Drug Detail:Latanoprost ophthalmic (Latanoprost ophthalmic [ la-tan-oh-prost ])
Drug Class: Ophthalmic glaucoma agents
Highlights of Prescribing Information
IYUZEH (latanoprost ophthalmic solution) 0.005%, for topical ophthalmic use
Initial U.S. Approval: 1996
Indications and Usage for Iyuzeh Eye Drops
IYUZEH is a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. ( 1)
Iyuzeh Eye Drops Dosage and Administration
One drop in the affected eye(s) once daily in the evening. ( 2)
Dosage Forms and Strengths
Ophthalmic solution containing latanoprost 0.005% (50 mcg/mL). ( 3)
Contraindications
Known hypersensitivity to latanoprost or any other ingredients in this product. ( 4)
Warnings and Precautions
- Pigmentation: Pigmentation of the iris, periorbital tissue (eyelid) and eyelashes can occur. Iris pigmentation likely to be permanent. ( 5.1)
- Eyelash Changes: Gradual change to eyelashes including increased length, thickness and number of lashes. Usually, reversible. ( 5.2)
Adverse Reactions/Side Effects
Most common adverse reactions (5% to 35%) for IYUZEH are: conjunctival hyperemia, eye irritation, eye pruritus, abnormal sensation in eye, foreign body sensation in eyes, vision blurred and lacrimation increased. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Thea Pharma Inc. at 1-833-838-4028 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2022
Full Prescribing Information
1. Indications and Usage for Iyuzeh Eye Drops
IYUZEH ™ (latanoprost ophthalmic solution) 0.005% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2. Iyuzeh Eye Drops Dosage and Administration
The recommended dosage is one drop in the affected eye(s) once daily in the evening. If one dose is missed, treatment should continue with the next dose as normal.
The dosage of IYUZEH should not exceed once daily; the combined use of two or more prostaglandins, or prostaglandin analogs including IYUZEH is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
Reduction of the IOP starts approximately 3 to 4 hours after administration and the maximum effect is reached after 8 to 12 hours.
IYUZEH may be used concomitantly with other topical ophthalmic drug products to lower IOP. In vitro studies have shown that precipitation occurs when eye drops containing thimerosal are mixed with the preserved 0.005% latanoprost reference product. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart. Contact lenses should be removed prior to the administration of IYUZEH and may be reinserted 15 minutes after administration.
The solution from one individual unit is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual unit is opened, the remaining contents should be discarded immediately after administration.
3. Dosage Forms and Strengths
Ophthalmic solution: opalescent, white to slightly yellow solution containing latanoprost 0.005% (50 mcg/mL).
4. Contraindications
Known hypersensitivity to latanoprost or any other ingredients in this product.
5. Warnings and Precautions
5.1 Pigmentation
Topical latanoprost ophthalmic products, including IYUZEH have been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes. Pigmentation is expected to increase as long as latanoprost is administered.
The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of latanoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long-term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with IYUZEH can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
5.2 Eyelash Changes
Latanoprost ophthalmic products, including IYUZEH may gradually change eyelashes and vellus hair in the treated eye; these changes include increased length, thickness, pigmentation, the number of lashes or hairs, and misdirected growth of eyelashes. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Intraocular Inflammation
IYUZEH should be used with caution in patients with a history of intraocular inflammation (iritis/uveitis) and should generally not be used in patients with active intraocular inflammation because inflammation may be exacerbated.
5.4 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with latanoprost ophthalmic products, including IYUZEH. IYUZEH should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
5.5 Herpetic Keratitis
Reactivation of herpes simplex keratitis has been reported during treatment with latanoprost. IYUZEH should be used with caution in patients with a history of herpetic keratitis. IYUZEH should be avoided in cases of active herpes simplex keratitis because inflammation may be exacerbated.
6. Adverse Reactions/Side Effects
The following adverse reactions have been reported with the use of topical latanoprost products and are discussed in greater detail in other sections of the label:
- Iris pigmentation changes [see Warnings and Precautions (5.1)]
- Eyelid skin darkening [see Warnings and Precautions (5.1)]
- Eyelash changes (increased length, thickness, pigmentation, and number of lashes) [see Warnings and Precautions (5.2)]
- Intraocular inflammation (iritis/uveitis) [see Warnings and Precautions (5.3)]
- Macular edema, including cystoid macular edema [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the two clinical trials conducted with IYUZEH (latanoprost ophthalmic solution) 0.005% comparing it to XALATAN the preserved 0.005% latanoprost reference product, the most frequently reported ocular adverse reactions were conjunctival hyperemia and eye irritation ( Table 1).
| Symptom/Finding | Adverse Reactions (incidence (%)) | |
|---|---|---|
| IYUZEH
(n=378) | XALATAN
(n=358) |
|
| Conjunctival hyperemia | 129 (34) | 133 (37) |
| Eye irritation | 72 (19) | 112 (31) |
| Eye pruritus | 57 (15) | 58 (16) |
| Abnormal sensation in eye | 51 (14) | 52 (15) |
| Foreign body sensation in eyes | 44 (12) | 36 (10) |
| Vision blurred | 28 (7) | 30 (8) |
| Lacrimation increased | 19 (5) | 14 (4) |
| Photophobia | 13 (3) | 17 (5) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of topical latanoprost products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to ophthalmic latanoprost products, or a combination of these factors, include:
- Nervous System Disorders: Dizziness; headache; toxic epidermal necrolysis
- Eye Disorders: Eyelash and vellus hair changes of the eyelid (increased length, thickness, pigmentation, and number of eyelashes); keratitis; corneal edema and erosions; intraocular inflammation (iritis/uveitis); macular edema, including cystoid macular edema; trichiasis; periorbital and lid changes resulting in deepening of the eyelid sulcus; iris cyst; eyelid skin darkening; localized skin reaction on the eyelids; conjunctivitis; pseudopemphigoid of the ocular conjunctiva.
- Respiratory, Thoracic and Mediastinal Disorders: Asthma and exacerbation of asthma; dyspnea
- Skin and Subcutaneous Tissue Disorders: Pruritis
- Infections and Infestations: Herpes keratitis
- Cardiac Disorders: Angina; palpitations; angina unstable
- General Disorders and Administration Site Conditions: Chest pain
7. Drug Interactions
The combined use of two or more prostaglandins, or prostaglandin analogs including IYUZEH is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of IYUZEH administration in pregnant women to inform drug-associated risks.
In animal reproduction studies, intravenous (IV) administration of latanoprost to pregnant rabbits and rats throughout the period of organogenesis produced malformations, embryofetal lethality and spontaneous abortion at clinically relevant doses (equivalent to 1.3 – 324 times the maximum recommended human ophthalmic dose, on a mg/m 2 basis, assuming 100% absorption) (see Data) .
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Embryofetal studies were conducted in pregnant rats administered latanoprost daily by IV injection on gestation days 6 through 15, to target the period of organogenesis. A NOAEL for rat developmental toxicity was not established. Cleft palate was observed at 1 mcg/kg (equivalent to 3.2 times the maximum RHOD, on a mg/m 2 basis, assuming 100% absorption). Brain porencephalic cyst(s) were observed ≥50 mcg/kg (162 times the maximum RHOD). Skeletal anomalies were observed at 250 mcg/kg (811 times the maximum RHOD). No maternal toxicity was detectable at 250 mcg/kg/day.
Prenatal and postnatal development was assessed in rats. Pregnant rats were administered latanoprost daily by IV injection from gestation day 15, through delivery, until weaning (lactation Day 21). No adverse effects on rat offspring were observed at doses up to 10 mcg/kg/day (32 times the maximum RHOD, on a mg/m2 basis, assuming 100% absorption). At 100 mcg/kg/day (324 times the maximum RHOD), maternal deaths and pup mortality occurred.
8.2 Lactation
Risk Summary
It is not known whether this drug or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when IYUZEH is administered to a nursing woman.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for IYUZEH and any potential adverse effects on the breastfed child from IYUZEH.
10. Overdosage
Intravenous infusion of up to 3 mcg/kg of latanoprost in healthy volunteers produced mean plasma concentrations 200 times higher than during clinical treatment with latanoprost ophthalmic solution and no adverse reactions were observed. IV dosages of 5.5 to 10 mcg/kg caused abdominal pain, dizziness, fatigue, hot flushes, nausea, and sweating.
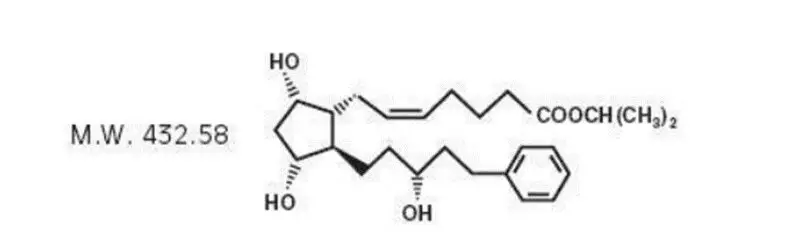
11. Iyuzeh Eye Drops Description
Latanoprost is a prostaglandin F 2α analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is C 26H 40O 5 and its chemical structure is:
 |
Latanoprost is a colorless to yellow oil that is very soluble in acetonitrile and freely soluble in ethanol, ethyl acetate, and methanol. It is practically insoluble in water and hexanes.
IYUZEH (latanoprost ophthalmic solution) 0.005% is supplied as a sterile, isotonic, aqueous solution of latanoprost with a pH of approximately 7 and an osmolality of approximately 280 mOsmol/kg. Each mL of IYUZEH contains 50 mcg of latanoprost. The inactive ingredients are: polyoxyl 40 hydrogenated castor oil, sorbitol, carbomer 974P, polyethylene glycol 4000, disodium edetate, sodium hydroxide (for pH-adjustment) and water for injections. One drop contains approximately 1.5 mcg of latanoprost.
IYUZEH does not contain a preservative.
12. Iyuzeh Eye Drops - Clinical Pharmacology
12.1 Mechanism of Action
Latanoprost is a prostaglandin F 2α analogue that is believed to reduce the IOP by increasing the outflow of aqueous humor. Studies in animals and man suggest that the main mechanism of action is increased uveoscleral outflow. Elevated IOP represents a major risk factor for glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss.
12.2 Pharmacodynamics
Reduction of the IOP in man starts about 3-4 hours after administration and maximum effect is reached after 8-12 hours. IOP reduction is present for at least 24 hours.
12.3 Pharmacokinetics
Absorption
Latanoprost is absorbed through the cornea where the isopropyl ester prodrug is hydrolyzed to the acid form to become biologically active.
Distribution
The distribution volume in humans is 0.16 ± 0.02 L/kg. The acid of latanoprost can be measured in aqueous humor during the first 4 hours, and in plasma only during the first hour after local administration. Studies in man indicate that the peak concentration in the aqueous humor is reached about two hours after topical administration.
Elimination
Metabolism
Latanoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to the biologically active acid. The active acid of latanoprost reaching the systemic circulation is primarily metabolized by the liver to the 1,2-dinor and 1,2,3,4-tetranor metabolites via fatty acid β-oxidation.
Excretion
The elimination of the acid of latanoprost from human plasma is rapid (t
1/2 = 17 min) after both IV and topical administration. Systemic clearance is approximately 7 mL/min/kg. Following hepatic β-oxidation, the metabolites are mainly eliminated via the kidneys. Approximately 88% and 98% of the administered dose are recovered in the urine after topical and IV dosing, respectively.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Latanoprost was not carcinogenic in either mice or rats when administered by oral gavage at doses of up to 170 mcg/kg/day (approximately 2800 times the recommended maximum human dose) for up to 20 and 24 months, respectively.
Mutagenesis
Latanoprost was not mutagenic in bacteria, in mouse lymphoma, or in mouse micronucleus tests. Chromosome aberrations were observed in vitro with human lymphocytes. Additional in vitro and in vivo studies on unscheduled DNA synthesis in rats were negative.
Impairment of Fertility
Latanoprost has not been found to have any effect on male or female fertility in rat studies at IV doses up to 250 mcg/kg/day (811 times the maximum RHOD, on a mg/m 2 basis, assuming 100% absorption).
14. Clinical Studies
14.1 Elevated Baseline IOP
In randomized, controlled clinical trials of patients with open angle glaucoma or ocular hypertension with mean baseline IOP of 19 - 24 mmHg, IYUZEH lowered IOP by 3 – 8 mmHg versus 4 – 8 mmHg by latanoprost ophthalmic solution preserved with benzalkonium chloride. Latanoprost ophthalmic solution preserved with benzalkonium chloride was approximately 1 mmHg more effective than IYUZEH.
16. How is Iyuzeh Eye Drops supplied
IYUZEH (latanoprost ophthalmic solution) is an opalescent, isotonic, white to slightly yellow solution of latanoprost 50 mcg/mL (0.005%) practically free from foreign particles.
It is supplied as a sterile solution in translucent low-density polyethylene single-dose container packaged in foil pouches (5 single-dose containers per pouch).
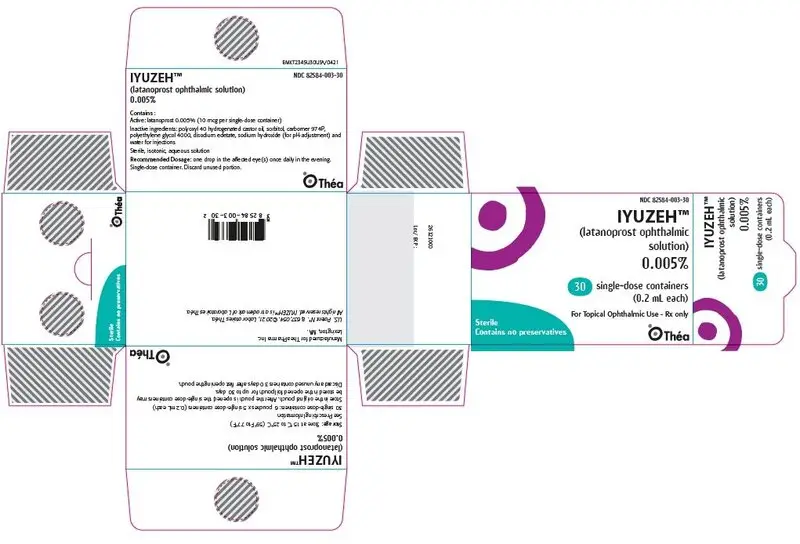
NDC 82584-003-30; Unit-of-Use Carton of 30
Storage:
Store at 15°C to 25°C (59°F to 77°F).
Store in the original pouch. After the pouch is opened, the single-dose containers may be stored in the opened foil pouch for up to 30 days at room temperature 15°C to 25°C (59°F to 77°F).
Patient should be advised to write down the date the foil pouch is opened in the space provided on the pouch.
Discard any unused containers 30 days after first opening the pouch.
17. Patient Counseling Information
Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Inform patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of IYUZEH.
Potential for Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with latanoprost ophthalmic solution. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
Handling the Container
Advise patients that IYUZEH is a sterile solution that does not contain a preservative. The drops are supplied in single-dose container. The solution from one individual container is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual container is opened, the remaining contents should be discarded immediately after administration. Open a new single-dose container every time you use IYUZEH.
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of IYUZEH.
Contact Lens Use
Advise patients that contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of IYUZEH.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
If a Dose is Missed
Advise patients that if one dose is missed, treatment should continue with the next dose as normal.
Manufactured for: Thea Pharma Inc. Waltham, MA.
All rights reserved.
U.S. Patent N°. 8,637,054. ©2021, Laboratoires Théa. All rights reserved. IYUZEH ™ is a trademark of Laboratoires Théa.
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 82584-003-30
IYUZEH™
(latanoprost ophthalmic
solution)
0.005%
30 single-dose containers
(0.2 mL each)
For Topical Ophthalmic Use - Rx only
Sterile
Contains no preservatives
Théa
PRINCIPAL DISPLAY PANEL
NDC 82584-003-81
Recommended Dosage: one drop in the affected eye(s) once
daily in the evening. Single-dose container. Discard unused portion.
See carton for inactive ingredients
Storage: Store at 15°C to 25°C (59°F to 77°F)
Store in the original pouch. After the pouch is opened, the single-dose containers may be stored
in the opened foil pouch for up to 30 days.
Discard any unused containers 30 days after first opening the pouch.
Write down the date you open the pouch
__ / __ / __
IYUZEH™
(latanoprost ophthalmic solution) 0.005%
For Topical Ophthalmic Use
5 single-dose containers (0.2 mL each)
Rx Only
Sterile – Contains no preservatives
Manufactured for Thea Pharma Inc.
Lexington, MA
Théa

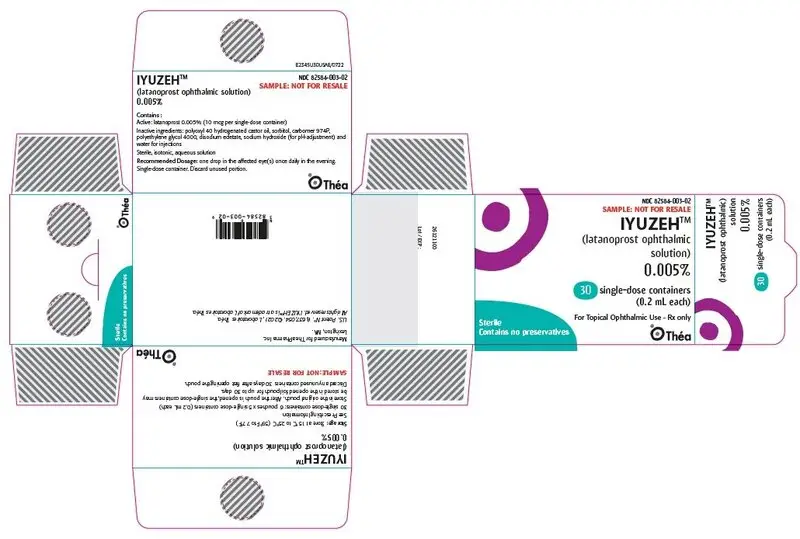
PRINCIPAL DISPLAY PANEL
NDC 82584-003-02
SAMPLE: NOT FOR RESALE
IYUZEH™
(latanoprost ophthalmic
solution)
0.005%
30 single-dose containers
(0.2 mL each)
For Topical Ophthalmic Use - Rx only
Sterile
Contains no preservatives
Théa
PRINCIPAL DISPLAY PANEL
NDC 82584-003-01
Recommended Dosage: one drop in the affected eye(s) once
daily in the evening. Single-dose container. Discard unused portion.
See carton for inactive ingredients
Storage: Store at 15°C to 25°C (59°F to 77°F)
Store in the original pouch. After the pouch is opened, the single-dose containers may be stored
in the opened foil pouch for up to 30 days.
Discard any unused containers 30 days after first opening the pouch.
Write down the date you open the pouch
__ / __ / __
IYUZEH™
(latanoprost ophthalmic solution) 0.005%
For Topical Ophthalmic Use
5 single-dose containers (0.2 mL each)
Rx Only
Sterile – Contains no preservatives
SAMPLE: NOT FOR RESALE
Manufactured for Thea Pharma Inc.
Lexington, MA
Théa

| IYUZEH
latanoprost ophthalmic solution 0.005% solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Thea Pharma Inc. (117787029) |