Drug Class: Miscellaneous antihypertensive combinations
Highlights of Prescribing Information
ISOSORBIDE DINITRATE AND HYDRALAZINE HYDROCHLORIDE tablet, for oral use
Initial U.S. Approval: 2005
Indications and Usage for Isosorbide and Hydralazine tablets
Isosorbide dinitrate and hydralazine hydrochloride tablets are a combination of isosorbide dinitrate, a nitrate vasodilator, and hydralazine hydrochloride, an arteriolar vasodilator, indicated for:
- the treatment of heart failure as an adjunct therapy to standard therapy in self-identified black patients to improve survival, prolong time to hospitalization for heart failure and to improve patient-reported functional status (1.1)
Limitations of use:
- There is little experience in patients with NYHA class IV heart failure (1.2)
Isosorbide and Hydralazine tablets Dosage and Administration
One tablet three times a day titrated to a maximum tolerated dose up to two tablets three times a day (2)
Dosage may be decreased to as little as one-half tablet three times a day if intolerable side effects occur. Efforts should be made to titrate up as soon as side effects subside (2)
Dosage Forms and Strengths
Tablets (scored): 20 mg isosorbide dinitrate and 37.5 mg hydralazine hydrochloride (3)
Contraindications
- Patients who are allergic to organic nitrates (4)
- Use of phosphodiesterase type 5 (PDE5) inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil, or soluble guanylate cyclase (sGC) stimulator (riociguat). (4)
Warnings and Precautions
- May cause symptomatic hypotension (5.1)
- Symptomatic Lupus Erythematosus Syndromes: Consider discontinuation if clinically appropriate (5.2)
- Myocardial ischemia and angina (5.3)
- Peripheral Neuritis: May be treated with Pyridoxine (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions (≥ 5% more on isosorbide dinitrate and hydralazine hydrochloride tablets than on placebo) were headache and dizziness (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Wilshire Pharmaceuticals, Inc. at 1-877-495-6856 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Geriatric Use: Isosorbide dinitrate and hydralazine may be eliminated more slowly in elderly patients. Initiate therapy at the low end of the dosing range (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2020
Full Prescribing Information
1. Indications and Usage for Isosorbide and Hydralazine tablets
1.1 Treatment of Heart Failure in Self-identified Black Patients
Isosorbide dinitrate and hydralazine hydrochloride tablets are indicated for the treatment of heart failure as an adjunct to standard therapy in self-identified black patients to improve survival, to prolong time to hospitalization for heart failure, and to improve patient-reported functional status.
2. Isosorbide and Hydralazine tablets Dosage and Administration
Isosorbide dinitrate and hydralazine hydrochloride tablets should be initiated at a dose of one isosorbide dinitrate and hydralazine hydrochloride tablet, three times a day. Titrate to a maximum of two tablets three times daily, if tolerated.
Although titration of isosorbide dinitrate and hydralazine hydrochloride tablets can be rapid (3-5 days), some patients may experience side effects and may take longer to reach their maximum tolerated dose. The dosage may be decreased to as little as one-half isosorbide dinitrate and hydralazine hydrochloride tablet three times a day if intolerable side effects occur. Efforts should be made to titrate up as soon as side effects subside.
3. Dosage Forms and Strengths
The isosorbide dinitrate and hydralazine hydrochloride tablets (20 mg isosorbide dinitrate and 37.5 mg hydralazine hydrochloride) are orange, biconvex, approximately 8 mm in diameter, scored, film-coated, and debossed with "20" on one side over the score and "N" on the other side.
4. Contraindications
Isosorbide dinitrate and hydralazine hydrochloride tablets are contraindicated in patients who are allergic to organic nitrates.
Do not use isosorbide dinitrate and hydralazine hydrochloride tablets in patients who are taking PDE-5 inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil. Concomitant use can cause severe hypotension, syncope, or myocardial ischemia [see Drug Interactions (7.1)].
Do not use isosorbide dinitrate and hydralazine hydrochloride tablets in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use can cause hypotension.
5. Warnings and Precautions
5.1 Hypotension
Symptomatic hypotension, particularly with upright posture, may occur with even small doses of isosorbide dinitrate and hydralazine hydrochloride tablets. Hypotension is most likely to occur in patients who have been volume or salt depleted; correct prior to initiation of isosorbide dinitrate and hydralazine hydrochloride tablets [see Adverse Reactions (6.1)].
5.2 Systemic Lupus Erythematosus
Hydralazine hydrochloride has been reported to cause a drug-induced systemic lupus erythematosus (SLE) syndrome. Symptoms and signs usually regress when hydralazine hydrochloride is discontinued.
5.3 Worsening Ischemic Heart Disease
Hydralazine hydrochloride can cause tachycardia and hypotension potentially leading to myocardial ischemia and angina, particularly in patients with hypertrophic cardiomyopathy.
5.4 Peripheral Neuritis
Hydralazine hydrochloride has been associated with peripheral neuritis, evidenced by paresthesia, numbness, and tingling, which may be related to an antipyridoxine effect. Pyridoxine should be added to isosorbide dinitrate and hydralazine hydrochloride tablets therapy if such symptoms develop.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Isosorbide dinitrate and hydralazine hydrochloride tablets have been evaluated for safety in 517 heart failure patients in A-HeFT. A total of 317 of these patients received isosorbide dinitrate and hydralazine hydrochloride tablets for at least 6 months, and 220 received isosorbide dinitrate and hydralazine hydrochloride tablets for at least 12 months. In A-HeFT, 21% of the patients discontinued isosorbide dinitrate and hydralazine hydrochloride tablets for adverse reactions compared to 12% who discontinued placebo. Overall, adverse reactions were more common in isosorbide dinitrate and hydralazine hydrochloride tablets-treated than in placebo-treated patients. Table 1 lists adverse reactions reported with an incidence, after rounding, ≥ 2% higher on isosorbide dinitrate and hydralazine hydrochloride tablets than on placebo in A-HeFT, regardless of causality. The most common reasons for discontinuing isosorbide dinitrate and hydralazine hydrochloride tablets in the A-HeFT trial was headache (7%).
| Isosorbide dinitrate and hydralazine hydrochloride tablets (N=517) % | Placebo (N=527) % |
|
|---|---|---|
| Headache | 50 | 21 |
| Dizziness | 32 | 14 |
| Asthenia | 14 | 11 |
| Nausea | 10 | 6 |
| Hypotension | 8 | 4 |
| Sinusitis | 4 | 2 |
| Ventricular tachycardia | 4 | 2 |
| Paresthesia | 4 | 2 |
| Vomiting | 4 | 2 |
| Amblyopia | 3 | 1 |
In the V-HeFT I and II clinical studies, a total of 587 patients with heart failure were treated with the combination of isosorbide dinitrate and hydralazine hydrochloride. The type, pattern, frequency and severity of adverse reactions reported in these studies were similar to those reported in A-HeFT, described above and no unusual adverse reactions were reported.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of isosorbide dinitrate and hydralazine hydrochloride tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7. Drug Interactions
7.1 Phosphodiesterase Inhibitors
Isosorbide dinitrate and hydralazine hydrochloride tablets are contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), PDE5 inhibitors such as avanafil, sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. Do not use isosorbide dinitrate and hydralazine hydrochloride tablets in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use can cause hypotension [see Contraindications (4)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of isosorbide dinitrate and hydralazine hydrochloride tablets in children have not been established.
8.5 Geriatric Use
Clinical studies of isosorbide dinitrate and hydralazine hydrochloride tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between elderly and younger patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic and renal function, and of concomitant disease or other drug therapies.
Isosorbide dinitrate, its active metabolites, and hydralazine may be eliminated more slowly in elderly patients.
8.6 Renal Impairment
There are no studies of renal impairment using isosorbide dinitrate and hydralazine hydrochloride tablets. No dose adjustment is required for hydralazine or isosorbide dinitrite [see Clinical Pharmacology (12.3)].
Dialyzability of hydralazine has not been determined. Dialysis is not an effective method for removing isosorbide dinitrate or its metabolite isosorbide-5-mononitrate from the body.
8.7 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of hydralazine alone has not been determined. Isosorbide dinitrate concentrations increase in patients with cirrhosis. There are no studies of hepatic impairment using isosorbide dinitrate and hydralazine hydrochloride tablets.
10. Overdosage
The signs and symptoms of overdosage with isosorbide dinitrate and hydralazine hydrochloride tablets are expected to be those of excessive pharmacologic effect, i.e., vasodilatation, reduced cardiac output and hypotension, and signs and symptoms include headache, confusion, tachycardia, and generalized skin flushing. Complications can include myocardial ischemia and subsequent myocardial infarction, cardiac arrhythmia, and profound shock. Syncope, coma and death may ensue without appropriate treatment.
11. Isosorbide and Hydralazine tablets Description
Isosorbide dinitrate and hydralazine hydrochloride tablets are a fixed-dose combination of isosorbide dinitrate, a vasodilator with effects on both arteries and veins, and hydralazine hydrochloride, a predominantly arterial vasodilator.
Isosorbide dinitrate is described chemically as 1,4:3,6-dianhydro-D-glucitol dinitrate and its structural formula is:
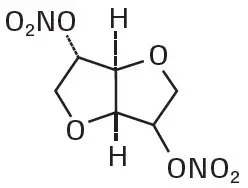
Isosorbide dinitrate is a white to off-white, crystalline powder with the empirical formula C6H8N2O8 and a molecular weight of 236.14. It is freely soluble in organic solvents such as alcohol, chloroform and ether, but is only sparingly soluble in water.
Hydralazine hydrochloride is described chemically as 1-hydrazinophthalazine monohydrochloride, and its structural formula is:
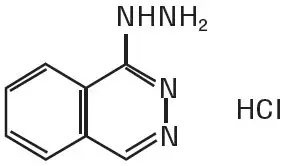
Hydralazine hydrochloride is a white to off-white, crystalline powder with the empirical formula C8H8N4∙HCl and a molecular weight of 196.64. It is soluble in water, slightly soluble in alcohol, and very slightly soluble in ether.
Each isosorbide dinitrate and hydralazine hydrochloride tablet for oral administration contains 20 mg of isosorbide dinitrate and 37.5 mg of hydralazine hydrochloride.
The inactive ingredients in isosorbide dinitrate and hydralazine hydrochloride tablets include: anhydrous lactose, microcrystalline cellulose, sodium starch glycolate, colloidal silicon dioxide, magnesium stearate, hypromellose, FD&C Yellow No. 6 aluminum lake, polyethylene glycol, titanium dioxide, polysorbate 80.
12. Isosorbide and Hydralazine tablets - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action underlying the beneficial effects of isosorbide dinitrate and hydralazine hydrochloride tablets in the treatment of heart failure has not been established.
Isosorbide dinitrate is a vasodilator affecting both arteries and veins. Its dilator properties result from the release of nitric oxide and the subsequent activation of guanylyl cyclase, and ultimate relaxation of vascular smooth muscle.
Several well-controlled clinical trials have used exercise testing to assess the anti-anginal efficacy of chronically-delivered nitrates. In the large majority of these trials, active agents were no more effective than placebo after 24 hours (or less) of continuous therapy. Attempts to overcome nitrate tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates have been absent from the body for several hours is response to nitrates restored.
Hydralazine hydrochloride is a selective dilator of arterial smooth muscle. Animal data suggests that hydralazine may also mitigate tolerance to nitrates.
12.2 Pharmacodynamics
The basis for the beneficial clinical effects of isosorbide dinitrate and hydralazine hydrochloride tablets is not known. In a small study of patients with chronic heart failure administered single doses of hydralazine 75 mg, isosorbide dinitrate 20 mg, and the combination, the combination elicited a statistically significant decrease in pulmonary capillary wedge pressure compared to hydralazine alone. The increase in cardiac output, renal blood flow and limb blood flow with the combination, however, was not greater than with hydralazine alone. There is no study of hemodynamic effects following multiple dosing.
12.3 Pharmacokinetics
14. Clinical Studies
Isosorbide dinitrate and hydralazine hydrochloride tablets or a combination of isosorbide dinitrate and hydralazine hydrochloride was studied in two placebo-controlled clinical trials in 1,692 patients with mild to severe heart failure (mostly NYHA class II and III) and one active control trial (vs. enalapril) in 804 patients. The results of the trials follow:
Placebo-controlled Study: The A-HeFT trial evaluated isosorbide dinitrate and hydralazine hydrochloride tablets vs. placebo among 1,050 self-identified black patients (over 95% NYHA class III) at 169 centers in the United States. All patients had stable symptomatic heart failure. Patients were required to have LVEF ≤ 35% or left ventricular internal diastolic dimension > 2.9 cm/m2 plus LVEF < 45%. Patients were maintained on stable background therapy and randomized to isosorbide dinitrate and hydralazine hydrochloride tablets (n=518) or placebo (n=532). Isosorbide dinitrate and hydralazine hydrochloride tablets were initiated at 20 mg isosorbide dinitrate/37.5 mg hydralazine hydrochloride three times daily and titrated to a target dose of 40/75 mg three times daily or to the maximum tolerated dose. Patients were treated for up to 18 months.
The randomized population was 60% male, 1% NYHA class II, 95% NYHA class III and 4% NYHA class IV, with a mean age of 57 years, and was generally treated with standard treatments for heart failure including diuretics (94%, almost all loop diuretics), beta-blockers (87%), angiotensin converting enzyme inhibitors (ACE-I; 78%), angiotensin II receptor blockers (ARBs; 28%), either ACE-I or ARB (93%), digitalis glycosides (62%) and aldosterone antagonists (39%).
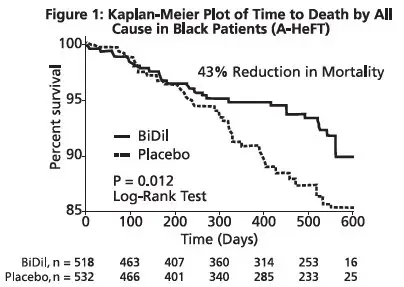
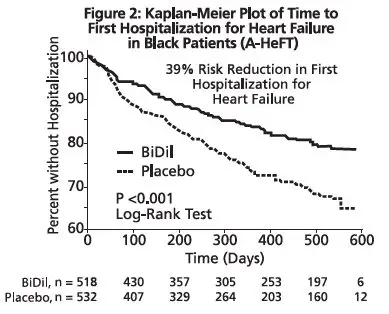
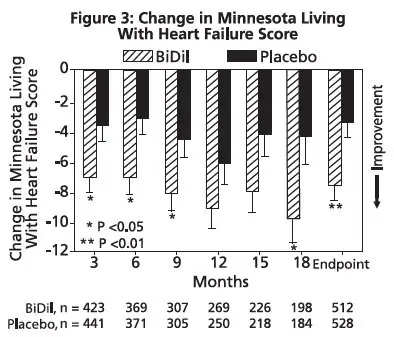
The primary endpoint was a composite score consisting of all-cause mortality, first hospitalization for heart failure, and responses to the Minnesota Living with Heart Failure questionnaire. The trial was terminated early, at a mean follow-up of 12 months, primarily because of a statistically significant 43% reduction in all-cause mortality in the isosorbide dinitrate and hydralazine hydrochloride tablets-treated group (p=0.012; see Table 2 and Figure 1). The primary endpoint was also statistically in favor of Isosorbide dinitrate and hydralazine hydrochloride tablets (p ≤ 0.021). The isosorbide dinitrate and hydralazine hydrochloride tablets-treated group also showed a 39% reduction in the risk of a first hospitalization for heart failure (p<0.001; see Table 2 and Figure 2) and had statistically significant improvement in response to the Minnesota Living with Heart Failure questionnaire, a self-report of the patient's functional status, at most time points (see Figure 3). Patients in both treatment groups had mean baseline questionnaire scores of 51 (out of a possible 105).
| Isosorbide dinitrate and hydralazine hydrochloride tablets (N=518) | Placebo (N=532) | Hazard Ratio (95% CI) | P | |
|---|---|---|---|---|
| Composite | -0.16±1.93 | -0.47±2.04 | — | 0.021 |
| All-cause mortality | 6.2% | 10.2% | 0.57 (0.37, 0.89) | 0.012 |
| Hospitalization for heart failure | 16.4% | 24.4% | 0.61 (0.46, 0.80) | <0.001 |
 |
 |
 |
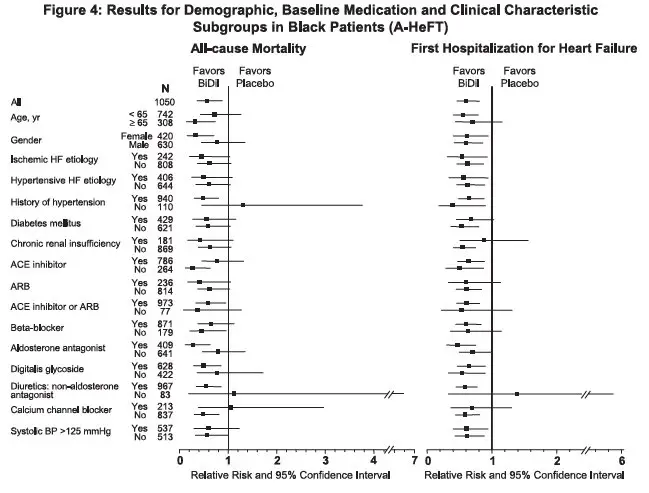
Effects on survival and hospitalization for heart failure were similar in subgroups by age, gender, baseline disease, and use of concomitant medications, as shown in Figure 4.
 |
Patients treated with isosorbide dinitrate and hydralazine hydrochloride tablets in the A-HeFT study had randomly measured blood pressures on average 3/3 mmHg lower than did patients on placebo. The contribution of the difference in blood pressure to the overall outcome difference is unknown. Whether both hydralazine and isosorbide dinitrate contribute to the overall outcome difference has not been studied in outcome trials. Isosorbide dinitrate and hydralazine have not been systematically studied for the treatment of heart failure as separate agents, and neither drug is indicated for heart failure.
16. How is Isosorbide and Hydralazine tablets supplied
Isosorbide dinitrate and hydralazine hydrochloride tablets contain 20 mg of isosorbide dinitrate and 37.5 mg of hydralazine hydrochloride. They are biconvex, approximately 8 mm in diameter, scored, film-coated, orange tablets debossed "20" on one side over the score and "N" on the other side.
- NDC 52536-006-09: Bottles of 90
| ISOSORBIDE DINITRATE AND HYDRALAZINE HYDROCHLORIDE
hydralazine hydrochloride and isosorbide dinitrate tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Wilshire Pharmaceuticals (078657245) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lannett Company Inc. | 006422406 | MANUFACTURE(52536-006) | |





