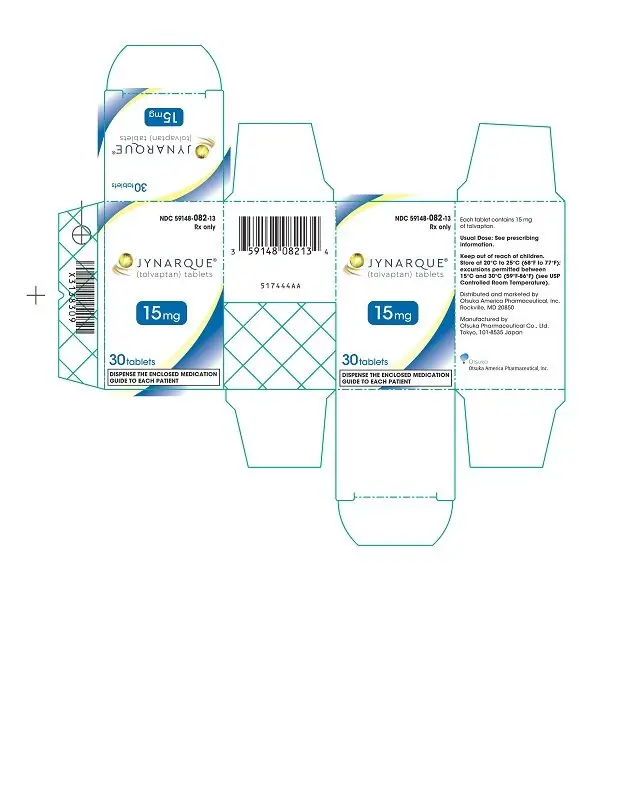
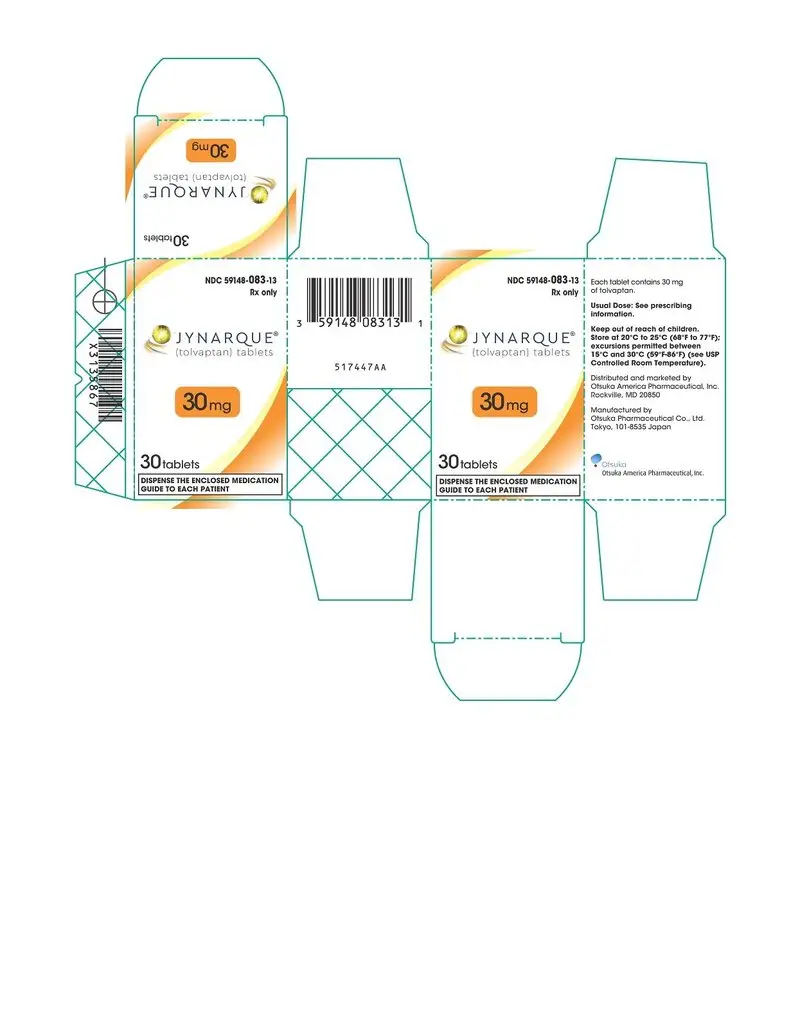
Drug Detail:Jynarque (Tolvaptan [ tol-vap-tan ])
Drug Class:
Highlights of Prescribing Information
JYNARQUE ® (tolvaptan) tablets for oral use
Initial U.S. Approval: 2009
WARNING: RISK OF SERIOUS LIVER INJURY
See full prescribing information for complete boxed warning.
- JYNARQUE (tolvaptan) can cause serious and potentially fatal liver injury. Acute liver failure requiring liver transplantation has been reported ( 5.1)
- Measure transaminases and bilirubin before initiating treatment, at 2 weeks and 4 weeks after initiation, then continuing monthly for the first 18 months and every 3 months thereafter ( 5.1)
- JYNARQUE is available only through a restricted distribution program called the JYNARQUE REMS Program ( 5.2)
Indications and Usage for Jynarque
JYNARQUE is a selective vasopressin V 2-receptor antagonist indicated to slow kidney function decline in adults at risk of rapidly progressing autosomal dominant polycystic kidney disease (ADPKD) ( 1)
Jynarque Dosage and Administration
- Recommended dosage ( 2.1)
| Initial Dosage | Titration Step | Target Dosage | |||
|---|---|---|---|---|---|
| 1st Dose | 45 mg | 1st Dose | 60 mg | 1st Dose | 90 mg |
| 2nd Dose
(8 hours later) | 15 mg | 2nd Dose
(8 hours later) | 30 mg | 2nd Dose
(8 hours later) | 30 mg |
| Total Daily Dose | 60 mg | Total Daily Dose | 90 mg | Total Daily Dose | 120 mg |
- Dose adjustment is recommended for patients taking moderate CYP 3A inhibitors ( 2.4, 5.4, 7.1)
Dosage Forms and Strengths
- Tablets: 15 mg, 30 mg, 45 mg, 60 mg and 90 mg ( 3)
Contraindications
- History of signs or symptoms of significant liver impairment or injury, does not include uncomplicated polycystic liver disease ( 4)
- Concomitant use of strong CYP 3A inhibitors is contraindicated ( 4)
- Uncorrected abnormal blood sodium concentrations ( 4, 5.3)
- Unable to sense or respond to thirst ( 4)
- Hypovolemia ( 4)
- Hypersensitivity to tolvaptan or any of its components ( 4)
- Uncorrected urinary outflow obstruction ( 4)
- Anuria ( 4)
Warnings and Precautions
- Hypernatremia, dehydration and hypovolemia: May require intervention ( 5.3)
Adverse Reactions/Side Effects
Most common observed adverse reactions with JYNARQUE (incidence >10% and at least twice that for placebo) were thirst, polyuria, nocturia, pollakiuria and polydipsia ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Otsuka America Pharmaceutical, Inc. at 1-800-438-9927 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Drug Interactions
Avoid concomitant use with:
- Strong CYP 3A Inducers ( 7.1)
- OATP1B1/3 and OAT3 Transporter Substrates ( 7.2)
- BCRP Transporter Substrates ( 7.3)
- V 2-Receptor Agonists ( 7.4)
Use In Specific Populations
- Pregnancy: May cause fetal harm ( 8.1)
- Lactation: Breastfeeding not recommended ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2018
Full Prescribing Information
WARNING: RISK OF SERIOUS LIVER INJURY
JYNARQUE (tolvaptan) can cause serious and potentially fatal liver injury. Acute liver failure requiring liver transplantation has been reported [see Warnings and Precautions (5.1)] .
Measure ALT, AST and bilirubin before initiating treatment, at 2 weeks and 4 weeks after initiation, then monthly for the first 18 months and every 3 months thereafter [see Warnings and Precautions (5.1)] . Prompt action in response to laboratory abnormalities, signs, or symptoms indicative of hepatic injury can mitigate, but not eliminate, the risk of serious hepatotoxicity.
Because of the risks of serious liver injury, JYNARQUE is available only through a restricted distribution program under a Risk Evaluation and Mitigation Strategy (REMS) called the JYNARQUE REMS Program [see Warnings and Precautions (5.2)] .
1. Indications and Usage for Jynarque
JYNARQUE is indicated to slow kidney function decline in adults at risk of rapidly progressing autosomal dominant polycystic kidney disease (ADPKD).
2. Jynarque Dosage and Administration
2.1 Recommended Dosage
The initial dosage for JYNARQUE is 60 mg orally per day as 45 mg taken on waking and 15 mg taken 8 hours later. Titrate to 60 mg plus 30 mg then to 90 mg plus 30 mg per day if tolerated with at least weekly intervals between titrations. Patients may down-titrate based on tolerability. Encourage patients to drink enough water to avoid thirst or dehydration.
2.2 Monitoring
To mitigate the risk of significant or irreversible liver injury, perform blood testing for ALT, AST and bilirubin prior to initiation of JYNARQUE, at 2 and 4 weeks after initiation, monthly for 18 months and every 3 months thereafter . Monitor for concurrent symptoms that may indicate liver injury [see Warnings and Precautions (5.1)] .
2.3 Missed Doses
If a dose of JYNARQUE is not taken at the scheduled time, take the next dose at its scheduled time.
3. Dosage Forms and Strengths
JYNARQUE (tolvaptan) is supplied as non-scored, blue, shallow-convex, immediate release tablets, debossed with "OTSUKA" and the tablet strength (mg) on one side.
JYNARQUE 15 mg tablets are triangular, 30 mg tablets are round, 45 mg tablets are square, 60 mg tablets are rectangular, and 90 mg tablets are pentagonal.
4. Contraindications
JYNARQUE is contraindicated in patients:
- With a history, signs or symptoms of significant liver impairment or injury. This contraindication does not apply to uncomplicated polycystic liver disease [see Warnings and Precautions (5.1)]
- Taking strong CYP 3A inhibitors
- With uncorrected abnormal blood sodium concentrations [see Warnings and Precautions (5.3)]
- Unable to sense or respond to thirst [see Warnings and Precautions (5.3)]
- Hypovolemia [see Warnings and Precautions (5.3)]
- Hypersensitivity (e.g., anaphylaxis, rash) to tolvaptan or any component of the product [see Adverse Reactions (6)]
- Uncorrected urinary outflow obstruction
- Anuria
5. Warnings and Precautions
5.1 Serious Liver Injury
JYNARQUE can cause serious and potentially fatal liver injury. Acute liver failure requiring liver transplantation has been reported in the post-marketing ADPKD experience. Discontinuation in response to laboratory abnormalities or signs or symptoms of liver injury (such as fatigue, anorexia, nausea, right upper abdominal discomfort, vomiting, fever, rash, pruritus, icterus, dark urine or jaundice) can reduce the risk of severe hepatotoxicity.
In a 3‑year placebo-controlled trial and its open-label extension (in which patients' liver tests were monitored every 4 months), evidence of serious hepatocellular injury (elevations of hepatic transaminases of at least 3 times ULN combined with elevated bilirubin at least 2 times the ULN) occurred in 0.2% (3/1487) of tolvaptan treated patients compared to none of the placebo treated patients.
To reduce the risk of significant or irreversible liver injury, assess ALT, AST and bilirubin prior to initiation of JYNARQUE, at 2 weeks and 4 weeks after initiation, then monthly for 18 months and every 3 months thereafter.
At the onset of signs or symptoms consistent with hepatic injury or if ALT, AST, or bilirubin increase to >2 times ULN, immediately discontinue JYNARQUE, obtain repeat tests as soon as possible (within 48-72 hours), and continue testing as appropriate. If laboratory abnormalities stabilize or resolve, JYNARQUE may be reinitiated with increased frequency of monitoring as long as ALT and AST remain below 3 times ULN.
Do not restart JYNARQUE in patients who experience signs or symptoms consistent with hepatic injury or whose ALT or AST ever exceeds 3 times ULN during treatment with tolvaptan, unless there is another explanation for liver injury and the injury has resolved.
In patients with a stable, low baseline AST or ALT, an increase above 2 times baseline, even if less than 2 times upper limit of normal, may indicate early liver injury. Such elevations may warrant treatment suspension and prompt (48-72 hours) re-evaluation of liver test trends prior to reinitiating therapy with more frequent monitoring.
5.2 JYNARQUE REMS Program
JYNARQUE is available only through a restricted distribution program under a Risk Evaluation and Mitigation Strategy (REMS) called the JYNARQUE REMS Program, because of the risks of liver injury [see Warnings and Precautions (5.1)] .
Notable requirements of the JYNARQUE REMS Program include the following:
- Prescribers must be certified by enrolling in the REMS program.
- Prescribers must inform patients receiving JYNARQUE about the risk of hepatotoxicity associated with its use and how to recognize the signs and symptoms of hepatotoxicity and the appropriate actions to take if it occurs.
- Patients must enroll in the REMS program and comply with ongoing monitoring requirements [see Warnings and Precautions (5.1)] .
- Pharmacies must be certified by enrolling in the REMS program and must only dispense to patients who are authorized to receive JYNARQUE.
Further information, including a list of qualified pharmacies/distributors, is available at www.JYNARQUEREMS.com or by telephone at 1-877-726-7220.
5.3 Hypernatremia, Dehydration and Hypovolemia
JYNARQUE increases free water clearance and, as a result, may cause dehydration, hypovolemia and hypernatremia. Therefore, ensure abnormalities in sodium concentrations are corrected prior to initiation of therapy.
Instruct patients to drink water when thirsty, and throughout the day and night if awake. Monitor for weight loss, tachycardia and hypotension because they may signal dehydration.
In the two double-blind, placebo-controlled trials of patients with ADPKD, hypernatremia (defined as any serum sodium concentration >150 mEq/L) was observed in 4.0% versus 0.6% and 1.4% versus 0% of tolvaptan-treated versus placebo-treated patients, respectively. The rate of dehydration and hypovolemia in the two studies was 2.1% versus 0.7% and 2.3% versus 0.4% for tolvaptan-treated versus placebo-treated patients, respectively.
During JYNARQUE therapy, if serum sodium increases above normal range or the patient becomes hypovolemic or dehydrated and fluid intake cannot be increased, then suspend JYNARQUE until serum sodium, hydration status and volume status is within the normal range.
5.4 Co-Administration with Inhibitors of CYP 3A
Concomitant use of JYNARQUE with drugs that are moderate or strong CYP 3A inhibitors (e.g., ketoconazole, itraconazole, lopinavir/ritonavir, indinavir/ritonavir, ritonavir, and conivaptan) increases tolvaptan exposure [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)] . Use with strong CYP 3A inhibitors is contraindicated; dose reduction of JYNARQUE is recommended for patients while taking moderate CYP 3A inhibitors [see Dosage and Administration (2.4) and Contraindications (4)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Serious Liver Injury [see Boxed Warning and Warnings and Precautions (5.1)]
- Hypernatremia, Dehydration and Hypovolemia [see Warnings and Precautions (5.3)]
- Drug Interactions with Inhibitors of CYP 3A [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. JYNARQUE has been studied in over 3000 patients with ADPKD. Long-term, placebo-controlled safety information of JYNARQUE in ADPKD is principally derived from two trials where 1,413 subjects received tolvaptan and 1,098 received placebo for at least 12 months across both studies.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of tolvaptan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or establish a causal relationship to drug exposure.
Hepatobiliary Disorders: Liver failure requiring transplant
Immune System Disorders: Anaphylaxis
7. Drug Interactions
7.2 OATP1B1/3 and OAT3 Transporter Substrates
The oxobutyric acid metabolite of tolvaptan is an inhibitor of OATP1B1/B3 and OAT3 in vitro. Patients who take JYNARQUE should avoid concomitant use with OATP1B1/B3 and OAT3 substrates (e.g., statins, bosentan, glyburide, nateglinide, repaglinide, methotrexate, furosemide), as the plasma concentrations of these substrates may be increased [see Clinical Pharmacology (12.3)] .
8. Use In Specific Populations
8.2 Lactation
Data
In lactating rats administration of radiolabeled tolvaptan, lacteal radioactivity concentrations reached the highest level at 8 hours after administration and then decreased gradually with time with a half-life of 27.3 hours. The level of activity in milk ranged from 1.5- to 15.8-fold those in blood over the period of 72 hours post-dose. In a prenatal and postnatal study in rats, maternal toxicity was noted at 100 mg/kg/day or higher (≥4.4 times the human exposure at the 90/30 mg dose). Increased perinatal death and decreased body weight of the offspring were observed during the lactation period and after weaning at approximately 17.3 times the human exposure at the 90/30 mg dose.
8.4 Pediatric Use
Safety and effectiveness of JYNARQUE in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of tolvaptan did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Use in Patients with Hepatic Impairment
Because of the risk of serious liver injury, use is contraindicated in patients with a history, signs or symptoms of significant liver impairment or injury. This contraindication does not apply to uncomplicated polycystic liver disease which was present in 60% and 66% of patients in TEMPO 3:4 and REPRISE, respectively. No specific exclusion for hepatic impairment was implemented in TEMPO 3:4. However, REPRISE excluded patients with ADPKD who had hepatic impairment or liver function abnormalities other than that expected for ADPKD with typical cystic liver disease [see Contraindications (4)].
8.7 Use in Patients with Renal Impairment
Efficacy studies included patients with normal and reduced renal function [see Clinical Studies (14)] . TEMPO 3:4 required patients to have an estimated creatinine clearance ≥60 mL/min, while REPRISE included patients with eGFR CKD-Epi 25 to 65 mL/min/1.73m 2.
10. Overdosage
Single oral doses up to 480 mg (4 times the maximum recommended daily dose) and multiple doses up to 300 mg once daily for 5 days have been well tolerated in trials in healthy subjects. There is no specific antidote for tolvaptan intoxication. The signs and symptoms of an acute overdose can be anticipated to be those of excessive pharmacologic effect: a rise in serum sodium concentration, polyuria, thirst, and dehydration/hypovolemia.
No mortality was observed in rats or dogs following single oral doses of 2000 mg/kg (maximum feasible dose). A single oral dose of 2000 mg/kg was lethal in mice, and symptoms of toxicity in affected mice included decreased locomotor activity, staggering gait, tremor and hypothermia.
In patients with suspected JYNARQUE overdosage, assessment of vital signs, electrolyte concentrations, ECG and fluid status is recommended. Continue replacement of water and electrolytes until aquaresis abates. Dialysis may not be effective in removing JYNARQUE because of its high binding affinity for human plasma protein (>98%).
11. Jynarque Description
JYNARQUE contains tolvaptan, a selective vasopressin V2-receptor antagonist in immediate release tablets for oral administration available in 15 mg, 30 mg, 45 mg, 60 mg and 90 mg strengths. Tolvaptan is (±)-4'-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1 H-1-benzazepin-1-yl) carbonyl]- o-tolu- m-toluidide. The empirical formula is C 26H 25ClN 2O 3. Molecular weight is 448.94. The chemical structure is:
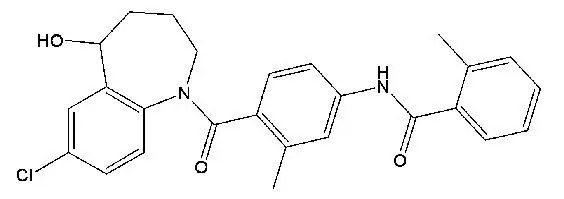
Inactive ingredients include corn starch, hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate and microcrystalline cellulose and FD&C Blue No. 2 Aluminum Lake as colorant.
12. Jynarque - Clinical Pharmacology
12.1 Mechanism of Action
Tolvaptan is a selective vasopressin V 2-receptor antagonist with an affinity for the V 2-receptor that is 1.8 times that of native arginine vasopressin (AVP). Tolvaptan affinity for the V 2-receptor is 29 times that for the V 1a-receptor. Decreased binding of vasopressin to the V 2-receptor in the kidney lowers adenylate cyclase activity resulting in a decrease in intracellular adenosine 3', 5'-cyclic monophosphate (cAMP) concentrations. Decreased cAMP concentrations prevent aquaporin 2 containing vesicles from fusing with the plasma membrane, which in turn causes an increase in urine water excretion, an increase in free water clearance (aquaresis) and a decrease in urine osmolality. In human ADPKD cyst epithelial cells, tolvaptan inhibited AVP-stimulated in vitro cyst growth and chloride-dependent fluid secretion into cysts. In animal models, decreased cAMP concentrations were associated with decreases in the rate of growth of total kidney volume and the rate of formation and enlargement of kidney cysts. Tolvaptan metabolites have no or weak antagonist activity for human V 2-receptors compared with tolvaptan.
12.2 Pharmacodynamics
In healthy subjects or patients with eGFRs as low as 10 mL/min/1.73m 2 receiving a single dose of tolvaptan, the onset of the aquaretic effects occurs within 1 to 2 hours post-dose. In healthy subjects, single doses of 60 mg and 90 mg produce a peak effect of about a 9 mL/min increase in urine excretion rate is observed between 4 and 8 hours post-dose. Higher doses of tolvaptan do not increase the peak effect in urine excretion rate but sustain the effect for a longer period of time.
Urine excretion rate returns to baseline within 24 hours following the maximum recommended 90 mg dose of tolvaptan.
Changes in free water clearance mirror the changes in urine excretion rate. Increased free water clearance causes an increase in serum sodium concentration unless fluid intake is increased to match urine output.
Increases in urine excretion rate and free water clearance are positively correlated with baseline glomerular filtration rate with increases in both values observed in patients with creatinine clearance as low as 15 mL/min.
With the recommended split-dose regimens, tolvaptan inhibits vasopressin from binding to the V 2-receptor in the kidney for the entire day, as indicated by increased urine output and decreased urine osmolality. Following a 90/30 mg split-dose regimen in patients with eGFR >60 mL/min/1.73 m 2, the change in mean daily urine volume was about 4 L for a mean total daily volume of about 7 L. In patients with eGFR <30 mL/min/1.73 m 2, the mean change in daily urine volume was about 2 L for a total daily urine volume of about 5 L.
Plasma concentrations of native AVP may increase (avg. 2-9 pg/mL) with tolvaptan treatment and return to baseline levels when treatment is stopped.
During tolvaptan treatment, small changes in renal function are expected and the changes are independent of baseline renal function. Glomerular filtration rate is decreased about 6%-10% and uric acid clearance is decreased about 20%-25%. Percent changes in renal plasma flow are highly correlated to percent changes in GFR. These changes are reversed upon discontinuation of tolvaptan.
12.3 Pharmacokinetics
In healthy subjects, the pharmacokinetics of tolvaptan after single doses of up to 480 mg and multiple doses up to 300 mg once daily have been studied. In ADPKD patients, single doses up to 120 mg and multiple split-doses up to 90/30 mg have been studied.
14. Clinical Studies
JYNARQUE was shown to slow the rate of decline in renal function in patients at risk of rapidly progressing ADPKD in two trials; TEMPO 3:4 in patients at earlier stages of disease and REPRISE in patients at later stages. The findings from these trials, when taken together, suggest that JYNARQUE slows the loss of renal function progressively through the course of the disease.
17. Patient Counseling Information
As part of patient counseling, healthcare providers must review the JYNARQUE Medication Guide with every patient [see Medication Guide].
| MEDICATION GUIDE
JYNARQUE ® (jin-AR-kew) (tolvaptan) Tablets |
|||
|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Issued: XX/201X | ||
| What is the most important information I should know about JYNARQUE?
JYNARQUE can cause serious side effects, including:
|
|||
|
| ||
| To help reduce your risk of liver problems, your healthcare provider will do a blood test to check your liver: | |||
|
|||
| It is important to stay under the care of your healthcare provider during treatment with JYNARQUE.
Because of the risk of serious liver problems JYNARQUE is only available through a restricted distribution program called the JYNARQUE Risk Evaluation and Mitigation Strategy (REMS) Program.
|
|||
| What is JYNARQUE?
JYNARQUE is a prescription medicine used to slow kidney function decline in adults who are at risk of rapidly progressing autosomal dominant polycystic kidney disease (ADPKD). It is not known if JYNARQUE is safe and effective in children. |
|||
Do not take JYNARQUE if you:
|
|||
Before taking JYNARQUE, tell your healthcare provider about all your medical conditions, including if you:
|
|||
How should I take JYNARQUE?
|
|||
| What are the possible side effects of JYNARQUE?
JYNARQUE may cause serious side effects, including: See " What is the most important information I should know about JYNARQUE?"
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
| How should I store JYNARQUE?
JYNARQUE comes in a child-resistant package. Store JYNARQUE between 68°F to 77°F (20°C to 25°C). Keep JYNARQUE and all medicines out of the reach of children. |
|||
| General information about the safe and effective use of JYNARQUE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use JYNARQUE for a condition for which it was not prescribed. Do not give JYNARQUE to other people, even if they have the same symptoms you have. It may harm them. |
|||
| What are the ingredients in JYNARQUE?
Active ingredient: tolvaptan Inactive ingredients: corn starch, hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate and microcrystalline cellulose, and FD&C Blue no. 2 Aluminum Lake as colorant. |
|||
| Manufactured by Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan
Distributed and marketed by Otsuka America Pharmaceutical, Inc., Rockville, MD 20850 USA JYNARQUE is a registered trademark of Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan ©2018, Otsuka Pharmaceutical Co., Ltd., Tokyo, 101-8535 Japan For more information about JYNARQUE, go to www. JYNARQUEREMS.com or call 1-877-726-7220. |
|||
| JYNARQUE
tolvaptan kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| JYNARQUE
tolvaptan kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| JYNARQUE
tolvaptan kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| JYNARQUE
tolvaptan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| JYNARQUE
tolvaptan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Otsuka America Pharmaceutical, Inc. (008314390) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Corporation | 002346625 | label(59148-087, 59148-088, 59148-089) , pack(59148-087, 59148-088, 59148-089) , relabel(59148-087, 59148-088, 59148-089) , repack(59148-087, 59148-088, 59148-089) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc | 069777290 | analysis(59148-087, 59148-088, 59148-089) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Packaging Coordinators, LLC | 078525133 | label(59148-087, 59148-088, 59148-089) , pack(59148-087, 59148-088, 59148-089) , repack(59148-087, 59148-088, 59148-089) , sterilize(59148-087, 59148-088, 59148-089) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boston Analytical Inc | 080408849 | analysis(59148-087, 59148-088, 59148-089) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Corporation | 143696495 | manufacture(59148-089, 59148-087, 59148-088) , pack(59148-087, 59148-088, 59148-089) , relabel(59148-087, 59148-088, 59148-089) , repack(59148-087, 59148-088, 59148-089) , label(59148-087, 59148-088, 59148-089) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Otsuka Pharmaceutical Co Ltd | 694877866 | analysis(59148-087, 59148-088, 59148-089) , manufacture(59148-087, 59148-088, 59148-089, 59148-082, 59148-083) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Otsuka Pharmaceutical Co Ltd | 695314484 | analysis(59148-087, 59148-088, 59148-089) , manufacture(59148-087, 59148-088, 59148-089) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Otsuka Pharmaceutical Co Ltd | 695733295 | analysis(59148-087, 59148-088, 59148-089) , api manufacture(59148-087, 59148-088, 59148-089) , manufacture(59148-087, 59148-088, 59148-089) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Otsuka Pharmaceutical Co Ltd | 711003178 | analysis(59148-087, 59148-088, 59148-089) , api manufacture(59148-087, 59148-088, 59148-089) | |