Drug Detail:Jevtana (Cabazitaxel [ ka-baz-i-tax-el ])
Drug Class: Mitotic inhibitors
Highlights of Prescribing Information
JEVTANA® (cabazitaxel) injection, for intravenous use
Initial U.S. Approval: 2010
WARNING: NEUTROPENIA AND HYPERSENSITIVITY
See full prescribing information for complete boxed warning.
- Neutropenic deaths have been reported. Obtain frequent blood counts to monitor for neutropenia. JEVTANA is contraindicated in patients with neutrophil counts of ≤1,500 cells/mm3. Primary prophylaxis with G-CSF is recommended in patients with high-risk clinical features. Consider primary prophylaxis with G-CSF in all patients receiving a dose of 25 mg/m2 (4, 5.1, 5.2)
- Severe hypersensitivity can occur and may include generalized rash/erythema, hypotension and bronchospasm. Discontinue JEVTANA immediately if severe reactions occur and administer appropriate therapy. (2.1, 5.2)
- Contraindicated if history of severe hypersensitivity reactions to cabazitaxel or to drugs formulated with polysorbate 80. (4)
Recent Major Changes
| Dosage and Administration (2.5) | 07/2023 |
| Warnings and Precautions (5.9) | 07/2023 |
Indications and Usage for Jevtana
JEVTANA is a microtubule inhibitor indicated in combination with prednisone for treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. (1)
Jevtana Dosage and Administration
Recommended Dose: JEVTANA 20 mg/m2 administered every three weeks as a one-hour intravenous infusion in combination with oral prednisone 10 mg administered daily throughout JEVTANA treatment. (2.1)
A dose of 25 mg/m2 can be used in select patients at the discretion of the treating healthcare provider. (2.1, 5.1, 5.2, 6.1, 14)
- JEVTANA requires two dilutions prior to administration. (2.5)
- Use the entire contents of the accompanying diluent to achieve a concentration of 10 mg/mL JEVTANA. (2.5)
- PVC equipment should not be used. (2.5)
-
Premedication Regimen: Administer intravenously 30 minutes before each dose of JEVTANA:
- Antihistamine (dexchlorpheniramine 5 mg or diphenhydramine 25 mg or equivalent antihistamine)
- Corticosteroid (dexamethasone 8 mg or equivalent steroid)
- H2 antagonist (2.1)
- Dosage Modifications: See full prescribing information (2.2, 2.3, 2.4)
Dosage Forms and Strengths
- Single dose vial 60 mg/1.5 mL, supplied with diluent (5.7 mL) for JEVTANA (3)
Contraindications
- Neutrophil counts of ≤1,500/mm3 (2.2, 4)
- History of severe hypersensitivity to JEVTANA or polysorbate 80 (4)
- Severe hepatic impairment (Total Bilirubin >3 × ULN) (4)
Warnings and Precautions
- Bone marrow suppression (particularly neutropenia) and its clinical consequences (febrile neutropenia, neutropenic infections, and death): Monitor blood counts frequently to determine if dosage modification or initiation of G-CSF is needed. Closely monitor patients with hemoglobin <10 g/dL. (2.2, 4, 5.1)
- Increased toxicities in elderly patients: Patients ≥65 years of age were more likely to experience fatal outcomes and certain adverse reactions, including neutropenia and febrile neutropenia. Monitor closely. (5.2, 8.5)
- Hypersensitivity: Severe hypersensitivity reactions can occur. Premedicate with corticosteroids and H2 antagonists. Discontinue infusion immediately if hypersensitivity is observed and treat as indicated. (4, 5.3)
- Gastrointestinal disorders: Nausea, vomiting, and diarrhea may occur. Mortality related to diarrhea has been reported. Rehydrate and treat with antiemetics and antidiarrheals as needed. If experiencing Grade ≥3 diarrhea, dosage should be modified. (2.2) Deaths have occurred due to gastrointestinal hemorrhage, perforation and neutropenic enterocolitis. Delay or discontinue JEVTANA and treat as indicated. (5.4)
- Renal failure, including cases with fatal outcomes, has been reported. Identify cause and manage aggressively. (5.5)
- Urinary disorders including cystitis: Cystitis, radiation cystitis, and hematuria may occur. Monitor patients who previously received pelvic radiation for signs and symptoms of cystitis. Interrupt or discontinue JEVTANA and provide medical or surgical supportive care, as needed, in patients experiencing severe hemorrhagic cystitis. (5.6)
- Respiratory disorders: Interstitial pneumonia/pneumonitis, interstitial lung disease and acute respiratory distress syndrome, including fatal outcomes, have been reported. Delay or discontinue JEVTANA and treat as indicated. (5.7)
- Hepatic impairment: Administer JEVTANA at a dose of 20 mg/m2 in patients with mild hepatic impairment. Administer JEVTANA at a dose of 15 mg/m2 in patients with moderate hepatic impairment. (2.3, 5.8)
- Embryo-fetal toxicity: JEVTANA can cause fetal harm and loss of pregnancy. Advise males with female partners of reproductive potential to use effective contraception. (5.9, 8.1, 8.3)
Adverse Reactions/Side Effects
Most common all grades adverse reactions and laboratory abnormalities (≥10%) with JEVTANA 20 mg/m2 or 25 mg/m2 are neutropenia, anemia, diarrhea, nausea, fatigue, asthenia, vomiting, hematuria, constipation, decreased appetite, back pain, and abdominal pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Avoid coadministration of JEVTANA with strong CYP3A inhibitors. If patients require coadministration of a strong CYP3A inhibitor, consider a 25% JEVTANA dose reduction. (2.4, 7.1, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2023
Full Prescribing Information
1. Indications and Usage for Jevtana
JEVTANA® is indicated in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen.
2. Jevtana Dosage and Administration
2.1 Dosing Information
The recommended dose of JEVTANA is based on calculation of the Body Surface Area (BSA), and is 20 mg/m2 administered as a one-hour intravenous infusion every three weeks in combination with oral prednisone 10 mg administered daily throughout JEVTANA treatment.
A dose of 25 mg/m2 can be used in select patients at the discretion of the treating healthcare provider [see Warnings and Precautions (5.1, 5.2), Adverse Reactions (6.1), and Clinical Studies (14)].
Primary prophylaxis with G-CSF is recommended in patients with high-risk clinical features. Consider primary prophylaxis with G-CSF in all patients receiving a dose of 25 mg/m2 [see Contraindications (4) and Warnings and Precautions (5.1, 5.2)].
Premedicate at least 30 minutes prior to each dose of JEVTANA with the following intravenous medications to reduce the risk and/or severity of hypersensitivity [see Warnings and Precautions (5.3)]:
- antihistamine (dexchlorpheniramine 5 mg, or diphenhydramine 25 mg or equivalent antihistamine),
- corticosteroid (dexamethasone 8 mg or equivalent steroid),
- H2 antagonist.
Antiemetic prophylaxis is recommended and can be given orally or intravenously as needed [see Warnings and Precautions (5.3)].
JEVTANA injection single-dose vial requires two dilutions prior to administration [see Dosage and Administration (2.5)].
2.2 Dose Modifications for Adverse Reactions
Reduce or discontinue JEVTANA dosing for adverse reactions as described in Table 1.
| Toxicity | Dosage Modification |
|---|---|
| Prolonged grade ≥3 neutropenia (greater than 1 week) despite appropriate medication including granulocyte-colony stimulating factor (G-CSF) | Delay treatment until neutrophil count is >1,500 cells/mm3, then reduce dosage of JEVTANA by one dose level. Use G-CSF for secondary prophylaxis. |
| Febrile neutropenia or neutropenic infection | Delay treatment until improvement or resolution, and until neutrophil count is >1,500 cells/mm3, then reduce dosage of JEVTANA by one dose level. Use G-CSF for secondary prophylaxis. |
| Grade ≥3 diarrhea or persisting diarrhea despite appropriate medication, fluid and electrolytes replacement | Delay treatment until improvement or resolution, then reduce dosage of JEVTANA by one dose level. |
| Grade 2 peripheral neuropathy | Delay treatment until improvement or resolution, then reduce dosage of JEVTANA by one dose level. |
| Grade ≥3 peripheral neuropathy | Discontinue JEVTANA. |
Patients at a 20 mg/m2 dose who require dose reduction should decrease dosage of JEVTANA to 15 mg/m2 [see Adverse Reactions (6.1)].
Patients at a 25 mg/m2 dose who require dose reduction should decrease dosage of JEVTANA to 20 mg/m2. One additional dose reduction to 15 mg/m2 may be considered [see Adverse Reactions (6.1)].
2.3 Dose Modifications for Hepatic Impairment
- Mild hepatic impairment (total bilirubin >1 to ≤1.5 × Upper Limit of Normal (ULN) or AST >1.5 × ULN): Administer JEVTANA at a dose of 20 mg/m2.
- Moderate hepatic impairment (total bilirubin >1.5 to ≤3 × ULN and AST = any): Administer JEVTANA at a dose of 15 mg/m2 based on tolerability data in these patients; however, the efficacy of this dose is unknown.
- Severe hepatic impairment (total bilirubin >3 × ULN): JEVTANA is contraindicated in patients with severe hepatic impairment [see Warning and Precautions (5.8) and Clinical Pharmacology (12.3)].
2.4 Dose Modifications for Use with Strong CYP3A Inhibitors
Concomitant drugs that are strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase plasma concentrations of cabazitaxel. Avoid the coadministration of JEVTANA with these drugs. If patients require coadministration of a strong CYP3A inhibitor, consider a 25% JEVTANA dose reduction [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
2.5 Preparation and Administration
JEVTANA is a hazardous anticancer drug. Follow applicable special handling and disposal procedures [see References (15)]. If JEVTANA first diluted solution, or second (final) dilution for intravenous infusion should come into contact with the skin or mucous, immediately and thoroughly wash with soap and water.
Do not use PVC infusion containers or polyurethane infusions sets for preparation and administration of JEVTANA infusion solution.
JEVTANA should not be mixed with any other drugs.
3. Dosage Forms and Strengths
JEVTANA (cabazitaxel) injection is supplied as a kit consisting of the following:
- Cabazitaxel injection: 60 mg/1.5 mL; a clear yellow to brownish-yellow viscous solution
- Diluent: 5.7 mL of 13% (w/w) ethanol in water; a clear colorless solution
4. Contraindications
JEVTANA is contraindicated in patients with:
- neutrophil counts of ≤1,500/mm3 [see Warnings and Precautions (5.1)]
- history of severe hypersensitivity reactions to cabazitaxel or to other drugs formulated with polysorbate 80 [see Warnings and Precautions (5.3)]
- severe hepatic impairment (total bilirubin >3 × ULN) [see Warnings and Precautions (5.8)]
5. Warnings and Precautions
5.1 Bone Marrow Suppression
JEVTANA is contraindicated in patients with neutrophils ≤1,500/mm3 [see Contraindications (4)]. Closely monitor patients with hemoglobin <10 g/dL.
Bone marrow suppression manifested as neutropenia, anemia, thrombocytopenia and/or pancytopenia may occur. Neutropenic deaths have been reported.
5.2 Increased Toxicities in Elderly Patients
In a randomized trial (TROPIC), 2% of patients (3/131) <65 years of age and 6% (15/240) ≥65 years of age died of causes other than disease progression within 30 days of the last JEVTANA dose. Patients ≥65 years of age are more likely to experience certain adverse reactions, including neutropenia and febrile neutropenia. The incidence of the following grade 3–4 adverse reactions was higher in patients ≥65 years of age compared to younger patients; neutropenia (87% vs 74%), and febrile neutropenia (8% vs 6%).
In a randomized clinical trial (PROSELICA) comparing two doses of JEVTANA, deaths due to infection within 30 days of starting JEVTANA occurred in 0.7% (4/580) patients on the 20 mg/m2 arm and 1.3% (8/595) patients on the 25 mg/m2 arm; all of these patients were >60 years of age.
In PROSELICA, on the 20 mg/m2 arm, 3% (5/178) of patients <65 years of age and 2% (9/402) ≥65 years of age died of causes other than disease progression within 30 days of the last JEVTANA dose. On the 25 mg/m2 arm, 2% (3/175) patients <65 years of age and 5% (20/420) ≥65 years of age died of causes other than disease progression within 30 days of the last JEVTANA dose [see Adverse Reactions (6) and Use in Specific Populations (8.5)].
In CARD, a death due to infection within 30 days of starting JEVTANA occurred in 0.8% (1/126) patient who was >75 years of age. There were 2.4% (3/126) of patients who died of causes other than disease progression within 30 days of the last JEVTANA dose; all of these patients were >75 years of age.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions may occur within a few minutes following the initiation of the infusion of JEVTANA, thus facilities and equipment for the treatment of hypotension and bronchospasm should be available. Severe hypersensitivity reactions can occur and may include generalized rash/erythema, hypotension and bronchospasm.
Premedicate all patients prior to the initiation of the infusion of JEVTANA [see Dosage and Administration (2.1)]. Observe patients closely for hypersensitivity reactions, especially during the first and second infusions. Severe hypersensitivity reactions require immediate discontinuation of the JEVTANA infusion and appropriate therapy. JEVTANA is contraindicated in patients with a history of severe hypersensitivity reactions to cabazitaxel or to other drugs formulated with polysorbate 80 [see Contraindications (4)].
5.4 Gastrointestinal Adverse Reactions
Nausea, vomiting and severe diarrhea, at times, may occur. Deaths related to diarrhea and electrolyte imbalance occurred in the randomized clinical trials. Intensive measures may be required for severe diarrhea and electrolyte imbalance. Antiemetic prophylaxis is recommended. Treat patients with rehydration, antidiarrheal or antiemetic medications as needed. Treatment delay or dosage reduction may be necessary if patients experience Grade ≥3 diarrhea [see Dosage and Administration (2.2)].
Gastrointestinal (GI) hemorrhage and perforation, ileus, enterocolitis, neutropenic enterocolitis, including fatal outcome, have been reported in patients treated with JEVTANA [see Adverse Reactions (6.2)]. Risk may be increased with neutropenia, age, steroid use, concomitant use of NSAIDs, antiplatelet therapy or anticoagulants, and patients with a prior history of pelvic radiotherapy, adhesions, ulceration and GI bleeding.
Abdominal pain and tenderness, fever, persistent constipation, diarrhea, with or without neutropenia, may be early manifestations of serious gastrointestinal toxicity and should be evaluated and treated promptly. JEVTANA treatment delay or discontinuation may be necessary.
The incidence of gastrointestinal adverse reactions is greater in the patients who have received prior radiation. In PROSELICA, diarrhea was reported in 41% (297/732) of patients who had received prior radiation and in 27% (118/443) of patients without prior radiation. Of the patients who had previously received radiation, more patients on the 25 mg/m2 arm reported diarrhea, compared to patients on the 20 mg/m2 arm.
5.5 Renal Failure
In the randomized clinical trial (TROPIC), renal failure of any grade occurred in 4% of the patients being treated with JEVTANA, including four cases with fatal outcome. Most cases occurred in association with sepsis, dehydration, or obstructive uropathy [see Adverse Reactions (6.1)]. Some deaths due to renal failure did not have a clear etiology. Appropriate measures should be taken to identify causes of renal failure and treat aggressively.
5.6 Urinary Disorders Including Cystitis
Cystitis, radiation cystitis, and hematuria, including that requiring hospitalization, has been reported with JEVTANA in patients who previously received pelvic radiation [see Adverse Reactions (6.2)]. In PROSELICA, cystitis and radiation cystitis were reported in 1.2% and 1.5% of patients who received prior radiation, respectively. Hematuria was reported in 19.4% of patients who received prior radiation and in 14.4% of patients who did not receive prior radiation. Cystitis from radiation recall may occur late in treatment with JEVTANA. Monitor patients who previously received pelvic radiation for signs and symptoms of cystitis while on JEVTANA. Interrupt or discontinue JEVTANA in patients experiencing severe hemorrhagic cystitis. Medical and/or surgical supportive treatment may be required to treat severe hemorrhagic cystitis.
5.7 Respiratory Disorders
Interstitial pneumonia/pneumonitis, interstitial lung disease and acute respiratory distress syndrome have been reported and may be associated with fatal outcome [see Adverse Reactions (6.2)]. Patients with underlying lung disease may be at higher risk for these events. Acute respiratory distress syndrome may occur in the setting of infection.
Interrupt JEVTANA if new or worsening pulmonary symptoms develop. Closely monitor, promptly investigate, and appropriately treat patients receiving JEVTANA. Consider discontinuation. The benefit of resuming JEVTANA treatment must be carefully evaluated.
5.8 Use in Patients with Hepatic Impairment
Cabazitaxel is extensively metabolized in the liver.
JEVTANA is contraindicated in patients with severe hepatic impairment (total bilirubin >3 × ULN) [see Contraindications (4)]. Dose should be reduced for patients with mild (total bilirubin >1 to ≤1.5 × ULN or AST >1.5 × ULN) and moderate (total bilirubin >1.5 to ≤3.0 × ULN and any AST) hepatic impairment, based on tolerability data in these patients [see Dosage and Administration (2.3) and Use in Specific Populations (8.7)]. Administration of JEVTANA to patients with mild and moderate hepatic impairment should be undertaken with caution and close monitoring of safety.
5.9 Embryo-Fetal Toxicity
Based on findings in animal reproduction studies and its mechanism of action, JEVTANA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, intravenous administration of cabazitaxel in pregnant rats during organogenesis caused embryonic and fetal death at doses lower than the maximum recommended human dose (approximately 0.06 times the Cmax in patients at the recommended human dose). Advise males with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of JEVTANA [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in another section of the label:
- Bone Marrow Suppression [see Warnings and Precautions (5.1)]
- Increased Toxicities in Elderly Patients [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.4)]
- Renal Failure [see Warnings and Precautions (5.5)]
- Urinary Disorders Including Cystitis [see Warnings and Precautions (5.6)]
- Respiratory Disorders [see Warnings and Precautions (5.7)]
- Use in Patients with Hepatic Impairment [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified from clinical trials and/or postmarketing surveillance. Because they are reported from a population of unknown size, precise estimates of frequency cannot be made.
Gastrointestinal: Gastritis, intestinal obstruction.
Respiratory: Interstitial pneumonia/pneumonitis, interstitial lung disease and acute respiratory distress syndrome.
Renal and urinary disorders: Radiation recall hemorrhagic cystitis.
7. Drug Interactions
7.1 CYP3A Inhibitors
Cabazitaxel is primarily metabolized through CYP3A [see Clinical Pharmacology (12.3)]. Strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase plasma concentrations of cabazitaxel. Avoid the coadministration of JEVTANA with strong CYP3A inhibitors. If patients require coadministration of a strong CYP3A inhibitor, consider a 25% JEVTANA dose reduction [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of JEVTANA in pediatric patients have not been established.
JEVTANA was evaluated in 39 pediatric patients (ages 3 to 18 years) receiving prophylactic G-CSF. The maximum tolerated dose (MTD) was 30 mg/m2 intravenously over 1 hour on Day 1 of a 21 day cycle in pediatric patients with solid tumors based on the dose-limiting toxicity (DLT) of febrile neutropenia. No objective responses were observed in 11 patients with refractory high grade glioma (HGG) or diffuse intrinsic pontine glioma (DIPG). One patient had a partial response among the 9 patients with ependymoma.
Infusion related/hypersensitivity reactions were seen in 10 patients (26%). Three patients experienced serious adverse events of anaphylactic reaction. The incidence of infusion related/hypersensitivity reactions decreased with steroid premedication. The most frequent treatment-emergent adverse events were similar to those reported in adults.
Based on the population pharmacokinetics analysis conducted with data from 31 pediatric patients with cancer (ages 3 to 18 years), the clearances by body surface area were comparable to those in adults.
8.5 Geriatric Use
In the TROPIC study, of the 371 patients with prostate cancer treated with JEVTANA every three weeks plus prednisone, 240 patients (64.7%) were 65 years of age and over, while 70 patients (18.9%) were 75 years of age and over. No overall differences in effectiveness were observed between patients ≥65 years of age and younger patients. Elderly patients (≥65 years of age) may be more likely to experience certain adverse reactions. The incidence of death due to causes other than disease progression within 30 days of the last cabazitaxel dose were higher in patients who were 65 years of age or greater compared to younger patients [see Warnings and Precautions (5.2)]. The incidence of grade 3–4 neutropenia and febrile neutropenia were higher in patients who were 65 years of age or greater compared to younger patients. The following grade 1–4 adverse reactions were reported at rates ≥5% higher in patients 65 years of age or older compared to younger patients: fatigue (40% vs 30%), neutropenia (97% vs 89%), asthenia (24% vs 15%), pyrexia (15% vs 8%), dizziness (10% vs 5%), urinary tract infection (10% vs 3%), and dehydration (7% vs 2%), respectively.
In the PROSELICA study, the grade 1–4 adverse reactions reported at rates of at least 5% higher in patients 65 years of age or older compared to younger patients were diarrhea (43% vs 33%), fatigue (30% vs 19%), asthenia (22% vs 13%), constipation (20% vs 13%), clinical neutropenia (13% vs 6%), febrile neutropenia (11% vs 5%), and dyspnea (10% vs 3%).
In the CARD study, the grade 1–4 adverse reactions reported at rates of at least 5% higher in patients 65 years of age or older compared to younger patients were decreased appetite (16% vs 7%), hypertension (5% vs 0), constipation (18% vs 7%), paresthesia (6% vs 0), stomatitis (10% vs 3%), musculoskeletal pain (5% vs 0), fatigue (31% vs 23%), asthenia (30% vs 19%), and edema peripheral (11% vs 0).
Based on a population pharmacokinetic analysis, no significant difference was observed in the pharmacokinetics of cabazitaxel between patients <65 years (n=100) and older (n=70).
8.6 Renal Impairment
No dose adjustment is necessary in patients with renal impairment not requiring hemodialysis. Patients presenting with end-stage renal disease (creatinine clearance CLCR <15 mL/min/1.73 m2), should be monitored carefully during treatment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Cabazitaxel is extensively metabolized in the liver. Patients with mild hepatic impairment (total bilirubin >1 to ≤1.5 × ULN or AST >1.5 × ULN) should have JEVTANA dose of 20 mg/m2. Administration of cabazitaxel to patients with mild hepatic impairment should be undertaken with caution and close monitoring of safety [see Clinical Pharmacology (12.3)]. The maximum tolerated dose in patients with moderate hepatic impairment (total bilirubin >1.5 to ≤3.0 × ULN and AST = any) was 15 mg/m2, however, the efficacy at this dose level was unknown. JEVTANA is contraindicated in patients with severe hepatic impairment (total bilirubin >3 × ULN) [see Contraindications (4)].
10. Overdosage
There is no known antidote for JEVTANA overdose. Overdose has resulted from improper preparation [see Dosage and Administration (2.5)]. Read the entire section Dosage and Administration (2) carefully before mixing or diluting. Complications of overdose include exacerbation of adverse reactions such as bone marrow suppression and gastrointestinal disorders. Overdose has led to fatal outcome.
In case of overdose, the patient should be kept in a specialized unit where vital signs, chemistry and particular functions can be closely monitored. Patients should receive therapeutic G-CSF as soon as possible after discovery of overdose. Other appropriate symptomatic measures should be taken, as needed.
11. Jevtana Description
JEVTANA (cabazitaxel) injection is an antineoplastic agent belonging to the taxane class that is for intravenous use. It is prepared by semi-synthesis with a precursor extracted from yew needles.
The chemical name of cabazitaxel is (2α,5β,7β,10β,13α)-4-acetoxy-13-({(2R,3S)-3-[(tertbutoxycarbonyl) amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1-hydroxy-7,10-dimethoxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate – propan-2-one (1:1).
Cabazitaxel has the following structural formula:
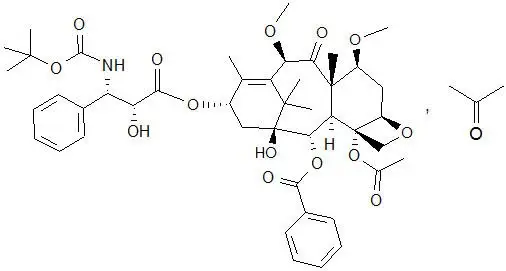
Cabazitaxel is a white to almost-white powder with a molecular formula of C45H57NO14C3H6O and a molecular weight of 894.01 (for the acetone solvate) / 835.93 (for the solvent free). It is lipophilic, practically insoluble in water and soluble in alcohol.
JEVTANA (cabazitaxel) injection 60 mg/1.5 mL is a sterile, non-pyrogenic, clear yellow to brownish-yellow viscous solution and is available in single-dose vials containing 60 mg cabazitaxel (anhydrous and solvent free) and 1.56 g polysorbate 80 (citric acid monohydrate is used to adjust the pH of the polysorbate 80 between 3.3 to 3.8).
Each mL contains 40 mg cabazitaxel (anhydrous) and 1.04 g polysorbate 80.
DILUENT for JEVTANA is a clear, colorless, sterile, and non-pyrogenic solution containing 13% (w/w) ethanol in water for injection, approximately 5.7 mL.
JEVTANA requires two dilutions prior to intravenous infusion. JEVTANA injection should be diluted only with the supplied DILUENT for JEVTANA, followed by dilution in either 0.9% sodium chloride solution or 5% dextrose solution.
12. Jevtana - Clinical Pharmacology
12.1 Mechanism of Action
Cabazitaxel is a microtubule inhibitor. Cabazitaxel binds to tubulin and promotes its assembly into microtubules while simultaneously inhibiting disassembly. This leads to the stabilization of microtubules, which results in the inhibition of mitotic and interphase cellular functions.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of cabazitaxel.
Cabazitaxel was positive for genotoxicity by an aneugenic mechanism in the in vivo micronucleus test, inducing an increase of micronuclei in rats at doses ≥0.5 mg/kg. Cabazitaxel increased numerical aberrations with or without metabolic activation in an in vitro test in human lymphocytes though no induction of structural aberrations was observed. Cabazitaxel did not induce mutations in the bacterial reverse mutation (Ames) test. The positive in vivo genotoxicity findings are consistent with the pharmacological activity of the compound (inhibition of tubulin depolymerization).
In a fertility study performed in female rats at cabazitaxel doses of 0.05, 0.1, or 0.2 mg/kg/day there was no effect of administration of the drug on mating behavior or the ability to become pregnant. In repeat-dose toxicology studies in rats with intravenous cabazitaxel administration once every three weeks for up to 6 months, atrophy of the uterus was observed at the 5 mg/kg dose level (approximately the AUC in patients with cancer at the recommended human dose) along with necrosis of the corpora lutea at doses ≥1 mg/kg (approximately 0.2 times the AUC at the clinically recommended human dose).
In a fertility study in male rats, cabazitaxel did not affect mating performances or fertility at doses of 0.05, 0.1, or 0.2 mg/kg/day. In repeat-dose toxicology studies with intravenous cabazitaxel administration once every three weeks for up to 9 months, degeneration of seminal vesicle and seminiferous tubule atrophy in the testis were observed in rats at a dose of 1 mg/kg (approximately 0.2 times the AUC in patients at the recommended human dose), and minimal testicular degeneration (minimal epithelial single cell necrosis in epididymis) was observed in dogs treated at a dose of 0.5 mg/kg (approximately 0.1 times the AUC in patients at the recommended human dose).
14. Clinical Studies
14.1 TROPIC Trial (JEVTANA + prednisone compared to mitoxantrone)
The efficacy and safety of JEVTANA in combination with prednisone were evaluated in a randomized, open-label, international, multi-center study in patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen (TROPIC, NCT00417079).
A total of 755 patients were randomized to receive either JEVTANA 25 mg/m2 intravenously every 3 weeks for a maximum of 10 cycles with prednisone 10 mg orally daily (n=378), or to receive mitoxantrone 12 mg/m2 intravenously every 3 weeks for 10 cycles with prednisone 10 mg orally daily (n=377) for a maximum of 10 cycles.
This study included patients over 18 years of age with hormone-refractory metastatic prostate cancer either measurable by RECIST criteria or non-measurable disease with rising PSA levels or appearance of new lesions, and ECOG (Eastern Cooperative Oncology Group) performance status 0–2. Patients had to have neutrophils >1,500 cells/mm3, platelets >100,000 cells/mm3, hemoglobin >10 g/dL, creatinine <1.5 × upper limit of normal (ULN), total bilirubin <1 × ULN, AST <1.5 × ULN, and ALT <1.5 × ULN. Patients with a history of congestive heart failure, or myocardial infarction within the last 6 months, or patients with uncontrolled cardiac arrhythmias, angina pectoris, and/or hypertension were not included in the study.
Demographics, including age, race, and ECOG performance status (0–2) were balanced between the treatment arms. The median age was 68 years (range 46–92) and the racial distribution for all groups was 83.9% Caucasian, 6.9% Asian, 5.3% Black, and 4% Others in the JEVTANA group.
Efficacy results for the JEVTANA arm versus the control arm are summarized in Table 5 and Figure 1.
| JEVTANA + Prednisone n=378 | Mitoxantrone + Prednisone n=377 |
|
|---|---|---|
|
||
| Overall Survival | ||
| Number of deaths (%) | 234 (61.9%) | 279 (74.0%) |
| Median survival (month) (95% CI) | 15.1 (14.1–16.3) | 12.7 (11.6–13.7) |
| Hazard Ratio* (95% CI) | 0.70 (0.59–0.83) | |
| p-value | <0.0001 | |
Figure 1: Kaplan-Meier Overall Survival Curves (TROPIC)
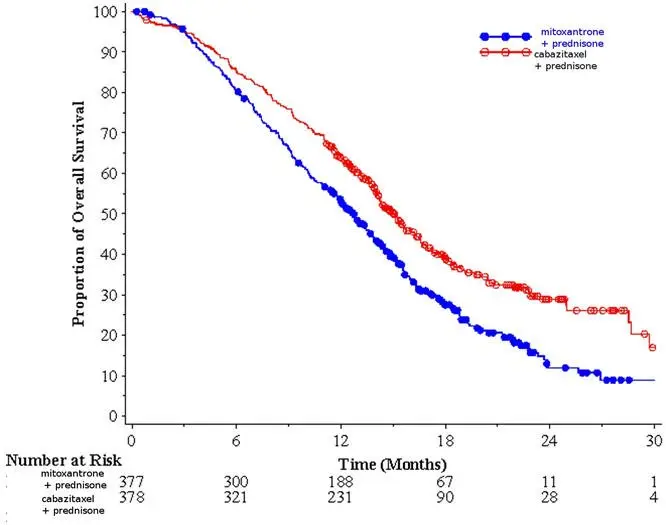
Investigator-assessed tumor response of 14.4% (95% CI: 9.6–19.3) was higher for patients in the JEVTANA arm compared to 4.4% (95% CI: 1.6–7.2) for patients in the mitoxantrone arm, p=0.0005.
14.2 PROSELICA Trial (comparison of two doses of JEVTANA)
The efficacy and safety of JEVTANA were evaluated in a noninferiority, multicenter, randomized, open-label study (PROSELICA, NCT01308580). A total of 1200 patients with metastatic castration-resistant prostate cancer, previously treated with a docetaxel-containing regimen were randomized to receive either JEVTANA 25 mg/m2 (n=602) or 20 mg/m2 (n=598) dose. Overall survival (OS) was the major efficacy outcome.
Demographics, including age, race, and ECOG performance status (0–2) were balanced between the treatment arms. The median age was 68 years (range 45–89) and the racial distribution for all groups was 87% Caucasian, 6.9% Asian, 2.3% Black, and 3.8% Others in the JEVTANA 20 mg/m2 group. The median age was 69 years (range 45–88) and the racial distribution for all groups was 88.7% Caucasian, 6.6% Asian, 1.8% Black, and 2.8% Others in the JEVTANA 25 mg/m2 group.
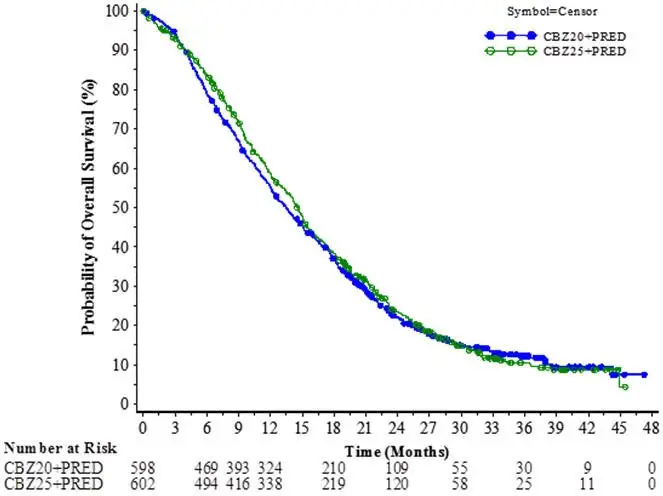
The study demonstrated noninferiority in overall survival (OS) of JEVTANA 20 mg/m2 in comparison with JEVTANA 25 mg/m2 in an intent-to-treat population (see Table 6 and Figure 2). Based on the per-protocol population, the estimated median OS was 15.1 months on JEVTANA 20 mg/m2 and 15.9 months on JEVTANA 25 mg/m2, the observed hazard ratio (HR) of OS was 1.042 (97.78% CI: 0.886, 1.224). Among the subgroup analyses intended for assessing the heterogeneity, no notable difference in OS was observed on the JEVTANA 25 mg/m2 arm compared to the JEVTANA 20 mg/m2 arm in subgroups based on the stratification factors of ECOG performance status score, measurability of disease, or region.
| CBZ20+PRED n=598 | CBZ25+PRED n=602 |
|
|---|---|---|
| CBZ20=Cabazitaxel 20 mg/m2, CBZ25=Cabazitaxel 25 mg/m2, PRED=Prednisone/Prednisolone. | ||
| CI=confidence interval. | ||
|
||
| Overall Survival | ||
| Number of deaths, n (%) | 497 (83.1%) | 501 (83.2%) |
| Median survival (95% CI) (months) | 13.4 (12.2 to 14.9) | 14.5 (13.5 to 15.3) |
| Hazard Ratio* (97.78% CI†) | 1.024 (0.886, 1.184) | |
Figure 2: Kaplan-Meier Overall Survival Curves (intent-to-treat population) (PROSELICA)
14.3 CARD Trial (JEVTANA 25 mg/m2 + prednisone/prednisolone + primary prophylaxis with G-CSF compared to abiraterone acetate + prednisone/prednisolone or enzalutamide)
The efficacy and safety of JEVTANA were evaluated in a multinational, randomized, active-controlled, open-label study (CARD: NCT02485691) in patients with metastatic castration resistant prostate cancer (mCRPC) previously treated with a docetaxel containing regimen and had progressed within 12 months of initiating either abiraterone or enzalutamide. A total of 255 patients were randomized to receive either JEVTANA 25 mg/m2 every 3 week plus prednisone/prednisolone 10 mg daily (n=129), abiraterone 1000 mg once daily plus prednisone/prednisolone 5 mg twice daily or enzalutamide 160 mg once daily depending on prior therapy received (n=126). Primary prophylactic G-CSF was administered at each cycle for patients in the JEVTANA arm. This study included patients over 18 years of age with ECOG performance status 0–2. Patients had to have neutrophils >1,500 cells/mm3, platelets >100,000 cells/mm3, hemoglobin >10 g/dL, creatinine <1.5 × upper limit of normal (ULN), total bilirubin <1 × ULN, AST <1.5 × ULN, and ALT <1.5 × ULN. Patients with a history of congestive heart failure, or myocardial infarction within the last 6 months, or patients with uncontrolled cardiac arrhythmias, angina pectoris, and/or hypertension were not included in the study. Randomization was stratified by ECOG performance status (0 or 1 vs 2), time from abiraterone acetate or enzalutamide to disease progression, and receipt of abiraterone acetate or enzalutamide before or after docetaxel containing regimen.
The major efficacy outcome measure was radiographic progression free-survival (rPFS) as defined by Prostate Cancer Working Group-2 (PCWG2) assessed by study investigators. Other efficacy outcome measures included overall survival and objective response rate.
Demographics and baseline disease characteristics were balanced between treatment arms. The overall median age was 70 years (range 45 to 88), 95% of patients had an ECOG PS of 0 to 1 and median Gleason score was 8. A majority of the patients (61%) had their prior treatment with abiraterone acetate or enzalutamide after docetaxel. There were 36% of patients on the cabazitaxel arm with visceral disease (liver 8%, lung 8%, other 20%) and 57% with bone-only disease. Race and ethnicity data were not collected. Approximately 92% of the patients on the cabazitaxel arm received primary prophylaxis with G-CSF therapy during the first 3 cycles and, overall, 90% of the patients on the cabazitaxel arm received primary prophylaxis with G-CSF therapy at each cycle.
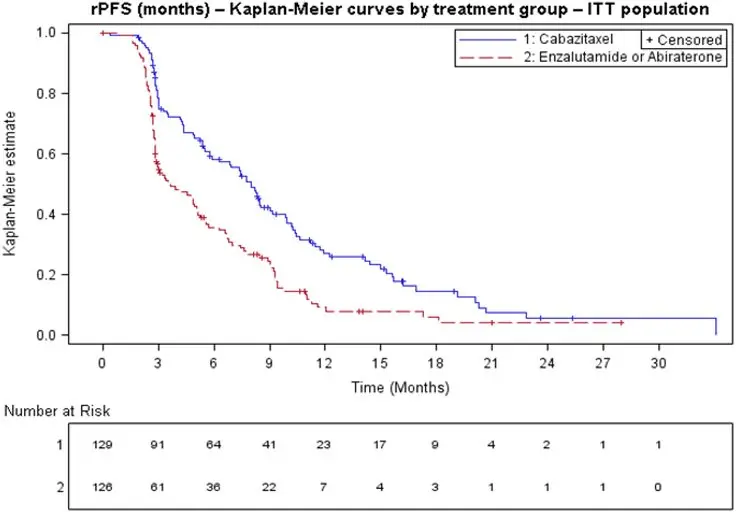
Efficacy results from the CARD trial are summarized in Table 7 and Figure 3.
| JEVTANA + prednisone/prednisolone + G-CSF n=129 | Abiraterone + prednisone/prednisolone or Enzalutamide n=126 |
|
|---|---|---|
|
||
| Radiographic Progression Free Survival (rPFS) | ||
| Number of events (%)* | 95 (73.6%) | 101 (80.2%) |
| Median rPFS (months) (95% CI) | 8.0 (5.7 to 9.2) | 3.7 (2.8 to 5.1) |
| Hazard Ratio (95% CI) | 0.54 (0.40 to 0.73) | |
| p-value† | <0.0001 | |
| Overall Survival (OS)‡ | ||
| Median OS [95% CI] (months) | 13.6 [11.5; 17.5] | 11.0 [9.2; 12.9] |
| Hazard Ratio (95% CI) | 0.64 [0.46; 0.89] | |
| p-value | 0.0078 | |
Figure 3: Kaplan-Meier of Radiographic PFS (ITT Population)
In terms of therapy sequence prior to randomization, rPFS was consistent across the subgroups of patients who received abiraterone acetate/enzalutamide prior to docetaxel (HR=0.61, 95% CI: 0.39, 0.96) and those who received abiraterone acetate/enzalutamide after docetaxel (HR=0.48, 95% CI: 0.32, 0.70).
Objective tumor response rate assessed by study investigators was 36.5% (95% CI: 26.6 to 48.4) for JEVTANA arm versus 11.5% (95% CI: 2.9 to 20.2) for abiraterone acetate plus prednisone/prednisolone or enzalutamide arm, p=0.004.
16. How is Jevtana supplied
16.1 How Supplied
JEVTANA is supplied as a kit, NDC 0024-5824-11, that contains the following:
- One single-dose vial of JEVTANA (cabazitaxel) injection: a clear yellow to brownish-yellow viscous solution of 60 mg/1.5 mL in a clear glass vial with a grey rubber closure, aluminum cap, and light green plastic flip-off cap (JEVTANA vial NDC 0024-5823-15).
- One single-dose vial of Diluent for JEVTANA: a clear colorless solution of 13% (w/w) ethanol in water for injection in a clear glass vial with a grey rubber closure, gold-color aluminum cap, and colorless plastic flip-off cap (diluent vial NDC 0024-5822-01).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| JEVTANA
cabazitaxel kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Sanofi-Aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Chimie | 291592785 | ANALYSIS(0024-5824) , API MANUFACTURE(0024-5824) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi-Aventis Deutschland GmbH | 313218430 | ANALYSIS(0024-5824) , LABEL(0024-5824) , MANUFACTURE(0024-5824) , PACK(0024-5824) | |





