARTERIOGRAPHY | IA-DSA1 | Maximum Total Dose | ||||
|---|---|---|---|---|---|---|
| Intra-arterial Injection Sites | 320 mg Iodine/mL | 270 mg Iodine/mL | 320 mg Iodine/mL | |||
| Carotid Arteries | 10 mL to 14 mL | 5 mL to 8 mL | Usually Not to Exceed 175 mL | |||
| Vertebral Arteries | 10 mL to 12 mL | 5 mL to 8 mL | ||||
| Right Coronary Artery | 3 mL to 8 mL | Usually Not to Exceed 200 mL | ||||
| Left Coronary Artery | 3 mL to 10 mL | |||||
| Left Ventricle | 20 mL to 45 mL | |||||
| Renal Arteries | 8 mL to 18 mL | 10 mL to 25 mL | -- | Usually Not to Exceed 250 mL | ||
| Aortography | 30 mL to 70 mL | 20 mL to 50 mL | 10 mL to 50 mL | |||
| Major Branches of Aorta | 10 mL to 70 mL | 5 mL to 30 mL | 2 mL to 10 mL | |||
| Aortofemoral Runoffs | 20 mL to 90 mL | -- | 6 mL to 15 mL | |||
| Peripheral Arteries | 15 mL to 30 mL | -- | 3 mL to 15 mL | |||
1 IA-DSA = Intra-arterial Digital Subtraction Angiography
2.3 Intravenous Dosage and Administration
- Computed Tomography of the Head or Body (270 mg Iodine/mL and 320 mg Iodine/mL)
- Excretory Urography (270 mg Iodine/mL and 320 mg Iodine/mL)
- Peripheral Venography (270 mg Iodine/mL)
- Coronary Computed Tomography Angiography (CCTA) (320 mg Iodine/mL)
Recommended dosage of iodixanol injection is dependent on: the administration procedure, patient weight, and CT device factors, as detailed in Table 2. Calibrate the intravenous injection rate so that image acquisition coincides with peak arterial concentration. The time between iodixanol injection and peak arterial concentration varies between patients. Selected dosing for different indications in adults and pediatric patients over 12 years of age are shown in Table 2.
TABLE 2
| ADULTS and PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER IODIXANOL INJECTION DOSING RECOMMENDATIONS FOR INTRAVENOUS CONTRAST ADMINISTRATION |
||||
|---|---|---|---|---|
| Study Type | Comment | 270 mg Iodine/mL | 320 mg Iodine/mL | Maximum Total Volume |
| CT of Head or Body1 |
Bolus | 75 mL to 150 mL | 75 mL to 150 mL | 150 mL |
| Infusion | 100 mL to 150 mL | 100 mL to 150 mL | ||
| Excretory Urography | Normal Renal Function | 1 mL/kg | 1 mL/kg | 100 mL |
| Venography | Per lower extremity | 50 mL to 150 mL | 250 mL | |
| CCTA1,2 | Bolus injection with test bolus3 or bolus tracking | 50 mL to 150 mL4
(4 mL to 7 mL per second) | 150 mL | |
1 For CT of the head and body, iodixanol injection may be used with an automated contrast injection system or contrast media management system cleared for use with iodixanol injection.
2 For pediatric patients aged 12 to 17, recommended dose is 1 mL/kg to 2 mL/kg.
3 The main iodixanol injection volume may be preceded by a test bolus consisting of 20 mL iodixanol injection, immediately followed by a 20 mL saline flush, both injected at rate of 4 mL/sec to 7 mL/sec.
4 Injection of iodixanol injection with saline can be either biphasic (without dilution phase) or triphasic (with dilution phase). Alternatively, a dose of 1 mL/kg may be used to calculate total iodixanol injection dose (excluding any test bolus). For CCTA acquired at < 120 kVp, the dose of iodixanol injection may be reduced by up to 15% in patients < 85 kg and BMI < 30 kg/m2. For CCTA acquired on a scanner with more than 64 detector rows, the dose of iodixanol injection may be reduced in proportion to the scan duration.
2.4 Dosage in Pediatric Patients Less Than 12 Years of Age
Intra-arterial Dosage and Administration
Angiocardiography, cerebral arteriography, or visceral arteriography (320 mg Iodine/mL): The recommended dosage is 1 mL/kg to 2 mL/kg. The maximum dose should not exceed 4 mL/kg.
Intravenous Dosage and Administration
Computerized Tomography or Excretory Urography (270 mg Iodine/mL):
The recommended dosage is 1 mL/kg to 2 mL/kg. The maximum dose should not exceed 2 mL/kg.
2.5 Instructions for Use with an Automated Contrast Injection System or Contrast Management System for CT of the Head and Body
- Iodixanol injection may be used with an automated contrast injection system cleared for use with contrast media.
◦ See above Important Dosage and Administration Instructions for iodixanol injection (2.1).
◦ See device labeling for information on device indications, instructions for use, and techniques to help assure safe use.
- Iodixanol injection 320 mg Iodine/mL in 100 mL and 150 mL bottles may be used with a contrast media management system cleared for use with iodixanol injection 320 mg Iodine/mL in 100 mL and 150 mL bottles.
◦ See device labeling for information on device indications, instructions for use, and techniques to help assure safe use.
◦ Use sterile technique for penetrating the container closure of iodixanol injection 320 mg Iodine/mL and transferring iodixanol injection solution. Clean the stopper with a pad soaked in sporicidal solution followed by a pad soaked in alcohol, then puncture the stopper. The container closure may be penetrated only one time with a suitable sterile component of the contrast media management system cleared for use with iodixanol injection 320 mg Iodine/mL in 100 mL and 150 mL bottles.
◦ Once the iodixanol injection 320 mg Iodine/mL is punctured do not remove the bottle from the work area during the entire period of use.
◦ Maximum use time is 4 hours after initial puncture.
◦ Each bottle is for one procedure only. Discard unused portion.
3. Dosage Forms and Strengths
Injection: Non-ionic, isotonic, water-soluble, sterile, pyrogen-free, colorless to pale yellow solution in the following strengths:
- 270 mg of organically bound iodine per mL (550 mg Iodixanol per mL).
- 320 mg of organically bound iodine per mL (652 mg Iodixanol per mL).
Available in the following format: Single-dose polypropylene bottle.
4. Contraindications
Iodixanol is contraindicated for intrathecal use [see Warnings and Precautions (5.1)] :
5. Warnings and Precautions
5.1 Risks Associated with Inadvertent Intrathecal Administration
Iodixanol is for intravascular use only and is contraindicated for intrathecal use [see Contraindications (4) and Dosage and Administration (2.1)] . Inadvertent Intrathecal administration can cause death, convulsions/seizures, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, rhabdomyolysis, hyperthermia, and brain edema.
5.2 Hypersensitivity Reactions
Iodixanol can cause life-threatening or fatal hypersensitivity reactions including anaphylaxis. Manifestations include respiratory arrest, laryngospasm, bronchospasm, angioedema, and shock. Most severe reactions develop shortly after the start of the injection (within 3 minutes), but reactions can occur up to hours later. There is an increased risk in patients with a history of a previous reaction to contrast agent, and known allergies (i.e., bronchial asthma, drug, or food allergies) or other hypersensitivities. Premedication with antihistamines or corticosteroids does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
Obtain a history of allergy, hypersensitivity, or hypersensitivity reactions to iodinated contrast agents and always have emergency resuscitation equipment and trained personnel available prior to iodixanol administration. Monitor all patients for hypersensitivity reactions.
5.3 Contrast-Induced Acute Kidney Injury
Acute kidney injury, including renal failure, may occur after iodixanol administration. Risk factors include: pre-existing renal impairment, dehydration, diabetes mellitus, congestive heart failure, advanced vascular disease, elderly age, concomitant use of nephrotoxic or diuretic medications, multiple myeloma/paraproteinaceous diseases, repetitive and/or large doses of an iodinated contrast agent.
Use the lowest necessary dose of iodixanol in patients with renal impairment. Adequately hydrate patients prior to and following iodixanol administration. Do not use laxatives, diuretics, or preparatory dehydration prior to iodixanol administration.
5.4 Cardiovascular Adverse Reactions
Life-threatening or fatal cardiovascular reactions including hypotension, shock, cardiac arrest have occurred with the use of iodixanol. Most deaths occur during injection or five to ten minutes later, with cardiovascular disease as the main aggravating factor. Cardiac decompensation, serious arrhythmias, and myocardial ischemia or infarction can occur during coronary arteriography and ventriculography.
Based upon clinical literature reported deaths from the administration of iodinated contrast agents range from 6.6 per million (0.00066%) to 1 in 10,000 (0.01%). Use the lowest necessary dose of iodixanol in patients with congestive heart failure and always have emergency resuscitation equipment and trained personnel available. Monitor all patients for severe cardiovascular reactions.
5.5 Thromboembolic Events
Angiocardiography
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke can occur during angiocardiography procedures with both ionic and nonionic contrast media. During these procedures, increased thrombosis and activation of the complement system occurs. Risk factors for thromboembolic events include: length of procedure, catheter and syringe material, underlying disease state, and concomitant medications.
To minimize thromboembolic events, use meticulous angiographic techniques, and minimize the length of the procedure. Avoid blood remaining in contact with syringes containing iodinated contrast agents, which increases the risk of clotting. Avoid angiocardiography in patients with homocystinuria because of the risk of inducing thrombosis and embolism.
5.6 Extravasation and Injection Site Reactions
Extravasation of iodixanol injection may cause tissue necrosis and/or compartment syndrome, particularly in patients with severe arterial or venous disease. Ensure intravascular placement of catheters prior to injection. Monitor patients for extravasation and advise patients to seek medical care for progression of symptoms.
5.7 Thyroid Storm in Patients with Hyperthyroidism
Thyroid storm has occurred after the intravascular use of iodinated contrast agents in patients with hyperthyroidism, or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of iodixanol.
5.8 Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age
Thyroid dysfunction characterized by hypothyroidism or transient thyroid suppression has been reported after both single exposure and multiple exposures to iodinated contrast media (ICM) in pediatric patients 0 to 3 years of age.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after ICM exposure. Pediatric patients with congenital cardiac conditions may be at the greatest risk given that they often require high doses of contrast during invasive cardiac procedures.
An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to ICM, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
5.9 Hypertensive Crisis in Patients with Pheochromocytoma
Hypertensive crisis has occurred after the use of iodinated contrast agents in patient with pheochromocytoma. Monitor patients when administering iodixanol if pheochromocytoma or catecholamine-secreting paragangliomas are suspected. Inject the minimum amount of contrast necessary, assess the blood pressure throughout the procedure, and have measures for treatment of a hypertensive crisis readily available.
5.10 Sickle Cell Crisis in Patients with Sickle Cell Disease
Iodinated contrast agents when administered intravascularly may promote sickling in individuals who are homozygous for sickle cell disease. Hydrate patients prior to and following iodixanol administration and use iodixanol only if the necessary imaging information cannot be obtained with alternative imaging modalities.
5.11 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agents; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering iodixanol to patients with a history of a severe cutaneous adverse reaction to iodixanol.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risks Associated with Inadvertent Intrathecal Administration [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Contrast-Induced Kidney Injury [see Warnings and Precautions (5.3)]
- Cardiovascular Adverse Reactions [see Warnings and Precautions (5.4)]
- Thromboembolic Events [see Warnings and Precautions (5.5)]
- Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age [see Warnings and Precautions (5.8)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Iodixanol is often associated with sensations of discomfort, warmth or pain. In a subgroup of 1,259 patients; 30% who received iodixanol or a comparator had application site discomfort, pain, warmth or cold. Iodixanol had a trend toward fewer patient reports of moderate or severe pain or warmth. Pain was reported in 2% of patients receiving iodixanol and 10% of patients receiving a comparator. Heat was reported in 29% of patients receiving iodixanol and 51% of patients receiving a comparator.
Table 3 shows the incidence of events reported in blinded, controlled clinical studies of iodixanol in a total of 1,244 adult patients. Adverse events (AEs) are listed by body system and in decreasing order of occurrence greater than 0.5% of patients. One or more adverse events were reported in 20% of patients during the study period (24 to 72 hours). In a 757 patient subgroup, the number of women reporting adverse events was 83/299 (28%) and the number of men was 77/458 (16%). A total of 3% of women and 0.8% of men reported chest pain.
TABLE 3
| NUMBER OF PATIENTS EXPOSED | Iodixanol N (%)= 1,244 | Pooled Comparators N (%) = 861 |
|
|---|---|---|---|
| Number of Patients with Any Adverse Event | 248 (19.9) | 194 (22.5) | |
| Body As a Whole | Patients With Any Event | 41 (3.3) | 22 (2.6) |
| Edema (any location) | 7 (0.6) | 0 (0) | |
| Cardiovascular | Patients With Any Event | 37 (3.0) | 39 (4.5) |
| Angina Pectoris/Chest Pain | 28 (2.2) | 22 (2.6) | |
| Gastrointestinal | Patients With Any Event | 51 (4.1) | 46 (5.3) |
| Diarrhea | 7 (0.6) | 6 (0.7) | |
| Nausea | 35 (2.8) | 32 (3.7) | |
| Vomiting | 10 (0.8) | 11 (1.3) | |
| Nervous System | Patients With Any Event | 101 (8.1) | 60 (7.0) |
| Agitation, Anxiety, Insomnia, Nervousness | 10 (0.8) | 0 (0) | |
| Dizziness | 8 (0.7) | 8 (0.9) | |
| Headache/Migraine | 31 (2.5) | 15 (1.7) | |
| Paresthesia | 12 (1.0) | 1 (0.1) | |
| Sensory Disturbance | 10 (0.8) | 9 (1.0) | |
| Syncope | 8 (0.6) | 1 (0.1) | |
| Vertigo | 30 (2.4) | 20 (2.3) | |
| Skin (not including application site) | Patients With Any Event | 42 (4.6) | 18 (2.1) |
| Nonurticarial Rash or Erythema | 26 (2.1) | 4 (0.5) | |
| Pruritus | 20 (1.6) | 3 (0.3) | |
| Urticaria | 6 (0.5) | 10 (1.2) | |
| Special Senses | Patients With Any Event | 57 (4.6) | 38 (4.4) |
| Parosmia | 6 (0.5) | 4 (0.5) | |
| Taste Perversion | 43 (3.5) | 32 (3.7) | |
| Scotoma | 14 (1.1) | 2 (0.2) | |
The following selected adverse events were reported in ≤0.5% of the 1,244 patients.
Body as a Whole—General Disorders: back pain, fatigue, malaise
Cardiovascular Disorders: arrhythmias, cardiac failure, conduction abnormalities, hypotension, myocardial infarction
Gastrointestinal System Disorders: dyspepsia
Hypersensitivity Disorders: pharyngeal edema
Nervous System: cerebral vascular disorder, convulsions, hypoesthesia, stupor, confusion
Peripheral Vascular Disorders: flushing, peripheral ischemia
Renal System Disorders: abnormal renal function, acute renal failure, hematuria
Respiratory System Disorders: asthma, bronchitis, dyspnea, pulmonary edema, rhinitis
Skin and Appendage Disorders: hematoma, increased sweating
Special Senses, Other Disorders: tinnitus
Vision Disorders: abnormal vision
6.2 Post-marketing Experience
The following additional adverse reactions have been identified during post approval use of iodixanol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to exposure.
Cardiovascular Disorders: Cardiac arrest, palpitations, spasms of coronary arteries, hypertension, and flushing
Endocrine Disorders: Hyperthyroidism, hypothyroidism
Eye Disorders: Transient visual impairment including cortical blindness, diplopia, and blurred vision
Gastrointestinal Disorders: Abdominal pain, pancreatitis, salivary gland enlargement
General Disorders and Administration Site Conditions: Chills, pyrexia, pain and discomfort, administration site reactions including extravasation
Immune System Disorders: Hypersensitivity reactions, anaphylactic shock including, life-threatening or fatal anaphylaxis
Nervous System Disorders: Tremor (transient), coma, disturbance in consciousness, transient contrast-induced encephalopathy caused by extravasation of contrast media (including amnesia, hallucination, paralysis, paresis, transient speech disorder, aphasia, dysarthria)
Psychiatric Disorders: Anxiety, agitation
Respiratory, Thoracic, and Mediastinal Disorders: Cough, sneezing, throat irritation or tightness, laryngeal edema, pharyngeal edema, bronchospasm
Skin and subcutaneous tissue disorders: Reactions range from mild (e.g. rash, erythema, pruritus, urticaria, and skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)]
6.3 Pediatric Adverse Reactions
The overall character, quality, and severity of adverse reactions in pediatric patients is similar to that reported in adult patients from post marketing surveillance and other information.
Additional safety data was obtained in studies of iodixanol in 459 pediatric patients. A total of 26 patients ranged in age from birth to <29 days, 148 ranged from 29 days to 2 years, 263 from 2 to <12 years, and 22 from 12 to 18 years. A total of 252 (55%) of the patients were male. The racial distribution was: Caucasian-81%, Black-14%, Oriental-2%, and other or unknown-4%. The proportion of patients undergoing an intra-arterial procedure by age was: 92 % (<29 days), 55% (29 days to 6 months), and 29 % (>6 months). In these studies, adverse events were numerically higher in pediatric patients less than one year of age compared to older pediatric patients.
In pediatric patients who received intravenous injections of iodixanol for computerized tomography or excretory urography, a concentration of 270 mg Iodine/mL was used in 144 patients, and a concentration of 320 mg Iodine/mL in 154 patients. All patients received one intravenous injection of 1 mL/kg to 2 mL/kg.
In pediatric patients who received intra-arterial and intracardiac studies, a concentration of 320 mg Iodine/mL was used in 161 patients. Twenty-two patients were <29 days of age; 78 were 29 days to 2 years of age; and 61 were over 2 years. Most of these pediatric patients received initial volumes of 1 mL/kg to 2 mL/kg and most patients received a maximum of 3 injections.
7. Drug Interactions
7.1 Drug-Drug Interactions
- Metformin
In patients with renal impairment, metformin can cause lactic acidosis. Iodinated contrast agents appear to increase the risk of metformin-induced lactic acidosis, possibly as a result of worsening renal function. Stop metformin at the time of, or prior to, iodixanol administration in patients with an eGFR between 30 mL/min/1.73 m2 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and reinstitute metformin only after renal function is stable.
- Radioactive Iodine
Administration of iodinated contrast agents may interfere with thyroid uptake of radioactive iodine (I-131 and I-123) and decrease therapeutic and diagnostic efficacy in patients with carcinoma of the thyroid. The decrease in efficacy lasts for 6 to 8 weeks.
- Beta-adrenergic Blocking Agents
The use of beta-adrenergic blocking agents lowers the threshold for and increases the severity of contrast reactions, and reduces the responsiveness of treatment of hypersensitivity reactions with epinephrine. Because of the risk of hypersensitivity reactions, use caution when administering iodixanol to patients taking beta-blockers.
- Oral Cholecystographic Contrast Agents
Renal toxicity has been reported in patients with liver dysfunction who were given an oral cholecystographic agent followed by intravascular iodinated contrast agents. Postpone the administration of iodixanol in patients who have recently received an oral cholecystographic contrast agent.
7.2 Drug-Laboratory Test Interactions
- Effect on Thyroid Tests
The results of protein bound iodine and radioactive iodine uptake studies, which depend on iodine estimation, will not accurately reflect thyroid function for at least 16 days following administration of iodinated contrast agents. However, thyroid function tests which do not depend on iodine estimations (e.g., T3 resin uptake and total or free thyroxine T4 assays) are not affected.
- Effect on Urine Tests
As reported with other contrast agents, iodixanol may produce a false-positive result for protein in the urine using urine dip tests. However, the Coomassie blue method has been shown to give accurate results for the measurement of urine protein in the presence of iodixanol. In addition, care should be used in interpreting the results of urine specific gravity measurements in the presence of high levels of iodixanol and other contrast agents in the urine. Refractometry or urine osmolality may be substituted.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no data with iodixanol use in pregnant women to inform any drug-associated risks. In animal reproduction studies, no developmental toxicity occurred with intravenous iodixanol administration to rats and rabbits at doses up to 0.24 (rat) or 0.48 (rabbit) times the maximum recommended human intravenous dose (see Data).
All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Reproduction studies were performed in rats and rabbits with intravenous administration of iodixanol at doses up to 2 g Iodine/kg, daily, from implantation of the embryo (gestation day 7 in rat; 6 in rabbit) through closure of the hard palate (gestation day 17 in rats; 18 in rabbits). No maternal toxicity occurred, and no adverse effects occurred on fetal survival, embryo-fetal development, or the ability of dams to rear a litter.
8.2 Lactation
Risk Summary
There are no data on the presence of iodixanol in human milk, the effects on the breastfed infant or the effects on milk production. Iodinated contrast agents are poorly excreted into human milk and are poorly absorbed by the gastrointestinal tract of a breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for iodixanol and any potential adverse effects on the breastfed infant from iodixanol or from the underlying maternal condition.
Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast agents is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 10 hours (approximately 5 elimination half-lives) after iodixanol administration in order to minimize drug exposure to a breast fed infant.
8.4 Pediatric Use
The safety and efficacy of iodixanol have been established in pediatric patients down to birth for angiocardiography, cerebral arteriography, visceral arteriography, CT imaging of the head and body, and excretory urography. The safety and efficacy of iodixanol have also been established in pediatric patients 12 years and older for intra-arterial digital subtraction angiography, peripheral arteriography, peripheral venography and CCTA. Use of iodixanol is supported by evidence from adequate and well controlled studies of iodixanol in adults and additional safety data obtained in 459 pediatric patients. In general, the types of adverse reactions reported are similar to those of adults. A higher number of adverse events in patients less than 1 year of age compared to older patients were observed in a study of iodixanol [see Adverse Events (6.3)] . The elimination of iodixanol is slower in this age group [see Clinical Pharmacology (12.3)] .
Thyroid function tests indicative of thyroid dysfunction, characterized by hypothyroidism or transient thyroid suppression have been reported following iodinated contrast media administration in pediatric patients, including term and preterm neonates. Some patients were treated for hypothyroidism. After exposure to iodinated contrast media, individualize thyroid function monitoring in pediatric patients 0 to 3 years of age based on underlying risk factors, especially in term and preterm neonates [see Warnings and Precautions (5.8) and Adverse Reactions (6.2)].
Pediatric patients at higher risk of experiencing an adverse reaction during and after administration of any contrast agent may include those with asthma, hypersensitivity to other medication and/or allergens, cyanotic and acyanotic heart disease, congestive heart failure, or a serum creatinine greater than 1.5 mg/dL. Pediatric patients with immature renal function or dehydration may be at increased risk for adverse events due to slower elimination of iodinated contrast agents [see Clinical Pharmacology (12.3)] .
8.5 Geriatric Use
In clinical studies of iodixanol, 254/757 (34%) of patients were 65 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients. Other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
10. Overdosage
The adverse effects of overdosage of any contrast agent may be life-threatening and affect mainly the pulmonary and cardiovascular systems. Treatment of an overdosage is directed toward the support of all vital functions and prompt institution of symptomatic therapy. Iodixanol injection does not bind to plasma or serum protein and can be dialyzed.
11. Iodixanol Injection Description
11.1 Chemical Characteristics
Iodixanol injection, USP is a dimeric, iso-osmolar, nonionic, water-soluble, radiographic contrast medium for intravascular (intravenous and intra-arterial) use. It is provided as a ready-to-use sterile, pyrogen-free, and preservative free, colorless to pale yellow solution.
The chemical formula is 5,5´-[(2-hydroxy-1,3-propanediyl) bis(acetylimino)] bis[N,N´-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide] with a molecular weight of 1,550.18 (iodine content 49.1%).
Iodixanol (C 35H 44I 6N 6O 15) has the following structural formula:

Iodixanol injection, USP is available in two strengths:
- Iodixanol Injection, USP, 270 mg Iodine/mL (550 mg Iodixanol/mL), 0.074 mg calcium chloride dihydrate, 1.87 mg sodium chloride, 1.2 mg tromethamine, and 0.1 mg edetate calcium disodium.
- Iodixanol Injection, USP, 320 mg Iodine/mL (652 mg Iodixanol/mL), 0.044 mg calcium chloride dihydrate, 1.11 mg sodium chloride, 1.2 mg tromethamine and 0.1 mg edetate calcium disodium.
Sodium chloride and calcium chloride have been added, resulting in an isotonic solution for injection providing for both concentrations a sodium/calcium ratio equivalent to blood.
The pH is adjusted to 7.4 with hydrochloric acid and/or sodium hydroxide to achieve a range between pH 6.8 and 7.7 at 22°C.
11.2 Physical Characteristics
The two concentrations of iodixanol injection, USP (270 mg Iodine/mL and 320 mg Iodine/mL) have the following physical properties:
TABLE 4
| Parameter | Concentration (mg Iodine/mL) | ||
|---|---|---|---|
| 320 | 270 | ||
| Osmolality (mOsmol/kg water) | 290 | 290 | |
| Viscosity (cP) | @ 20°C | 26.6 | 12.7 |
| @ 37°C | 11.8 | 6.3 | |
| Density (g/mL) | @ 20°C | 1.369 | 1.314 |
| @ 37°C | 1.356 | 1.303 | |
12. Iodixanol Injection - Clinical Pharmacology
12.1 Mechanism of Action
Intravascular injection of iodixanol opacifies vessels in the path of flow of the contrast agent, permitting visualization of internal structures.
In imaging of the body, iodinated contrast agents diffuse from the vascular into the extravascular space. In a normal brain with an intact blood-brain barrier, contrast does not diffuse into the extravascular space. In patients with a disrupted blood- brain barrier, contrast agent accumulates in the interstitial space in the region of disruption.
12.2 Pharmacodynamics
Following administration of iodixanol, the degree of enhancement is directly related to the iodine content in an administered dose. Peak iodine plasma levels occur immediately following rapid injection. The time to maximum contrast enhancement can vary, depending on the organ, from the time that peak blood iodine concentrations are reached to one hour after intravenous bolus administration. When a delay between peak blood iodine concentrations and peak contrast is present, it suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine-containing medium within the lesion and outside the blood pool.
For angiography, contrast enhancement is greatest immediately (15 seconds to 120 seconds) after rapid injection. Iodinated contrast agents may be visualized in the renal parenchyma within 30 to 60 seconds following rapid intravenous injection. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1 to 3 minutes, with optimum contrast occurring within 5 to 15 minutes.
12.3 Pharmacokinetics
Distribution
In an in vitro human plasma study, iodixanol did not bind to protein. The volume of distribution in adults was 0.26 L/kg body weight, consistent with distribution to extracellular space.
Elimination
In 40 healthy, young male volunteers receiving a single intravenous administration of iodixanol in doses of 0.3 gram Iodine/kg to 1.2 gram Iodine/kg body weight, the elimination half-life was 2.1 hr. (± 0.1). Renal clearance was 110 ± 14 mL/min, equivalent to glomerular filtration (108 mL/min). These values were independent of the dose administered.
Excretion
In adults, approximately 97% of the injected dose of iodixanol is excreted unchanged in urine within 24 hours, with less than 2% excreted in feces within five days post-injection.
Specific Populations
Pediatric: Forty pediatric patients ≤12 years old, with renal function that is normal for their age, received multiple intra-arterial administrations of iodixanol in doses of 0.32 gram Iodine/kg to 3.2 gram Iodine/kg body weight. The elimination half-lives for these patients are shown in Table 5.
Dose adjustments to account for differences in elimination half-life in pediatric patients less than 6 months of age have not been studied.
TABLE 5
| Age Range | Number of Patients | Elimination half-life |
|---|---|---|
| (hr. ± SD) | ||
| Newborn to < 2 months | 8 | 4.1 ± 1.4 |
| 2 to 6 months | 8 | 2.8 ± 0.6 |
| 6 to 12 months | 9 | 2.4 ± 0.4 |
| 1 to 2 years | 5 | 2.3 ± 0.6 |
| 2 to 12 years | 10 | 2.3 ± 0.5 |
| Adults | 40 | 2.1 ± 0.1 |
Renal Impairment: In patients with significantly impaired renal function, the total clearance of iodixanol is reduced and the half-life is increased. In a study of 16 adult patients who were scheduled for renal transplant, the mean creatinine clearance was 13.6 ± 4.7 mL/min). In these patients, plasma half-life was 23 hours (t 1/2 for typical patients = 2.1 hours). Contrast enhancement time in kidneys increased from 6 hours to at least 24 hours. Dose adjustments in patients with renal impairment have not been studied. In patients with normal blood brain barriers and severe renal impairment, iodinated contrast agents have been associated with blood-brain barrier disruption and accumulation of contrast in the brain. Iodixanol has been shown to be dialyzable. In an in vitro hemodialysis study, after 4 hours of dialysis with a cellulose membrane, approximately 36% of iodixanol was removed from the plasma. After 4 hours of dialysis with polysulfone membranes, approximately 49% of iodixanol was removed.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed with iodixanol to evaluate carcinogenic potential. Iodixanol was not genotoxic in a series of studies including the Ames test, the CHO/HGPRT assay, a chromosome aberration assay in CHO cells, and a mouse micronucleus assay.
Iodixanol did not impair the fertility of male or female rats when administered at doses up to 0.24 times the maximum recommended human dose.
14. Clinical Studies
Iodixanol was studied in 1,244 adult patients. Approximately one-half (590) of the iodixanol patients were 60 years of age or older; the mean age was 56 years (range 18 to 90). A total of patients, 806 (65%) were male. The racial distribution was: Caucasian-85%, Black-12%, Oriental <1%, and other or unknown-3%.
A total of 1,235 patients were evaluable for efficacy. Efficacy assessment was based on quality of the radiographic diagnostic visualization (i.e., either: excellent, good, poor, or none) and on the ability to make a diagnosis (i.e., either: confirmed a previous diagnosis, found normal, or diagnosed new findings).
14.1 Intra-arterial Administration Studies
Angiocardiography, cerebral arteriography, peripheral arteriography, and visceral arteriography were studied with either one or both concentrations of iodixanol injection (270 mg Iodine/mL or 320 mg Iodine/mL). In these intra-arterial studies, diagnostic visualization ratings were good or excellent in all the patients and a radiologic diagnosis was made in all of the patients. In additional intra-arterial studies, overall quality of diagnostic visualization was rated optimal in the majority of patients and a radiologic diagnosis was made in all (100%) of the patients. The number of patients studied in each indication is provided below.
Angiocardiography was evaluated in two randomized, double-blind clinical studies in 101 adult patients given iodixanol 320 mg Iodine/mL. Seven additional angiocardiography studies were performed in 217 adult patients given iodixanol 320 mg Iodine/mL. Visualization ratings were good or excellent in all the patients given iodixanol; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Cerebral arteriography was evaluated in two randomized, double-blind clinical trials in 51 adult patients given iodixanol 320 mg Iodine/mL. Two additional cerebral arteriography studies were performed in 15 adult patients given iodixanol 270 mg Iodine/mL, 40 patients given iodixanol 320 mg Iodine/mL. Visualization ratings were good or excellent in all the patients a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Peripheral arteriography was evaluated in two randomized, double-blind clinical trials in 49 adult patients given iodixanol 320 mg Iodine/mL. Four additional peripheral arteriography studies were performed in 41 adult patients given iodixanol 270 mg Iodine/mL, 85 patients given iodixanol 320 mg Iodine/mL. Visualization ratings were good or excellent in 100% of the patients given iodixanol; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Visceral arteriography was evaluated in two randomized, double-blind clinical trials in 55 adult patients given iodixanol 320 mg Iodine/mL. Visualization ratings were good or excellent in all of the patients; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Similar studies with digital subtraction angiography (DSA) were completed with comparable findings noted in cerebral arteriography, peripheral arteriography, and visceral arteriography. Studies have not been conducted to determine the lowest effective concentration of iodixanol.
14.2 Intravenous Administration Studies
Excretory urography, computed tomography (CT) of the head, CT of the body, peripheral venography, and coronary computed tomography angiography (CCTA) were studied with either one or both iodixanol injection concentrations (270 mg Iodine/mL or 320 mg Iodine/mL). In the non-CCTA intravenous studies, diagnostic visualization ratings were good or excellent in 96% to 100% of the patients and a radiologic diagnosis was made in all of the patients given iodixanol. In the CCTA studies results were computed in terms of sensitivity and specificity compared to a standard of reference. The number of patients studied in each indication is provided below.
Excretory urography was evaluated in one uncontrolled, unblinded clinical trial in 40 patients, 20 given iodixanol 270 mg Iodine/mL and 20 given iodixanol 320 mg Iodine/mL, and in two randomized, double-blind clinical trials in 50 adult patients given iodixanol 270 mg Iodine/mL, 50 patients given iodixanol 320 mg Iodine/mL. Visualization ratings were good or excellent in all of the patients given iodixanol; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
CT of the head was evaluated in two randomized, double-blind clinical trials in 49 adult patients given iodixanol 270 mg Iodine/mL, in 50 patients given iodixanol 320 mg Iodine/mL. CT of the body was evaluated in three randomized, double-blind clinical trials in 104 adult patients given iodixanol 270 mg Iodine/mL, and 109 patients given iodixanol 320 mg Iodine/mL. In both CT of the head and body, visualization ratings were good or excellent in all of the patients given iodixanol; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Peripheral venography was evaluated in two randomized, double-blind clinical studies in 46 adult patients given iodixanol 270 mg Iodine/mL. Visualization ratings were good or excellent in all of the patients given iodixanol; a radiologic diagnosis was made in the majority of the patients. The results were similar to those of the active control. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Iodixanol 320 mg Iodine/mL for CCTA was evaluated in two prospective, multicenter clinical studies in a total of 1106 adult patients. The patient population consisted of stable outpatients with chest pain or other symptoms suggestive of coronary artery disease, and no known history of coronary disease. All the CCTAs were done using 64 detector row CT scanners. Most of the patients received beta-blocker medication for heart rate control and nitroglycerin for vasodilation. Patients with irregular cardiac rhythm or heart rate above 100 beats per minute were excluded. The mean patient age was 57 years in the first study and 59 years in the second study. Both studies had more men than women (59% male in the first study and 51% male in the second study), and more Caucasian patients (88% in the first study and 78% in the second study) than Black, Asian, or other patients. The BMI range was 17 to 50 with a mean of 31 in the first study and a BMI range of 15 to 71 with a mean of 30 in the second study.
In the first study, 230 patients (906 vessels) were evaluable for efficacy using the reference standard of invasive coronary angiography. Seventy-five vessels (8%, in 49 patients) were evaluated as positive for ≥50% stenosis. The CCTA images were randomized and read by three blinded, independent readers; the coronary angiography images were interpreted by an independent, blinded reader. Assuming independence between vessels, the vessel-level sensitivity (95% CI) for assessing ≥50% stenosis was 76% (63, 86) for reader 1, 89% (79, 95) for reader 2 and 77% (65, 86) for reader 3. The vessel-level specificity (95% CI) was 85% (81, 89) for reader 1, 84% (81, 87) for reader 2, and 89% (86, 91) for reader 3. The vessel-level sensitivity and specificity for assessing ≥70% stenosis were similar.
In a second study, 857 patients were evaluable for efficacy. Patients were followed up for 12 months after CCTA and the reference standard was a composite of pre-specified clinical outcomes (death, major adverse cardiac event, or coronary revascularization). Seventy-six patients (9%) experienced one or more of the pre-specified outcomes over 12 months of follow-up. The sensitivity (95% CI) and specificity (95% CI) of a positive CCTA finding (≥50% stenosis at the patient level) to predict one or more of the pre-specified clinical outcomes was 95% (87, 99) and 87% (84, 89), respectively.
16. How is Iodixanol Injection supplied
16.1 How Supplied
Iodixanol injection, USP is a ready-to-use sterile, pyrogen-free, preservative free, colorless to pale yellow solution available in two (2) strengths. It is supplied in the following configurations:
Iodixanol Injection, USP, 270 mg Iodine/mL:
| Product Code | Unit of Sale | Each |
| 381100 | NDC 65219-381-10 Unit of 10 | NDC 65219-381-03 100 mL single-dose polypropylene bottle |
| 381150 | NDC 65219-381-50 Unit of 10 | NDC 65219-381-05 150 mL single-dose polypropylene bottle |
Iodixanol Injection, USP, 320 mg Iodine/mL:
| Product Code | Unit of Sale | Each |
| 383105 | NDC 65219-383-05 Unit of 10 | NDC 65219-383-02 50 mL single-dose polypropylene bottle |
| 383110 | NDC 65219-383-10 Unit of 10 | NDC 65219-383-04 100 mL single-dose polypropylene bottle |
| 383150 | NDC 65219-383-50 Unit of 10 | NDC 65219-383-06 150 mL single-dose polypropylene bottle |
| 383200 | NDC 65219-383-70 Unit of 10 | NDC 65219-383-08 200 mL single-dose polypropylene bottle |
16.2 Storage and Handling
Protect iodixanol injection, USP from direct exposure to sunlight.
Store iodixanol injection, USP at controlled room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Iodixanol injection, USP may be stored in a contrast media warmer for up to one month at 37°C (98.6°F).
Do not freeze. Discard any product that is inadvertently frozen, as freezing may compromise the closure integrity of the immediate container.
DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING.
17. Patient Counseling Information
Hypersensitivity Reactions
Advise the patient concerning the risk of hypersensitivity reactions that can occur both during and after iodixanol injection administration. Advise the patient to report any signs or symptoms of hypersensitivity reactions during the procedure and to seek immediate medical attention for any signs or symptoms experienced after discharge [see Warnings and Precautions (5.2)].
Advise patients to inform their physician if they develop a rash after receiving iodixanol injection [see Warnings and Precautions (5.11)] .
Contrast-Induced Acute Kidney Injury
Advise the patient concerning appropriate hydration to decrease the risk of contrast-induced acute kidney injury [see Warnings and Precautions (5.3)] .
Extravasation
If extravasation occurs during injection, advise patients to seek medical care for progression of symptoms [see Warnings and Precautions (5.6)] .
Thyroid Dysfunction
Advise parents/caregivers about the risk of developing thyroid dysfunction after iodixanol injection administration. Advise parents/caregivers about when to seek medical care for their child to monitor for thyroid dysfunction [see Warnings and Precautions (5.8)].
PRINCIPAL DISPLAY PANEL – 320 mg Iodine/mL 50 mL Bottle Label
NDC 65219-383-02
Iodixanol Injection, USP
320 mg Iodine/mL
For Intra-arterial and Intravenous Use Only
in polypropylene bottle
50 mL Single-Dose Bottle
Discard Unused Portion
Sterile Aqueous Injection
NOT FOR INTRATHECAL USE
DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING
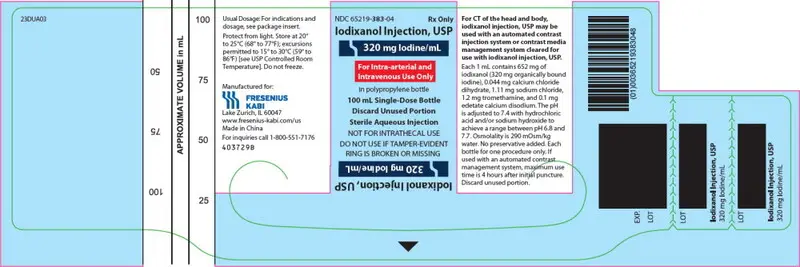
PRINCIPAL DISPLAY PANEL – 320 mg Iodine/mL 100 mL Bottle Label
NDC 65219-383-04
Iodixanol Injection, USP
320 mg Iodine/mL
For Intra-arterial and Intravenous Use Only
in polypropylene bottle
100 mL Single-Dose Bottle
Discard Unused Portion
Sterile Aqueous Injection
NOT FOR INTRATHECAL USE
DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING
PRINCIPAL DISPLAY PANEL – 320 mg Iodine/mL 150 mL Bottle Label
NDC 65219-383-06
Iodixanol Injection, USP
320 mg Iodine/mL
For Intra-arterial and Intravenous Use Only
in polypropylene bottle
150 mL Single-Dose Bottle
Discard Unused Portion
Sterile Aqueous Injection
NOT FOR INTRATHECAL USE
DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING
PRINCIPAL DISPLAY PANEL – 320 mg Iodine/mL 200 mL Bottle Label
NDC 65219-383-08
Iodixanol Injection, USP
320 mg Iodine/mL
For Intra-arterial and Intravenous Use Only
in polypropylene bottle
200 mL Single-Dose Bottle
Discard Unused Portion
Sterile Aqueous Injection
NOT FOR INTRATHECAL USE
DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING
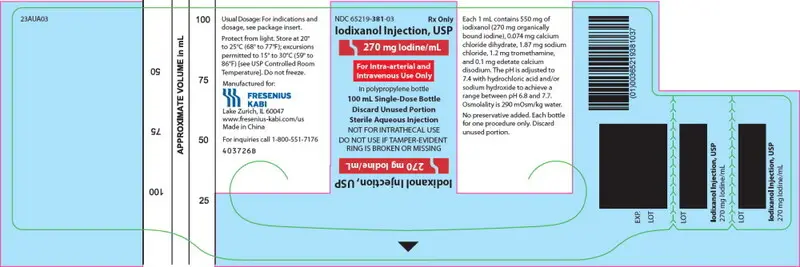
PRINCIPAL DISPLAY PANEL – 270 mg Iodine/mL 100 mL Bottle Label
NDC 65219-381-03
Iodixanol Injection, USP
270 mg Iodine/mL
For Intra-arterial and Intravenous Use Only
in polypropylene bottle
100 mL Single-Dose Bottle
Discard Unused Portion
Sterile Aqueous Injection
NOT FOR INTRATHECAL USE
DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING
PRINCIPAL DISPLAY PANEL – 270 mg Iodine/mL 150 mL Bottle Label
NDC 65219-381-05
Iodixanol Injection, USP
270 mg Iodine/mL
For Intra-arterial and Intravenous Use Only
in polypropylene bottle
150 mL Single-Dose Bottle
Discard Unused Portion
Sterile Aqueous Injection
NOT FOR INTRATHECAL USE
DO NOT USE IF TAMPER-EVIDENT RING IS BROKEN OR MISSING
| IODIXANOL
iodixanol injection, solution |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| IODIXANOL
iodixanol injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - FRESENIUS KABI USA, LLC (013547657) |