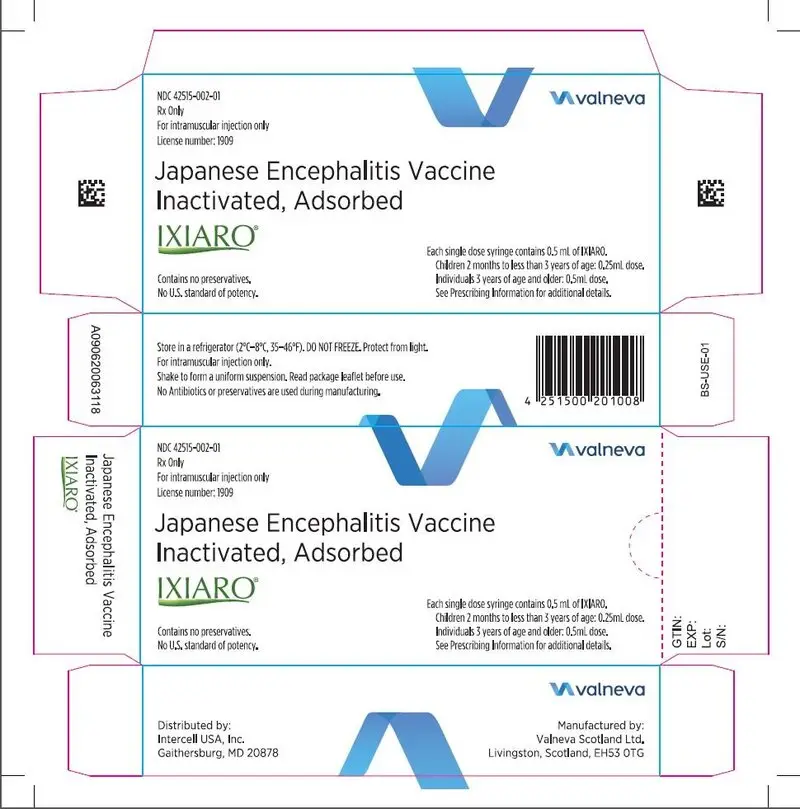
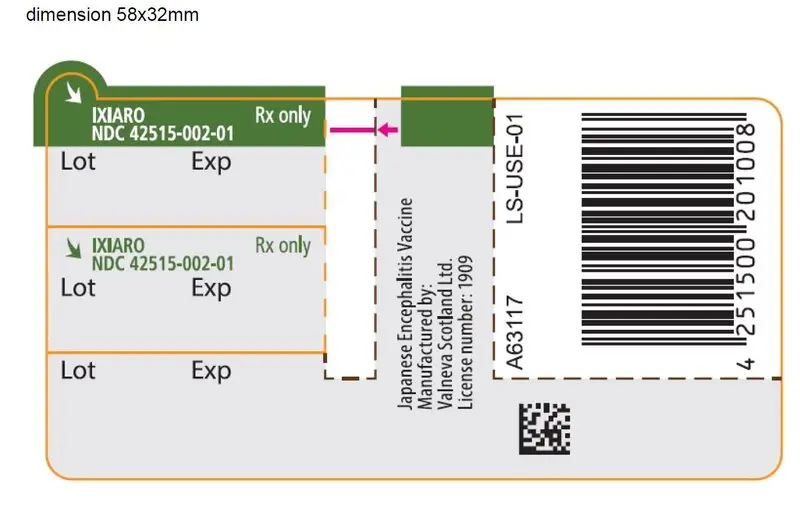
Drug Detail:Ixiaro (Japanese encephalitis virus vaccine (sa14-14-2) [ jap-a-neez-en-sef-a-lye-tis-vye-rus-vax-een ])
Drug Class: Viral vaccines
Highlights of Prescribing Information
IXIARO (Japanese Encephalitis Vaccine, Inactivated, Adsorbed)
Suspension for Intramuscular Injection
Initial U.S. Approval: 2009
Indications and Usage for Ixiaro
- IXIARO is a vaccine indicated for active immunization for the prevention of disease caused by Japanese encephalitis virus (JEV). IXIARO is approved for use in individuals 2 months of age and older. (1)
Ixiaro Dosage and Administration
For intramuscular administration only. (2)
|
Age (2) |
Dose* (2) |
Primary Series (2) |
|
Children 2 months to <3 years of age (2) |
0.25 mL (2) |
2 doses, |
|
Individuals 3 years of age and older (2) |
0.5 mL (2) |
2 doses, |
*To administer a 0.25 mL dose, expel and discard half of the volume from the 0.5 mL pre-filled syringe by pushing the plunger stopper up to the edge of the red line on the syringe barrel prior to injection. (2.3) (2)
Complete the primary immunization series at least 1 week prior to potential exposure to JEV. (2.1, 14) (2)
Individuals 17 years of age and older who have received a primary immunization series more than 1 year previously may be given a booster dose if ongoing exposure or re-exposure to JEV is expected. (2.1) (2)
(2)
Dosage Forms and Strengths
- Suspension for injection supplied in 0.5 mL single dose syringes.
Contraindications
Severe allergic reaction, (e.g., anaphylaxis,) after a previous dose of IXIARO, any other Japanese Encephalitis Virus vaccine, or any component of IXIARO, including protamine sulfate, is a contraindication to administration of IXIARO. (4) (4)
Warnings and Precautions
IXIARO contains protamine sulfate, a compound known to cause hypersensitivity reactions in some individuals. (5.1) (5)
Adverse Reactions/Side Effects
In infants 2 months to <1 year of age, the most common injection site reaction was redness (>15%); the most common solicited systemic adverse reactions were fever (>20%), irritability (>15%) and diarrhea (>10%). (6.1)
In children 1 to <3 years of age, the most common solicited systemic adverse reaction was fever (>20%). (6.1)
In children 3 to <12 years of age, the most common solicited systemic adverse reaction was fever (>10%). (6.1)
In adolescents 12 to <18 years of age, the most common injection site reactions were pain (15%) and tenderness (10%). (6.1)
In adults 18 years of age and older, the most common injection site reactions were pain (>25%) and tenderness (>25%); the most common solicited systemic adverse reactions were headache (>20%) and myalgia (>10%). (6.1) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Intercell USA Inc. at 844-349-4276 (8443-IXIARO) or VAERS at 1 800 822 7967 or www.vaers.hhs.gov. (6)
Use In Specific Populations
To report inadvertent use in pregnant women, contact Intercell USA Inc. at 1-844-349-4276 (8443-IXIARO).
See 17 for PATIENT COUNSELING INFORMATION and FDA‑approved patient labeling (Patient Information).
Revised 03/2017
See 17 for FDA-approved patient labeling.
Revised: 12/2022
Full Prescribing Information
1. Indications and Usage for Ixiaro
IXIARO is a vaccine indicated for the prevention of disease caused by Japanese encephalitis virus (JEV). IXIARO is approved for use in individuals 2 months of age and older.
2. Ixiaro Dosage and Administration
For intramuscular administration only.
2.1 Dosage and Schedule
Primary Series:
Children 2 months to <3 years of age: Primary immunization with IXIARO consists of two (2) 0.25 mL doses, administered 28 days apart.
Individuals 3 years of age and older: Primary immunization with IXIARO consists of two (2) 0.5 mL doses, administered 28 days apart.
Complete the primary immunization series at least 1 week prior to potential exposure to JEV.
Booster Dose:
Individuals 17 years of age and older: If the primary series of two doses was completed more than 1 year previously, a booster dose may be given if ongoing exposure or re-exposure to JEV is expected.
Infants, children and adolescents 2 months to <17 years of age: The safety and immunogenicity of a booster dose has not been evaluated.
2.2 Administration
IXIARO is administered intramuscularly. The preferred sites for intramuscular injection are the anterolateral aspect of the thigh in infants 2 to 11 months of age, the anterolateral aspect of the thigh (or the deltoid muscle if muscle mass is adequate) in children 1 to <3 years of age, or the deltoid muscle in individuals 3 years of age and older.
Do not administer intravenously, intradermally, or subcutaneously.
2.3 Preparation for Administration
Prior to agitation, IXIARO is a clear liquid with a white precipitate. Before administration, shake the syringe well to obtain a white, opaque, homogeneous suspension.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If either of these conditions exists, do not administer.
Preparation of a 0.25 mL Dose of IXIARO for Administration to Children 2 Months to <3 Years of Age:
1. Shake the pre-filled syringe containing 0.5 mL to obtain a homogeneous suspension.
2. Remove the syringe tip cap by gently twisting it. Do not attempt to snap or pull the tip off as this may damage the syringe.
3. Attach a sterile safety needle to the pre-filled syringe (needle is not provided with IXIARO).
4. Hold the syringe in an upright position and uncap the needle.
5. Push the plunger stopper up to the edge of the red line on the syringe barrel, indicated by a red arrow (see Figure1)*, and discard expelled volume into a medical waste container.
6. Lock the needle safety shield and remove the needle.
7. Attach a new sterile needle prior to injection of the remaining volume.
*If the plunger stopper is pushed beyond the red line, do not administer the vaccine. Repeat the procedure using a new pre-filled syringe.
Preparation of a 0.5 mL Dose of IXIARO for Administration to Individuals 3 Years of Age and Above:
1. Shake the pre-filled syringe containing 0.5 mL to obtain a homogeneous suspension.
2. Remove the syringe tip cap by gently twisting it. Do not attempt to snap or pull the tip off as this may damage the syringe.
3. Attach a sterile needle to the pre-filled syringe (needle is not provided with IXIARO).
3. Dosage Forms and Strengths
IXIARO is a suspension for injection supplied in 0.5 mL single dose syringes. For children 2 months to <3 years of age, a single dose is 0.25 mL. For individuals 3 years of age and older, a single dose is 0.5 mL. [See Dosage and Administration (2.1)]
4. Contraindications
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of IXIARO, any other Japanese Encephalitis Virus vaccine, or any component of IXIARO, including protamine sulfate, is a contraindication to administration of IXIARO [See Description (11)]. Alternatively, because of uncertainty as to which component of the vaccine may be responsible, individuals with a history of severe allergic reaction to another Japanese Encephalitis vaccine may be referred to an allergist for evaluation if immunization with IXIARO is considered.
5. Warnings and Precautions
6. Adverse Reactions/Side Effects
In infants 2 months to <1 year of age, the most common injection site reaction was redness (>15%); the most common solicited systemic adverse reactions were fever (>20%), irritability (>15%) and diarrhea (>10%). In children 1 to <3 years of age, the most common solicited systemic adverse reaction was fever (>20%). In children 3 to <12 years of age, the most common solicited systemic adverse reaction was fever (>10%). In adolescents 12 to <18 years of age, the most common solicited injection site reactions were pain (15%) and tenderness (10%). In adults 18 years of age and older, the most common injection site reactions were pain (>25%) and tenderness (>25%); the most common solicited systemic adverse reactions were headache (>20%) and myalgia (>10%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Clinical Studies in Children 2 Months to <18 Years of Age:
Adverse Events in a Pediatric Trial Comparing IXIARO to U.S.-Licensed Control Vaccines HAVRIX and PREVNAR:
The safety of IXIARO was evaluated in a randomized, controlled, open-label clinical trial in healthy male and female subjects 2 months to <18 years of age, conducted in the Philippines, a country where Japanese Encephalitis is endemic (Study 1)1. IXIARO was compared to two control vaccines: HAVRIX (Hepatitis A vaccine, pediatric 720 EL.U./0.5 mL formulation, GlaxoSmithKline Biologicals) and Prevnar (Pneumococcal 7-valent Conjugate Vaccine [Diphtheria CRM197 protein], Pfizer). A total of 1,769 subjects were randomized in an age-stratified scheme in a 3:1 ratio (2:1 ratio for ages <1 year) to receive intramuscular injections of either IXIARO (two 0.25 mL doses on Days 0 and 28 for infants and children 2 months to <3 years of age or two 0.5 mL doses on Days 0 and 28 for children 3 to <18 years of age) or HAVRIX (children 1 year of age and older, 2 doses on Day 0 and at Month 7) or Prevnar (infants 2 to <6 months of age, 3 doses on Days 0, 28, 56 and an optional 4th dose at Month 7 or later; infants 6 to <12 months of age, 3 doses on Days 0 and 56 and at Month 7). Subject numbers and dosing schemes by age group are displayed in Table 1.
Table 1. Subject Numbers and Dosing Schemes by Age Group (Safety Population, Study 1§,Philippines)
|
Treatment Group |
IXIARO* (N=1311) |
HAVRIX (N=394) |
PREVNAR (N=64) |
|
Subjects in Age Group 2 months to <1 year |
131 |
- |
64 |
|
Subjects in Age Group 1 year to <3 years |
640 |
213 |
- |
|
Subjects in Age Group 3 to <12 years |
300 |
101 |
- |
|
Subjects in Age Group 12 to <18 years |
240 |
80 |
- |
§NCT01041573
*Infants and children 2 months to <3 years of age received two 0.25 mL doses administered on Days 0 and 28. Individuals 3 years of age and older received two 0.5 mL doses administered on Days 0 and 28.
Analysis of safety in children was carried out using the safety population including 1,311 subjects receiving at least one dose of IXIARO, 394 subjects receiving the first dose of HAVRIX on Day 0, and 64 subjects receiving at least one dose of Prevnar on Day 0 (all infants <1 year of age). The IXIARO and control groups were similar with regard to demographics (mean age 5.48 years, range 2 months to 17 years; 49.5% female; ethnicity: Asian: 100% for Study 1 overall). Parents or subjects recorded adverse events on a diary card for the first seven days after each vaccination. Only those events considered to be assessable based on the subjects developmental status were recorded. Parents or subjects were queried regarding the occurrence of any unsolicited AEs following the previous vaccination at in-person visits, which included a medical exam, on Day 28, Day 56, and at Month 7.
Solicited Adverse Events
For an overview of solicited local and systemic reactions for pediatric age groups see Table 2 (infants 2 months to <1 year of age), Table 3 (toddlers 1 to <3 years of age) Table 4 (children 3 to <12 years of age), and Table 5 (adolescents 12 to <18 years of age). As children 1 year of age and older received the second dose of HAVRIX at the final study visit at Month 7, rates of solicited AEs among these subjects after the second vaccination are only available for IXIARO.
Table 2.Rates of Solicited Adverse Reactions on Days 0-7 After Each Vaccination in Infants 2 Months to <1 Year of Age, by Dose and Treatment Group, Study 1§,Philippines
|
Post Dose 1 (% of subjects) |
Post Dose 2 (% of subjects) |
|||
|
Injection Site Reactions |
IXIARO* (N=131‡) |
Prevnar (N=64‡) |
IXIARO* (N=131‡) |
Prevnar (N=64‡) |
|
Tenderness |
3.1 |
12.7 |
0.8 |
3.3 |
|
Hardening |
0.0 |
7.9 |
0.0 |
1.6 |
|
Swelling |
1.5 |
6.3 |
1.5 |
1.6 |
|
Redness |
17.6 |
25.4 |
5.3 |
16.4 |
|
Solicited Systemic Reactions | ||||
|
Irritability |
15.3 |
12.7 |
8.4 |
8.2 |
|
Vomiting |
7.6 |
6.3 |
3.8 |
1.6 |
|
Diarrhea |
11.5 |
6.3 |
8.4 |
4.9 |
|
Excessive fatigue |
3.1 |
7.9 |
1.5 |
3.3 |
|
Rash |
8.4 |
9.5 |
3.8 |
4.9 |
|
Loss of appetite |
5.3 |
9.5 |
5.3 |
6.6 |
|
Fever ≥37.7°C (≥99.9°F) |
23.7 |
25.4 |
14.5 |
23.3 |
|
37.7-38.6 °C (99.9-101.5°F) |
17.6 |
22.2 |
12.2 |
15.0 |
|
38.7-39.3 °C (101.6-102.7°F) |
6.1 |
1.6 |
1.5 |
6.7 |
|
39.4-40.5 °C (102.8-104.9°F) |
0.0 |
1.6 |
0.8 |
1.7 |
|
>40.5°C (>104.9°F) |
0.0 |
0.0 |
0.0 |
0.0 |
§NCT01041573
*IXIARO dose 0.25 mL.
‡N=number of subjects with available diary card data after each dose, used as the denominator to calculate percentages.
Table 3. Rates of Solicited Adverse Reactions on Days 0-7 After Each Vaccination in Children 1 Year to <3 Years of Age, by Dose and Treatment Group, Study 1§,Philippines
|
Post Dose 1 (% of subjects**) |
Post Dose 2 (% of subjects**) |
|||
|
Injection Site Reactions |
IXIARO* (N=640‡) |
Havrix (N=213‡) |
IXIARO* (N=637‡) |
|
|
Pain |
3.6 (6/165) |
7.4 (4/54) |
3.6 (6/166) |
|
|
Itching |
0.6 (1/180) |
0.0 (0/63) |
0.0 (0/184) |
|
|
Tenderness |
3.1 |
5.6 |
1.4 |
|
|
Hardening |
0.9 |
0.5 |
0.3 |
|
|
Swelling |
2.0 |
3.3 |
1.7 |
|
|
Redness |
6.1 |
7.5 |
2.5 |
|
|
Solicited Systemic Reactions | ||||
|
Irritability |
7.7 |
5.6 |
2.7 |
|
|
Nausea |
2.2 (5/228) |
1.3 (1/78) |
0.9 (2/229) |
|
|
Vomiting |
4.2 |
5.6 |
2.8 |
|
|
Diarrhea |
7.0 |
5.2 |
4.6 |
|
|
Flu-like symptoms |
7.7 (13/169) |
13.3 (8/60) |
4.0 (7/176) |
|
|
Excessive fatigue |
2.5 |
0.9 |
1.1 |
|
|
Muscle pain |
2.3 (3/130) |
0.0 (0/42) |
0.7 (1/136) |
|
|
Rash |
4.2 |
2.3 |
1.3 |
|
|
Headache |
1.5 (2/135) |
4.4 (2/45) |
1.4 (2/143) |
|
|
Loss of appetite |
5.6 |
4.2 |
2.5 |
|
|
Fever ≥37.7°C (≥99.9°F) |
20.2 |
15.5 |
12.7 |
|
|
37.7-38.6 °C (99.9-101.5°F) |
15.6 |
12.2 |
8.5 |
|
|
38.7-39.3 °C (101.6-102.7°F) |
3.0 |
1.4 |
2.5 |
|
|
39.4-40.5 °C (102.8-104.9°F) |
1.6 |
1.9 |
1.6 |
|
|
>40.5°C (>104.9°F) |
0.0 |
0.0 |
0.2 |
§NCT01041573
*IXIARO dose 0.25 mL.
‡N=number of subjects with available diary card data after each dose, used as the denominator to calculate percentages
** Where the number of subjects with available data for a particular symptom differed from the overall number of subjects with available diary card data, the rate (n/N) is provided; n is the number of subjects who reported that symptom, and N is the number of subjects with available data for that symptom.
Table 4. Rates of Solicited Adverse Reactions on Days 0-7 After Each Vaccination in Children 3 Years to <12 Years of Age, by Dose and Treatment Group, Study 1§,Philippines
|
Post Dose 1 (% of subjects) |
Post Dose 2 (% of subjects) |
||
|
Injection Site Reactions |
IXIARO* (N=291-300‡) |
Havrix (N=99-101‡) |
IXIARO* (N=293-300‡) |
|
Pain |
5.5 |
3.0 |
1.7 |
|
Itching |
1.4 |
0.0 |
0.0 |
|
Tenderness |
4.3 |
1.0 |
2.0 |
|
Hardening |
1.3 |
0.0 |
0.0 |
|
Swelling |
2.0 |
3.0 |
2.0 |
|
Redness |
3.0 |
1.0 |
0.7 |
|
Solicited Systemic Reaction | |||
|
Irritability |
0.0 |
1.0 |
0.3 |
|
Nausea |
0.3 |
0.0 |
0.3 |
|
Vomiting |
1.7 |
1.0 |
0.7 |
|
Diarrhea |
0.7 |
0.0 |
1.0 |
|
Flu-like symptoms |
1.4 |
2.0 |
0.3 |
|
Excessive fatigue |
1.0 |
1.0 |
0.7 |
|
Muscle pain |
2.4 |
3.0 |
0.3 |
|
Rash |
1.0 |
0.0 |
0.0 |
|
Headache |
3.8 |
4.0 |
1.4 |
|
Loss of appetite |
1.0 |
2.0 |
1.0 |
|
Fever ≥37.7°C (≥99.9°F) |
10.7 |
8.9 |
4.7 |
|
37.7-38.6 °C (99.9-101.5°F) |
7.7 |
6.9 |
3.3 |
|
38.7-39.3 °C (101.6-102.7°F) |
2.0 |
2.0 |
0.7 |
|
39.4-40.5 °C (102.8-104.9°F) |
1.0 |
0.0 |
0.7 |
|
>40.5°C (>104.9°F) |
0.0 |
0.0 |
0.0 |
§NCT01041573
*IXIARO dose 0.5 mL.
‡N=range of subjects with available diary card data after each dose, used as denominators to calculate percentages.
Table 5. Rates of Solicited Adverse Reactions on Days 0-7 After Each Vaccination in Children 12 Years to <18 Years of Age, by Dose and Treatment Group, Study 1§, Philippines
|
Post Dose 1 (% of subjects) |
Post Dose 2 (% of subjects) |
||
|
Injection Site Reactions |
IXIARO* (N=240‡) |
Havrix (N=80‡) |
IXIARO* (N=238‡) |
|
Pain |
15.0 |
12.5 |
6.7 |
|
Itching |
0.8 |
0.0 |
0.4 |
|
Tenderness |
10.0 |
13.8 |
4.6 |
|
Hardening |
1.3 |
0.0 |
0.4 |
|
Swelling |
0.4 |
1.3 |
0.8 |
|
Redness |
0.8 |
6.3 |
3.8 |
|
Solicited Systemic Reaction | |||
|
Irritability |
2.1 |
1.3 |
0.0 |
|
Nausea |
2.1 |
1.3 |
0.0 |
|
Vomiting |
1.3 |
0.0 |
0.0 |
|
Diarrhea |
0.4 |
2.5 |
0.0 |
|
Flu-like symptoms |
3.3 |
7.5 |
1.3 |
|
Excessive fatigue |
2.5 |
1.3 |
0.4 |
|
Muscle pain |
2.9 |
5.0 |
1.3 |
|
Rash |
0.8 |
1.3 |
0.0 |
|
Headache |
4.6 |
5.0 |
3.4 |
|
Loss of appetite |
2.1 |
2.5 |
0.4 |
|
Fever ≥37.7°C (≥99.9°F) |
3.8 |
6.3 |
5.0 |
|
37.7-38.6 °C (99.9-101.5°F) |
3.3 |
3.8 |
3.8 |
|
38.7-39.3 °C (101.6-102.7°F) |
0.4 |
1.3 |
1.3 |
|
39.4-40.5 °C (102.8-104.9°F) |
0.0 |
1.3 |
0.0 |
|
>40.5°C (>104.9°F) |
0.0 |
0.0 |
0.0 |
§NCT01041573
*IXIARO dose 0.5 mL.
‡N=number of subjects with available diary card data after each dose, used as the denominator to calculate percentages.
Serious Adverse Events
There was one death due to disseminated intravascular coagulation following suspected bacterial meningitis in a 12 year old male 4 months after the second dose of IXIARO. Forty serious adverse events (SAEs) were reported during the 7 month study period. Twenty-three subjects (1.6%) who received IXIARO, 1 subject (1.6%) who received Prevnar and 10 subjects (2.5%) who received HAVRIX experienced an SAE. Some subjects experienced more than one SAE.
The SAEs occurring most frequently in all study groups were febrile convulsions. A total of 12 febrile convulsions were reported (9 of them as SAEs), in 8 subjects (1.0% of children below the age of 3 years) receiving IXIARO, 3 subjects (1.4% of children below the age of 3 years) receiving HAVRIX and 1 subject (1.6%) receiving Prevnar. All febrile convulsions occurred in children below the age of 3 years. Onset of febrile convulsions ranged from 2 days to >5 months after doses of IXIARO with no apparent temporal clustering, 4 weeks after Prevnar and 9 days to >16 weeks after HAVRIX.
Adverse Events in a Pediatric Trial2 of IXIARO in Children Traveling from Western Countries:
The safety of IXIARO was evaluated in an ongoing, uncontrolled, open-label clinical trial conducted in the United States, Europe and Australiain healthy children with planned travel to JEV-endemic areas (Study 2)2. IXIARO (0.25 mL dose for children 2 months to <3 years of age, 0.5 mL dose for children and adolescents 3 to <18 years of age) was administered by intramuscular injection on Day 0 and Day 28. An analysis of safety was carried out after enrolment of 60 subjects (mean age: 12.50 years, range 10 months to 17 years; 56.7% female; ethnicity: White: 83.3%, Asian: 13.3%, Black: 3.3%). Parents or subjects recorded adverse events on a diary card for the first seven days after each vaccination. Only those events considered to be assessable based on the subjects developmental status were recorded. Parents or subjects were queried about the occurrence of unsolicited AEs through 6 months after the last vaccination (Month 7).
At the time of the analysis, 40% (2/5) subjects 2 months to <3 years of age experienced injection site hardening, injection site redness, and diarrhea following the first or second dose of IXIARO. Solicited adverse reactions among subjects 3 to <18 years of age are summarized in Table 6.
Table 6. Rates of Solicited Adverse Reactions on Days 0-7 After Each IXIARO 0.5 mL Vaccination in Children 3 Years to <18 Years of Age Traveling From Western Countries, Study 2§
|
Post Dose 1 (N=55) % of subjects |
Post Dose 2 (N=49) % of subjects |
|
|
Injection Site Reactions | ||
|
Pain |
18.2 |
16.3 |
|
Itching |
3.6 |
2.0 |
|
Tenderness |
30.9 |
24.5 |
|
Hardening |
0.0 |
2.0 |
|
Swelling |
0.0 |
0.0 |
|
Redness |
5.5 |
0.0 |
|
Solicited Systemic Reaction | ||
|
Irritability |
0.0 |
6.1 |
|
Nausea |
1.8 |
2.0 |
|
Vomiting |
0.0 |
2.0 |
|
Diarrhea |
1.8 |
0.0 |
|
Flu-like symptoms |
0.0 |
0.0 |
|
Excessive fatigue |
12.7 |
0.0 |
|
Muscle pain |
27.3 |
2.0 |
|
Rash |
1.8 |
2.0 |
|
Headache |
1.8 |
4.1 |
|
Loss of appetite |
1.8 |
0.0 |
|
Fever 37.7°C (99.9°F) |
5.5 |
2.0 |
|
37.7-38.6 °C (99.9-101.5°F) |
3.6 |
2.0 |
|
38.7-39.3 °C (101.6-102.7°F) |
1.8 |
0.0 |
|
39.4-40.5 °C (102.8-104.9°F) |
0.0 |
0.0 |
|
>40.5°C (>104.9°F) |
0.0 |
0.0 |
§NCT01047839
‡N=number of subjects with available diary card data after each dose, used as the denominator to calculate percentages.
Clinical Studies in Adults 18 Years of Age and Older:
In five randomized, controlled clinical studies3, 4, 5, 6, 7 conducted in North America, Europe, Australia and New Zealand, a total of 3,558 healthy adults 18 to 86 years of age received at least one dose of IXIARO and were followed-up for safety for 6 months after the first dose. In this pooled dataset of subjects who received IXIARO, one death occurred in a subject with metastatic lung adenocarcinoma four months after completing the two-dose regimen. About 1% of subjects who received IXIARO experienced a serious adverse event, including one case of multiple sclerosis. Approximately 1% of subjects who received IXIARO discontinued due to adverse events.
Adverse Events in a Clinical Trial Comparing IXIARO to a Control in Adults:
The safety of IXIARO was evaluated in a randomized, controlled, double‑blind clinical trial in healthy male and female subjects 18 years of age (Study 3)3. IXIARO was compared to a control: Phosphate Buffered Saline containing 0.1% aluminum hydroxide [PBS + Al(OH)3]. A total of 2,675 subjects were randomized in a 3:1 ratio to receive either an intramuscular injection of IXIARO (0.5 mL) each on Day 0 and Day 28, or an intramuscular injection of PBS + Al(OH)3 (0.5 mL) each on Day 0 and Day 28. Analysis of safety was carried out using the safety population including 1,993 subjects receiving at least one dose of IXIARO and 657 subjects receiving at least one dose of PBS + Al(OH)3 (mean age: 33.8 years, range 18 to 86 years; 55.3% female; ethnicity: White: 91.7%, Asian: 1.8%, Black: 3.4%, Other: 3.0%). The IXIARO and control groups were similar with regard to demographics. Subjects recorded adverse events on a diary card for the first seven days after each vaccination. In addition, the study investigator took a medical history and performed a physical exam to evaluate for adverse events on the day of each vaccination and at a visit 4 weeks after the second vaccination.
Serious Adverse Events
No deaths occurred during this trial. Sixteen serious adverse events (SAE) were reported during the study period. Ten subjects (0.5%) who received IXIARO and 6 subjects (0.9%) who received PBS + Al(OH)3 experienced a SAE. The serious adverse events occurring in the IXIARO group were as follows: Dermatomyositis, appendicitis, rectal hemorrhage, limb abscess (contralateral to the injected arm), chest pain, ovarian torsion, ruptured corpus luteal cyst, and three orthopedic injuries.
Systemic Adverse Events
Overall, the percentage of subjects who experienced at least one adverse event during the study period was 58.9% in the IXIARO group compared to 56.6% in the PBS + Al(OH)3 group. Adverse events of any severity grade occurring with an incidence of 1% of subjects are shown in Table 7. Most adverse events (>90%) were mild to moderate.
Table 7. Rates of Common Solicited and Unsolicited Systemic Adverse Events* in Adults Residing in Non-Endemic Areas After IXIARO or Control [PBS + Al(OH)3], Safety Population, Study 3§
|
Post Dose 1 % of subjects |
Post Dose 2 % of subjects |
Post Dose 1 or Dose 2 % of subjects |
||||
|
Adverse Event |
IXIARO |
PBS + Al(OH)3 N‡=657 |
IXIARO |
PBS + Al(OH)3 N‡=645 |
IXIARO |
PBS + Al(OH)3 N‡=657 |
|
Headache† |
21.6 |
20.2 |
13.4 |
13.0 |
27.9 |
26.2 |
|
Myalgia† |
13.3 |
12.9 |
5.6 |
5.3 |
15.6 |
15.5 |
|
Fatigue† |
8.6 |
8.7 |
5.2 |
5.9 |
11.3 |
11.7 |
|
Influenza-like Illness† |
8.2 |
8.5 |
5.8 |
4.3 |
12.3 |
11.7 |
|
Nausea† |
4.7 |
5.3 |
2.6 |
3.7 |
6.6 |
7.5 |
|
Nasopharyngitis |
2.3 |
1.8 |
2.6 |
2.3 |
4.7 |
4.0 |
|
Fever† |
1.9 |
2.1 |
1.5 |
1.7 |
3.2 |
3.0 |
|
Rhinitis |
1.0 |
0.8 |
0.5 |
0.6 |
1.4 |
1.4 |
|
Upper Respiratory Tract Infection |
0.9 |
0.9 |
0.8 |
0.9 |
1.7 |
2.0 |
|
Back Pain |
0.8 |
0.9 |
0.6 |
0.2 |
1.3 |
1.1 |
|
Pharyngolaryngeal Pain |
0.8 |
0.9 |
1.0 |
0.5 |
1.6 |
1.4 |
|
Rash† |
0.8 |
0.9 |
0.7 |
0.8 |
1.3 |
1.5 |
|
Diarrhea |
0.8 |
0.8 |
0.7 |
0.3 |
1.5 |
1.1 |
|
Cough |
0.8 |
0.8 |
0.6 |
0.6 |
1.2 |
1.2 |
|
Vomiting† |
0.6 |
0.8 |
0.8 |
0.9 |
1.4 |
1.7 |
§NCT00605085
*The adverse events in this table are those observed at an incidence of ≥1% in the IXIARO or PBS + Al(OH)3 groups.
† These symptoms were solicited in a subject diary card. Percentages also include unsolicited events that occurred after the 7 day period covered by the diary card.
‡N=number of subjects in the safety population (subjects treated with at least one dose) who received the respective dose
Injection Site Reactions
Injection site reactions after IXIARO were compared to reactions after PBS + Al(OH)3. Symptoms were recorded into a subject diary for the first seven days after each injection, and the injection site was assessed by the investigator at each visit. The rates of injection site reactions are shown in Table 8. Most injection site reactions (>90%) were mild to moderate.
Table 8. Rates of Injection Site Solicited Adverse Reactions* After IXIARO or Control [PBS + Al(OH)3], Adults Residing in Non-Endemic Areas, Safety Population With Evaluable Diary Cards, Study 3§
|
Post Dose 1 (% of subjects†) |
Post Dose 2 (% of subjects†) |
Post Dose 1 or Dose 2 (% of subjects†) |
||||
|
Adverse Reaction |
IXIARO |
PBS + Al(OH)3
|
IXIARO |
PBS + Al(OH)3
|
IXIARO |
PBS + Al(OH)3
|
|
Any Reaction |
48.5 |
47.7 |
32.6 |
32.2 |
55.4 |
56.2 |
|
Pain |
27.7 |
28.2 |
17.7 |
18.2 |
33.0 |
35.8 |
|
Tenderness |
28.8 |
26.9 |
22.5 |
18.1 |
35.9 |
32.6 |
|
Erythema |
6.8 |
5.4 |
4.6 |
4.1 |
9.6 |
7.4 |
|
Induration |
4.8 |
5.3 |
4.0 |
3.0 |
7.5 |
7.4 |
|
Edema |
2.4 |
3.3 |
2.3 |
1.6 |
4.2 |
4.6 |
|
Pruritus |
2.6 |
3.3 |
1.6 |
1.9 |
3.8 |
4.5 |
§NCT00605085
* Injection site reactions were assessed for 7 days after each dose.
† Denominators used to calculate percentages are based on the number of evaluable diary card entries (defined as documented presence on any day [i.e., entry of “yes”] or absence on all days [i.e., entry of “no”]) for each individual symptom and observation period.
‡N=number of subjects who returned diary cards after each dose
Adverse Events in a Clinical Trial Comparing IXIARO to JE-VAX in Adults:
The safety of IXIARO compared to another U.S.-licensed inactivated JE vaccine (JE-VAX) was evaluated in a randomized, double-blind clinical trial in subjects 18 years of age (Study 4)4.
No deaths occurred during this trial. One serious adverse event occurred in this trial in a subject with a history of myocardial infarction (MI) who experienced a MI three weeks after receiving the 2nd dose of IXIARO. The most common adverse events after immunization occurring in 1% of subjects were headache, myalgia, fatigue, influenza-like illness, nausea, nasopharyngitis, fever, pharyngolaryngeal pain, cough, rash, diarrhea, sinusitis, upper respiratory tract infection, back pain, migraine, vomiting and influenza, which occurred with similar frequency in both treatment groups. Local injection site reactions solicited in diary cards for 7 days after each vaccination were observed at a rate of 54% in the IXIARO group (N=428) compared to a rate of 69.1% in the JE-VAX group (N=435).
Adverse Events in a Clinical Trial Investigating a Booster Dose of IXIARO in Adults:
The safety of a booster dose of IXIARO administered 14 months after completion of the primary series was evaluated in an open-label, uncontrolled study in subjects 18 years of age (Study 8)8.
Within 28 days of booster vaccination, adverse events were reported by 35.4% of subjects (N=198). Within 12 months of booster vaccination, subjects who experienced at least one adverse event were 56.1%. Injection site reactions were reported in the subject diary for 30.8% of subjects within 7 days of booster vaccination. Adverse events considered by the investigators to be treatment-related were recorded for 11.6% of subjects (these related events were all observed within one month after the booster dose administration).
The most common injection site reactions (>10% of subjects) were pain (12.8%) and tenderness (19.2%); the most common systemic adverse events (>10%) were nasopharyngitis (15.2%) and headache (11.1%).
Safety in Concomitant Use with the Hepatitis A Vaccine, HAVRIX in Adults (Study 6)6
The safety of IXIARO when administered concomitantly with inactivated Hepatitis A Virus vaccine (HAVRIX) was evaluated in a controlled trial in which subjects 18 years of age were assigned randomly to one of three treatment groups: Group A (N=62) received IXIARO + HAVRIX; Group B (N=65) received IXIARO + control [PBS + Al(OH)3]; Group C (N=65) received HAVRIX + control [PBS + Al(OH)3]. One serious adverse event occurred in this trial in a subject with a history of alcoholism and seizure disorder who experienced a seizure three weeks after receiving the 2nd dose of IXIARO + control.
The percentage of subjects who experienced at least one adverse event was as follows: Group A: 38.7%; Group B: 41.5%; Group C: 47.7%. The most frequently reported injection site reaction on the day of the first vaccination in all three groups was injection site pain in 59.0% of subjects in Group A, in 48.4% of subjects in Group B and in 48.4% of subjects in Group C.
6.2 Post-Marketing Experience
The following additional adverse reactions have been identified during post approval use of IXIARO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to the vaccine.
Nervous system disorders: Paraesthesia, Neuritis.
7. Drug Interactions
7.1 Use with HAVRIX
In one clinical trial6 in adults, IXIARO was administered concomitantly with HAVRIX (Hepatitis A Vaccine) [See Adverse Reactions (6) and Clinical Studies (14)]. In this trial, there was no evidence for interference with the immune response to IXIARO or to HAVRIX when HAVRIX was administered concomitantly with dose 1 of IXIARO [See Clinical Studies (14)]. Data are not available on concomitant administration of IXIARO with other US-licensed vaccines.
When IXIARO is administered concomitantly with injectable vaccines, they should be given with separate syringes at different injection sites. IXIARO should not be mixed with any other vaccine in the same syringe or vial.
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy category B. Reproduction studies have been performed in female rats at doses approximately 300-fold excess relative to the projected human dose (on a mg/kg basis) and have revealed no evidence of impaired fertility or harm to the fetus due to IXIARO. There are, however, no adequate and well‑controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, IXIARO should be used during pregnancy only if clearly needed.
The effect of IXIARO vaccine on embryo-fetal and pre-weaning development was evaluated in a developmental toxicity study using pregnant rats. One group of rats was administered IXIARO twice prior to gestation and once during the period of organogenesis (gestation Day 6). A second group of pregnant rats was administered IXIARO once prior to gestation and once during the period of organogenesis (gestation Day 6). IXIARO was administered at 0.5 mL/rat/occasion (approximately 300-fold excess relative to the projected human dose on a mg/kg basis), by intramuscular injection. No adverse effects on mating, fertility, pregnancy, parturition, lactation, embryo-fetal or pre-weaning development were observed. There was a statistically significant finding of incomplete ossification in a few fetuses derived from the second group of pregnant rats. However, there are no data to suggest that this finding is vaccine related. There were no vaccine-related fetal malformations or other evidence of teratogenesis noted in this study.
Healthcare practitioners are encouraged to report inadvertent use in pregnant women to the distributor, Intercell USA Inc., at 1-844-349-4276 (8443-IXIARO).
8.3 Nursing Mothers
It is not known whether this vaccine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if IXIARO is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of IXIARO have not been established in infants younger than 2 months of age [See Adverse Reactions (6)and Clinical Studies (14)].
8.5 Geriatric Use
Clinical studies of IXIARO did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger subjects. In a study that included 24 subjects ≥65 years of age who received IXIARO per protocol (Study 6)6, the proportion of these subjects achieving a JEV neutralizing antibody titer ≥1:10 at 28 days after the second dose of IXIARO was 95.8% (geometric mean titer: 255.2).
In subjects 65 years of age and older who had been vaccinated in any of five trials3 ,4 ,5 ,6 ,7 included in a pooled dataset (N=161), adverse events were reported in 61.9% (73/118) of subjects in the IXIARO group, 57.7% (15/26) in the JE-VAX group, and 70.6% (12/17) in the control [PBS + Al(OH)3] group. Serious adverse events (SAE) were experienced by four subjects (3.4%) who received IXIARO, no subjects who received JE-VAX, and one subject (5.9%) who received the control [PBS + Al(OH)3]. The SAEs occurring in the IXIARO group were as follows: one case each of rectal hemorrhage, pancreatic adenocarcinoma, breast cancer, and one death in a subject with metastatic lung adenocarcinoma, which occurred four months after the subject completed the two-dose regimen.
11. Ixiaro Description
11 DESCRIPTION
IXIARO, Japanese Encephalitis Vaccine, Inactivated, Adsorbed is a sterile suspension for intramuscular injection. Each 0.5 mL dose of vaccine contains 6 antigen units of purified, inactivated JEV and approximately 250 mcg of aluminum hydroxide. The appearance of the liquid is a white, opaque, non-uniform suspension which becomes homogeneous upon shaking.
IXIARO is a vaccine prepared by propagating JEV strain SA14-14-2 in Vero cells. Multiple viral harvests are pooled, clarified and concentrated. The virus suspension is treated with protamine sulfate to remove contaminating DNA and proteins. The resulting partially purified virus is processed through a sucrose density gradient centrifugation step and fractionated. Each fraction is analyzed for the presence of virus, and fractions with the highest virus activity are pooled to give a purified virus suspension. The purified virus is then inactivated by treatment with formaldehyde. The preparation is adjusted to a specified antigen content and formulated by addition of aluminum hydroxide.
The formulated bulk vaccine is filled into syringes, at a volume of 0.5 mL per syringe. From the manufacturing process, IXIARO also contains: formaldehyde (not more than 200 ppm), bovine serum albumin (not more than 100 ng/mL), host cell DNA (not more than 200 pg/mL), sodium metabisulphite (not more than 200 ppm), host cell protein (not more than 100 ng/mL), and protamine sulfate (not more than 1µg/mL). No preservatives, stabilizers, or antibiotics are added to the formulation.
12. Ixiaro - Clinical Pharmacology
12.1 Mechanism of Action
Japanese encephalitis is a disease caused by the mosquito-borne Japanese encephalitis virus (JEV). IXIARO acts by inducing antibodies that neutralize live JEV. Accumulated data from animal studies, clinical trials of other JE vaccines, and human epidemiological studies, suggest that a virus neutralizing antibody response, as measured in vitro in a 50% plaque-reduction neutralization antibody test (PRNT50) with a threshold titer of ≥1:10, provides evidence of protective immunity10, 11. The evaluation of vaccine effectiveness of IXIARO was therefore based on neutralizing antibody response using a threshold PRNT50 titer of ≥1:10.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
IXIARO has not been evaluated for carcinogenic or mutagenic potential. IXIARO was found to have no effect on fertility of female rats at intramuscular doses of up to 300‑fold excess relative to the projected human dose (on a mg/kg basis) administered prior to and after mating [See Use in Specific Populations (8.1)]. The effect of IXIARO on male fertility has not been evaluated.
14. Clinical Studies
Immunogenicity of IXIARO in a Pediatric Clinical Trial in the Philippines
The immunogenicity of IXIARO was evaluated in a subgroup of children included in a randomized, controlled, open-label clinical trial in healthy children conducted in the Philippines (Study 1)1in which the safety of IXIARO was compared to control vaccines: HAVRIX (Hepatitis A vaccine, pediatric 720 EL.U./0.5 mL formulation) and Prevnar (Pneumococcal 7-valent Conjugate Vaccine [Diphtheria CRM197 protein]). Subjects in the IXIARO treatment arm received intramuscular doses on Day 0 and Day 28. A total of 396 subjects from the group receiving an age-appropriate dosage of IXIARO (0.25 mL for infants and children 2 months to <3 years of age or 0.5 mL for individuals 3 to <18 years of age) were randomized in an age-stratified scheme into the immunogenicity subgroup (mean age: 7.7 years; 49.6% female; ethnicity: 100% Asian). [ See Adverse Reactions (6.1)]
The immunogenicity evaluation included the proportion of subjects with PRNT50 titer ≥1:10 and geometric mean titer (GMT) at Day 56 and Month 7. The JEV-neutralizing antibody responses elicited by IXIARO in the Intent-to-Treat population (defined as all subjects who received at least one dose of IXIARO) are presented in Table 9.
Table 9.JEV-Neutralizing Antibody Response After IXIARO* Among Children 2 Months to <18 Years of Age Residing in the Philippines, Intent-To-Treat Population**, Study 1§
|
Age Group |
2 months – |
6 months – |
1 year – |
3 years - |
12 years - |
|
Time Point |
Proportion of Subjects with PRNT50 Titer ≥1:10 (n/N) [95% CI] |
||||
|
Pre-Vaccination Screen |
30% (3/10) [10.8, 60.3] |
0% (0/20) [0.0, 16.1] |
3.2% (4/125) [1.3, 7.9] |
16.8% (17/101) [10.8, 25.3] |
45.7% (64/140) [37.7, 54.0] |
|
Day 56 (28 days after vaccine dose 2) |
100%
[70.1, 100.0] |
100% (19/19) [83.2, 100.0] |
99.2% (119/120) [95.4, 99.9] |
100.0% (100/100) [96.3, 100.0] |
100% (137/137) [97.3, 100.0] |
|
Month 7 (6 months after vaccine dose 2) |
100% (10/10) [72.2, 100.0] |
100% (18/18) [82.4, 100.0] |
85.5% (106/124) [78.2, 90.6] |
91.0% (91/100) [83.8, 95.2] |
97.1% (133/137) [92.7, 98.9] |
|
Time Point |
Geometric Mean Titers◊ (N) [95% CI] |
||||
|
Pre-Vaccination Screen |
8.4 (10) [4.3, 16.7] |
5.0 (20) [5.0, 5.0] |
5.5 (124) [ 5.0, 6.1] |
6.5 (101) [ 5.8, 7.4] |
13.1 (140) [ 10.7, 16.1] |
|
Day 56 (28 days after vaccine dose 2) |
687.4 (9) |
377.8 (19) |
258.9 (121) [214.4, 312.6] |
213.7 (100) [175.6, 260.0] |
175.6 (137) [147.8, 208.7] |
|
Month 7 (6 months after vaccine dose 2) |
159.3 (10) |
64.0 (18) |
38.9 (125) [31.8, 47.7] |
43.6 (100) [35.6, 53.4] |
86.6 (137) |
§01041573
*Infants and children ≥2 months to <3 years of age received two 0.25 mL doses administered on Days 0 and 28. Individuals 3 years of age and older received two 0.5 mL doses administered on Days 0 and 28.
**The Intent to Treat population consisted of all subjects who received at least one dose of IXIARO.
N=number of subjects with data available
n=number of subjects with a PRNT50 titer ≥1:10
◊Reciprocal titers <10 were imputed to 5.
Immunogenicity of IXIARO in a Pediatric Clinical Study in Children 2 Months to <18 Years of Age Traveling from Non-Endemic Countries
The immunogenicity of IXIARO was evaluated in an uncontrolled, open-label clinical study conducted in the United States, Europe and Australia in healthy male and female children with planned travel to JEV-endemic areas (Study 2)2. IXIARO (0.25 mL dose for children 2 months to <3 years of age, 0.5 mL dose for children and adolescents 3 to <18 years of age) was administered by intramuscular injection on Day 0 and Day 28. An analysis was carried out on immunogenicity data for the first 54 subjects enrolled (median age: 15.2 years, range 10 months to 17 years; 59.3% female; ethnicity: White: 81.5%, Asian: 14.8%, Black: 3.7%). [ See Adverse Reactions (6.1)]
JEV neutralizing antibody titers were available for 51 subjects at Day 56 (5 subjects 2 months to <3 years of age and 46 subjects 3 to <18 years of age) and for 18 subjects at Month 7 (2 subjects 2 months to <3 years of age and 16 subjects 3 to <18 years of age). All subjects had PRNT50 titers ≥1:10 at Day 56 and Month 7, and GMTs were similar to those among children vaccinated with IXIARO in a study conducted in the Philippines, where JEV is endemic (see Table 9).
Immunogenicity of IXIARO in a Clinical Trial in Adults
Immunogenicity of the vaccine was evaluated in a randomized, active‑controlled, observer‑blinded clinical trial conducted in the U.S., Germany and Austria (Study 4)4 in 867 healthy adults 18 years of age and older (mean age: 41.3 years; 60.8% female; ethnicity: White 80.8%, Asian 0.8%, Black 13.1%, Other 5.3%). Subjects in the IXIARO treatment arm received the following schedule of three intramuscular doses: Day 0, IXIARO, Day 7, PBS + Al(OH)3 (0.5 mL phosphate buffered saline with 0.1% aluminum hydroxide), and on Day 28, IXIARO. Subjects in the comparator arm received a subcutaneous dose of 1.0 mL of the US‑licensed JEV vaccine, JE-VAX, on Days 0, 7 and 28. [ See Adverse Reactions (6.1)]
The proportion of subjects with, PRNT50 titer ≥1:10, and GMT were evaluated at Day 56 in the per protocol population which included all subjects who had no major protocol deviations and who had a PRNT50 titer <1:10 at baseline. The neutralizing antibody responses elicited by IXIARO met predefined statistical criteria for non-inferiority compared to those induced by JE‑VAX, and are presented in Table 10. All subjects were seronegative at baseline (PRNT50 titer <1:10).
Table 10. JEV-Neutralizing Antibody Response After IXIARO or JE‑VAX Among Adults Residing in Non-Endemic Areas, Per Protocol Population*, Study 4§
|
Proportion of Subjects with PRNT50 Titer ≥1:10 |
|||
|
Time Point |
IXIARO(n/N) [95% CI] |
JE‑VAX (n/N) [95% CI] |
Rate difference [95% CI] |
|
Day 56 (28 days after vaccine dose 2) |
96.4% (352/365) [94.0, 97.9] |
93.8% (347/370) [90.9, 95.8] |
2.6% [-0.5,6.0]† |
|
Geometric Mean Titers◊ |
|||
|
Time Point |
IXIARO (N**=361) [95% CI] |
JE‑VAX (N**=364) [95% CI] |
GMT ratio [95% CI] |
|
Day 56 (28 days after vaccine dose 2) |
243.6 [ 216.4, 274.1] |
102.0 [90.3, 115.2 ] |
2.33 [1.97, 2.75]‡ |
§00604708
*The Per Protocol population consisted of subjects with no major protocol deviations and a PRNT50 titer <1:10 at baseline
† Non-inferiority was demonstrated if the lower bound of the 2-sided 95% confidence interval (CI) for the difference in proportion of subjects with PRNT50 titer ≥1:10 (IXIARO minus JE‑VAX) was >‑10% at Day 56.
‡ Non-inferiority was demonstrated if the lower bound of the 2-sided 95% CI for the GMT ratio (IXIARO /JE‑VAX) was >1/1.5 at Day 56.
n=number of subjects with a PRNT50 titer ≥1:10
N=number of subjects in the Per Protocol Population
**N=Number of subjects with immunogenicity data
◊Reciprocal titers <10 were imputed to 5.
Temporal Evaluation of Immunogenicity of IXIARO During Vaccination Series in Adults
In a randomized, observer-blinded clinical study in 374 healthy adults 18 years of age and older (Study 5)5, the immunogenicity of IXIARO was evaluated on days 10, 28, 35, and 56 during the vaccination period. The proportion of subjects with PRNT50 titer ≥1:10 at each time point for the subjects randomized to the standard dosing regimen (IXIARO on days 0 and 28) are displayed in Table 11.
Table 11. JEV-Neutralizing Antibody Response During the Vaccination Series (IXIARO on Days 0 and 28) Among Adults Residing in Non-Endemic Areas, Per Protocol Population*, Study 5§
|
Time Point |
Proportion of Subjects with PRNT50 Titer ≥1:10 (n/N) [95% CI] |
|
Day 10 |
21.1% (24/114) [13.6%; 28.5%] |
|
Day 28 (28 days after vaccine dose 1) |
39.8% (45/113) [30.8%; 48.8%] |
|
Day 35 |
97.3% (110/113) [94.4%; 100.0%] |
|
Day 56 (28 days after vaccine dose 2) |
97.3% (110/113) [94.4%, 100%] |
§00505790
*The Per Protocol population consisted of all subjects with no major protocol deviations
n=number of subjects with a PRNT50 titer ≥1:10
N=number of subjects with immunogenicity data
Persistence of Neutralizing Antibody Response in Adults
The persistence of JEV-neutralizing antibody was evaluated in a subgroup of adult subjects recruited for follow-up after participation in one of two clinical trials (Study 4 and Study 5)4, 5. In the Intent-to-Treat population of subjects randomized to vaccination with IXIARO (N=181) and in the population of subjects who intended to stay in the study until month 36 (ITT36, N = 152), the proportion of subjects with PRNT50 titer ≥1:10 at 6, 12, 24 and 36 months after initiation of the two-dose series were 95% [95% CI 90.8, 97.4], 83.4% [95% CI 77.3, 88.1] , 81.8% [95% CI 75.5, 86.7] and 84.9% [95% CI 78.3, 89.7], respectively. Geometric mean titers (GMT) at 6, 12, 24 and 36 months after initiation of the two-dose series were 83.5 [95% CI 70.9, 98.4], 41.2 [95% CI 34.4, 49.3], 44.3; [95% CI 36.7, 53.4] and 43.8 [95% CI 36.5, 52.6], respectively.
In another study with 116 adults who completed the IXIARO primary immunization series (Study 9)9, the proportions of subjects with PRNT50 titers ≥1:10 at 11 and 23 months after completion of the primary series were 58.3% [95% CI 49.1, 66.9] and 48.3% [95% CI 39.4, 57.3], respectively, and GMTs were 18.0 [95% CI 14.3, 22.6] and 16.2 [95% CI 12.6, 20.8].
Immunogenicity of IXIARO Booster Doses in Adults
A single booster dose of IXIARO was evaluated in 198 adult subjects enrolled in an uncontrolled, open-label phase 3 study. IXIARO was administered 14 months after completion of the primary series (Study 8)8. The JEV neutralizing antibody responses from this study are presented in Table 12.
Table 12.JEV-Neutralizing Antibody ResponseFollowing a Booster Dose of IXIARO Administered 14 Months After Completion of the Primary Series Among Adults Residing in Non-Endemic Areas, Intent to Treat Population*, Study 8§
|
Time Point |
% PRNT Titer ≥1:10 (n/N) [95% CI] |
Geometric Mean Titers (N) [95% CI] |
|
Pre-booster, Day 0 |
69.2% (137/198) |
22.5 (198) |
|
Day 28 |
100.0% (198/198) |
900.1 (198) |
|
Month 6 |
98.5% (194/197) |
487.4 (197) |
|
Month 12 |
98.5% (191/194) |
361.4 (194) |
§00595309
*The Intent to Treat population consisted of all subjects who received the booster vaccination
n=number of subjects with a PRNT50 titer ≥1:10
N=number of subjects with immunogenicity data
The immunogenicity of booster doses was assessed in adult subjectsin another study investigating persistence of immunity following vaccination with the primary series of IXIARO (Study 5)5. Subjects with PRNT50 titers <1:10 at 11 months after completion of the primary series received a booster dose of IXIARO 11 months later (22 months after completion of the primary series). Among 27 subjects with available immunogenicity data at 4 weeks after the booster dose, the proportion of subjects with PRNT50 titer ≥1:10 was 100% [95% CI 87.5, 100.0], and the GMT was 2536.7 [95% CI 1467.7, 4384.4].
Concomitant Administration of IXIARO and Hepatitis A Vaccine, HAVRIX in Adults
The concomitant use of IXIARO with inactivated Hepatitis A Virus vaccine (HAVRIX) was evaluated in a randomized, controlled, single‑blind clinical trial including 192 healthy adults 18 to 61 years of age (Study 7)7. Subjects were divided into three treatment groups: Group A (N=62) received IXIARO (Day 0 and 28) + HAVRIX (Day 0); Group B (N=65) received IXIARO (Day 0 and 28) + control (0.5 mL phosphate buffered saline with 0.1% aluminum hydroxide by intramuscular injection on Day 0); Group C (N=65) received HAVRIX (Day 0) + control (Day 0 and 28). Anti-JEV GMT at Day 56 in Group A met non-inferiority criteria compared to anti-JEV GMT at Day 56 in Group B. In addition, anti-HAV GMT at Day 28 in Group A met non-inferiority criteria compared to anti-HAV GMT at Day 28 in Group C. Therefore, concomitant administration of IXIARO and HAVRIX did not adversely affect immunogenicity compared to administration of either vaccine individually. Safety results regarding co-administration of IXIARO with HAVRIX are summarized in Adverse Reactions (6.1).
Delayed Completion of Primary Immunization in Adults
The immunogenicity of a second dose of the primary series administered 11 months after dose 1 was assessed in 100 adults in a study investigating persistence of immunity following vaccination with different dose-schedules of IXIARO (Study 5)5. Four weeks after this delayed second dose, 99.0% of subjects (99/100) had a PRNT50 titer ≥1:10 (GMT 504.3 [95% CI: 367.3, 692.3]). One year later, 88.5% of subjects (85/96) had a PRNT50 titer ≥1:10 (GMT 121.0 [95% CI: 87.4, 167.6]).
15. References
- Clinical study referred to as NCT01041573 in the National Library of Medicine clinical trial database, also referred to as study IC51-323 in the Biologics License Application
- Clinical study referred to as NCT01047839 in the National Library of Medicine clinical trial database, also referred to as study IC51-322 in the Biologics License Application
- Clinical study referred to as NCT00605085 in the National Library of Medicine clinical trial database, also referred to as study IC51-302 in the Biologics License Application
- Clinical study referred to as NCT00604708 in the National Library of Medicine clinical trial database, also referred to as study IC51-301 in the Biologics License Application.
- Clinical study referred to as NCT00595790 in the National Library of Medicine clinical trial database, also referred to as study IC51-304 in the Biologics License Application.
- Clinical study referred to as NCT00596271 in the National Library of Medicine clinical trial database, also referred to as study IC51-308 in the Biologics License Application.
- Clinical study referred to as NCT00594958 in the National Library of Medicine clinical trial database, also referred to as study IC51-309 in the Biologics License Application.
- Clinical study referred to as NCT00595309 in the National Library of Medicine clinical trial database, also referred to as study IC51-311 in the Biologics License Application.
- Clinical study referred to as NCT00595270 in the National Library of Medicine clinical trial database, also referred to as study IC51-305 in the Biologics License Application.
- Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, Kotchasenee S, Gingrich JB, Latendresse J, Fukai K, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988 Sep 8;319(10):608-14.
- Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2-3 September, 2004. Vaccine. 2005;23:5205-11.
16. How is Ixiaro supplied
16.1 How Supplied
IXIARO is supplied as a sterile 0.5 mL suspension in a pre filled syringe (Type I glass) with a plunger stopper (chlorobutyl elastomer) in a pack size of 1 single-dose syringe with or without a separate needle. See Section 2.3 for information on preparing a 0.25 mL dose for children 2 months to <3 years of age and a 0.5 mL dose for individuals 3 years of age and older. NDC 42515-002-01.
Neither the syringe nor the packaging materials are made with natural rubber latex.
17 PATIENT COUNSELING INFORMATION
Question the vaccine recipient about reactions to previous vaccines, and inform the vaccine recipient of the benefits and risks of IXIARO.
Consult current U.S. and international advisories regarding prevalence of Japanese encephalitis in specific locations. Advise the parent, guardian, or recipient that IXIARO may not fully protect everyone who gets the vaccine and that personal precautions should be taken to reduce exposure to mosquito bites (adequate clothing, use of repellents, mosquito nets).
Give the parent, guardian, or recipient the Vaccine Information Statements, which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization, and inquire about previous reactions to vaccines.
Inform the parent, guardian, or recipient of the importance of completing the immunization series.
Instruct the parent, guardian, or recipient to immediately report any signs and/or symptoms of a severe adverse reaction, including anaphylaxis (difficulty breathing, wheezing, weakness or fast heart beat, hives).
Patient Information about
IXIARO (pronounced “ĭk-sē-ah-rō”)
Generic name: Japanese encephalitis vaccine, inactivated, adsorbed
Read this information about IXIARO before you are vaccinated. If you have any questions about IXIARO after reading this leaflet, ask your health care provider. This leaflet does not take the place of talking with your health care professional about IXIARO. Only your health care provider can decide if IXIARO is right for you.
What is IXIARO and how does it work?
- IXIARO is a vaccine for use in individuals 2 months of age and older to help protect against Japanese encephalitis (JE). You cannot get the disease from IXIARO.
- You will need 2 doses of the vaccine.
- You should consult your health care provider on the need for a booster dose of IXIARO
- You should still protect yourself from mosquito bites even if you have had the IXIARO vaccine.
- IXIARO may not fully protect everyone who gets the vaccine.
- IXIARO does not protect against encephalitis caused by other viruses/pathogens.
- IXIARO does not protect against other diseases transmitted by mosquitoes.
What is Japanese encephalitis virus (JEV) and what is the disease caused by JEV?
Japanese encephalitis (JE) is caused by the Japanese encephalitis virus, JEV, which is mainly found in Asia. JEV is transmitted to humans by mosquitoes that have bitten an infected animal (like pigs). Many infected people develop mild symptoms or no symptoms at all. In people who develop severe disease, JE usually starts as a flu-like illness, with fever, chills, tiredness, headache, nausea, and vomiting. Confusion and agitation also occur in the early stage. JE causes death in one out of every three people with overt encephalitis. One out of two survivors develops permanent brain damage. JE acquired during pregnancy may cause intrauterine infection and miscarriage.
Who is at risk for Japanese encephalitis?
- People who live in, or travel to, areas where JEV circulates.
- Laboratory personnel who work with JEV.
Who should not get IXIARO?
You should not get IXIARO if you:
- are allergic to any of the ingredients in the vaccine. A list of ingredients can be found at the end of this leaflet.
- had an allergic reaction after getting a dose of the vaccine or any other JEV vaccine.
IXIARO is not approved for use in infants below the age of 2 months.
What should I tell my health care professional before I am vaccinated with IXIARO?
It is very important to tell your health care provider if you:
- have had an allergic reaction to a previous dose of IXIARO or any other JEV vaccine.
- have a bleeding disorder or a reduction in blood platelets, which increases risk of bleeding or bruising (thrombocytopenia) and cannot receive injections in the arm.
- have a weakened immune system, for example, due to a genetic defect or HIV infection.
- are or may be pregnant, or are breast feeding. IXIARO has not been studied in pregnant women or nursing mothers.
- currently have any illness with a fever of more than 100°F (37.8°C).
- take any medicines, even those you can buy over the counter.
How is IXIARO given?
IXIARO is given as an injection in the upper arm muscle in individuals 3 years of age and older. Infants 2 to 11 months of age are given the vaccine into the thigh. Children 12 to 35 months of age may be given the vaccine into the arm muscle (if the muscle is large enough) or into the thigh.
You will get a total of 2 doses of the vaccine. Ideally, the doses are given as:
- First dose: at a date you and your health care provider choose.
- Second dose: 28 days after the first dose.
Make sure that you get both doses. If you miss the second dose, your health care provider will decide when to give the missed dose. Be aware that protection is not reliable until 1 week after you receive the second dose of IXIARO.
Adults 18 years of age and older: if the second dose was administered more than 1 year ago, you should consult your health care provider on the need for a booster dose of IXIARO prior to potential re-exposure to JEV.
What are the possible side effects of IXIARO?
The most common side effects in adolescents >12 years of age and adults are headache, muscle pain and injection site reactions (e.g., pain, swelling, tenderness, redness). Nausea, skin rash, fatigue, flu-like illness, fever, irritability and loss of appetite may also occur.
The most common side effects in children below the age of 12 years are fever, irritability, diarrhea, vomiting, loss of appetite, injection site pain and injection site redness.
Contact your health care provider right away if you get any symptoms after receiving IXIARO that concern you.
Tell your health care provider if you have any of the following problems because these may be signs of an allergic reaction:
- difficulty breathing
- hoarseness or wheezing
- hives
- dizziness, weakness or fast heart beat
What are the ingredients of IXIARO?
Active Ingredient: purified components of inactivated Japanese encephalitis virus (JEV).
Inactive Ingredients: aluminum hydroxide and phosphate buffered saline (sodium chloride, potassium dihydrogen phosphate, disodium hydrogen phosphate).
Minute amounts of other substances remain in the vaccine as a result of the manufacturing process. Refer to the package insert for a complete list.
What else should I know about IXIARO?
This leaflet is a summary of information about IXIARO. If you would like more information, please talk to your health care professional.U.S.and international agencies (such as cdc.gov and who.int) also provide additional information about JEV and related travel advisories.
Issued September 2022
License Holder:
Valneva Austria GmbH, Vienna, Austria
Manufacturer:
Valneva Scotland Ltd., Livingston, UK
and
Distributed by:
Valneva USA, Inc.
Bethesda, MD 20814 USA
| IXIARO
japanese encephalitis vaccine, inactivated, adsorbed injection, suspension |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Valneva Scotland Ltd. (737272380) |
| Registrant - Valneva Austria GmbH (300378693) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Valneva Scotland Ltd. | 737272380 | manufacture, analysis | |