Drug Detail:Isovue-250 (Iopamidol [ eye-oh-pam-ih-dol ])
Drug Class: Non-ionic iodinated contrast media
Isovue 250 Description
| Iopamidol | |||
|---|---|---|---|
| Parameter | 51% | 61% | 76% |
| Concentration (mgI/mL) | 250 | 300 | 370 |
| Osmolality @ 37° C (mOsm/kg water) | 524 | 616 | 796 |
| Viscosity (cP) @ 37° C | 3.0 | 4.7 | 9.4 |
| @ 20° C | 5.1 | 8.8 | 20.9 |
| Specific Gravity @ 37° C | 1.281 | 1.339 | 1.405 |
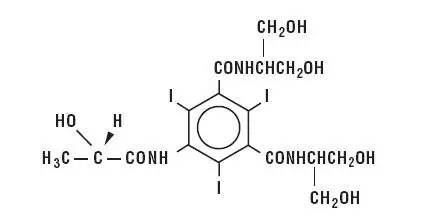 | MW 777.09 C17H22I3N3O8 CAS-60166-93-0 Organically Bound Iodine: 49% |
Related/similar drugs
diazepam, Valium, simethicone, hyoscyamine, Levsin, iopamidolIsovue 250 - Clinical Pharmacology
Iopamidol displays little tendency to bind to serum or plasma proteins.
No evidence of in vivo complement activation has been found in normal subjects.
Precautions
Information for Patients
Patients receiving injectable radiopaque diagnostic agents should be instructed to:
- Inform your physician if you are pregnant.
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease, or known thyroid disorder (see WARNINGS).
- Inform your physician if you are allergic to any drugs, food, or if you had any reactions to previous injections of substances used for x-ray procedures (see PRECAUTIONS-General).
- Inform your physician about any other medications you are currently taking, including nonprescription drugs, before you have this procedure.
- Advise patients to inform their physician if they develop a rash after receiving Isovue.
Adverse Reactions/Side Effects
| Adverse Reactions | ||
| Estimated Overall Incidence | ||
| System | > 1% | ≤ 1% |
| Cardiovascular | none | tachycardia hypotension hypertension myocardial ischemia circulatory collapse S-T segment depression bigeminy extrasystoles ventricular fibrillation angina pectoris bradycardia transient ischemic attack thrombophlebitis |
| Nervous | pain (2.8%) burning sensation (1.4%) | vasovagal reaction tingling in arms grimace faintness |
| Digestive | nausea (1.2%) | vomiting anorexia |
| Respiratory | none | throat constriction dyspnea pulmonary edema |
| Skin and Appendages | none | rash urticaria pruritus flushing |
| Body as a Whole | hot flashes (1.5%) | headache fever chills excessive sweating back spasm |
| Special Senses | warmth (1.1%) | taste alterations nasal congestion visual disturbances |
| Urogenital | none | urinary retention |
Isovue 250 Dosage and Administration
General
Patients should be well hydrated prior to and following ISOVUE (Iopamidol Injection) administration.
Pediatric Angiocardiography
The usual dose range for single injections is provided in the following table:
| Single Injection | |
| Usual Dose Range | |
| Age | mL |
| < 2 years | 10-15 |
| 2-9 years | 15-30 |
| 10-18 years | 20-50 |
The usual recommended dose for cumulative injections is provided in the following table:
| Cumulative Injection | |
| Usual Recommended Dose | |
| Age | mL |
| < 2 years | 40 |
| 2-4 years | 50 |
| 5-9 years | 100 |
| 10-18 years | 125 |
Storage and Handling
Directions for Proper Use of ISOVUE Pharmacy Bulk Package
- The transferring ISOVUE (Iopamidol Injection) from the Pharmacy Bulk Package should be performed in a suitable work area, such as a laminar flow hood, utilizing aseptic technique.
- The container closure may be penetrated only one time, utilizing a suitable transfer device. Once the Pharmacy Bulk Package is punctured, it should not be removed from the aseptic work area during the entire period of use.
- The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of 10 hours from initial closure entry is permitted to complete fluid transfer operation. Any unused ISOVUE Pharmacy Bulk Package injection must be discarded 10 hours after initial puncture of the bulk package.
- Storage temperature of container after the closure has been entered should not exceed 25° C (77° F).
How is Isovue 250 supplied
ISOVUE-250 (Iopamidol Injection 51%)
Ten 200 mL Pharmacy Bulk Packages
(NDC 0270-1317-41)
| ISOVUE
250
iopamidol injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ISOVUE
300
iopamidol injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ISOVUE
370
iopamidol injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - BIPSO GmbH (342104149) |
| Registrant - BIPSO GmbH (342104149) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BIPSO GmbH | 342104149 | MANUFACTURE(76381-315, 76381-317, 76381-316) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda GmbH | 313270016 | ANALYSIS(76381-317, 76381-316, 76381-315) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BRACCO IMAGING SPA | 434384007 | API MANUFACTURE(76381-315, 76381-316, 76381-317) | |







