Drug Detail:Klisyri ointment (Tirbanibulin topical [ tir-ban-i-bue-lin-top-i-kal ])
Drug Class: Topical antineoplastics
Highlights of Prescribing Information
KLISYRI (tirbanibulin) ointment, for topical use
Initial U.S. Approval: 2020
Indications and Usage for Klisyri Ointment
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp. (1)
Klisyri Ointment Dosage and Administration
- For topical use; not for oral or ophthalmic use. (2)
- Apply KLISYRI to the treatment field on the face or scalp once daily for 5 consecutive days using 1 single-dose packet per application. (2)
Dosage Forms and Strengths
Ointment: 1% tirbanibulin, single-dose packets. (3)
Contraindications
None. (4)
Warnings and Precautions
- May cause eye irritation upon ocular exposure. Avoid transfer of the drug into the eyes and to the periocular area. If accidental exposure occurs, flush eyes with water and seek medical care. (5.1)
- Local skin reactions can occur including severe reactions (e.g., vesiculation/pustulation, erosion/ulceration) in the treated area. Avoid use until skin is healed from any previous drug or surgical treatment. (5.2)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥2%) are local skin reactions, application site pruritus, and application site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Almirall, at 1-866-665-2782 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2021
Full Prescribing Information
1. Indications and Usage for Klisyri Ointment
KLISYRI is indicated for the topical treatment of actinic keratosis on the face or scalp.
2. Klisyri Ointment Dosage and Administration
For topical use only; not for oral or ophthalmic use.
Apply sufficient amount of KLISYRI to evenly cover up to 25 cm2 treatment field on the face or scalp once daily for 5 consecutive days using 1 single-dose packet per application.
Wash hands immediately with soap and water after application.
Avoid washing and touching the treated area for approximately 8 hours after application of KLISYRI. Following this time, the area may be washed with a mild soap.
Avoid transfer of KLISYRI to the periocular area [see Warnings and Precautions (5.1)].
Avoid application near and around the mouth and lips.
3. Dosage Forms and Strengths
Ointment: 1% white to off-white ointment in a single-dose packet (2.5 mg tirbanibulin in 250 mg).
5. Warnings and Precautions
5.1 Ophthalmic Adverse Reactions
KLISYRI may cause eye irritation.
Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
5.2 Local Skin Reactions
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation and erosion/ulceration) in the treated area can occur after topical application of KLISYRI [see Adverse Reactions (6.1)]. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment. Occlusion after topical application of KLISYRI is more likely to result in irritation.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Two double-blind, vehicle-controlled clinical trials were conducted in 702 adult subjects with actinic keratosis on the face or scalp. Subjects were randomized 1:1 to KLISYRI or vehicle. Subjects enrolled in the trials had 4 to 8 clinically typical, visible, and discrete AK lesions in a contiguous area of 25 cm2 on the face or scalp. Subjects had an average age of 70 years (range 45 to 96 years) and were predominantly Caucasian (99%), male (87%), with Fitzpatrick skin types I or II (72%) and actinic keratosis on the face (68%) or scalp (32%). Treatment groups were comparable across all demographics and baseline characteristics, including AK lesion count and distribution on the face or scalp.
In the controlled trials, local skin reactions (LSRs) were collected independent of adverse events. Local skin reactions including erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, erosions/ulcerations were assessed by the investigators using a grading scale of 0 = absent, 1 = mild (slightly, barely perceptible), 2 = moderate (distinct presence), and 3 = severe (marked, intense).
The percentages of subjects with the maximal post-baseline grades for each local skin reaction greater than baseline by treatment group are provided in Table 1. LSRs were mostly mild to moderate in degree (Table 1).
| KLISYRI
N = 353 | Vehicle
N = 349 |
|||||
| Local Skin Reactions | Mild
n (%) | Moderate
n (%) | Severe
n (%) | Mild
n (%) | Moderate
n (%) | Severe
n (%) |
| Erythema | 76 (22%) | 223 (63%) | 22 (6%) | 98 (28%) | 20 (6%) | 0 |
| Flaking/ Scaling | 92 (26%) | 166 (47%) | 31 (9%) | 86 (25%) | 33 (9%) | 1 (<1%) |
| Crusting | 107 (30%) | 50 (14%) | 7 (2%) | 31 (9%) | 8 (2%) | 0 |
| Swelling | 102 (29%) | 32 (9%) | 2 (<1%) | 15 (4%) | 1 (<1%) | 0 |
| Vesiculation/ Pustulation | 25 (7%) | 2 (<1%) | 2 (<1%) | 3 (<1%) | 0 | 0 |
| Erosion/ Ulceration | 32 (9%) | 9 (3%) | 0 | 10 (3%) | 0 | 0 |
Table 2 presents the adverse reactions experienced in ≥2% of subjects participating in the controlled clinical trials with KLISYRI. No subject withdrew from the trials due to adverse reactions.
|
a Application site pain includes pain, tenderness, stinging, and burning sensation at the application site. |
||
| Adverse Reaction
System Organ Class | KLISYRI
N = 353 | Vehicle
N = 349 |
| Number of Subjects (%) with any adverse reaction (possibly related to treatment) | 56 (16%) | 35 (10%) |
| Application site pruritus | 32 (9%) | 21 (6%) |
| Application site paina | 35 (10%) | 11 (3%) |
For the 51 subjects (45 KLISYRI, 6 vehicle) who maintained complete clearance through the 12-month follow-up period, no additional local adverse reactions were reported.
Dermal Safety Studies
Clinical studies in healthy subjects demonstrated KLISYRI did not cause contact sensitization (261 subjects), phototoxic skin reactions (31 subjects), or photoallergic skin reactions (64 subjects).
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data with KLISYRI use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
In animal reproduction studies, oral administration of tirbanibulin to pregnant rats during the period of organogenesis resulted in an increased incidence of fetal deaths and malformations at a systemic exposure that was at least 74 times the exposure associated with the maximum recommended human dose (MRHD). Oral administration of tirbanibulin to pregnant rabbits during the period of organogenesis resulted in reduced mean fetal weight and size at a systemic exposure that was 159 times the exposure associated with the MRHD (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
Tirbanibulin induced fetal deaths and external, visceral, and skeletal malformations when administered orally to pregnant rats during the period of organogenesis at doses greater than or equal to 1.25 mg/kg/day, which resulted in systemic exposures at least 74 times the exposure associated with the MRHD on an Area Under the Curve (AUC) comparison basis. Tirbanibulin had no apparent effects on fetal development in rats at a dose of 0.5 mg/kg/day, which resulted in systemic exposures 18 times the exposure associated with the MRHD.
Tirbanibulin reduced mean fetal weight and size (crown-rump length) when administered orally to pregnant rabbits during the period of organogenesis at a dose of 3 mg/kg/day, which resulted in a systemic exposure 159 times the exposure associated with the MRHD on an AUC comparison basis. Tirbanibulin had no apparent effects on fetal development in rabbits at a dose of 1 mg/kg/day, which resulted in systemic exposures 53 times the exposure associated with the MRHD.
Tirbanibulin was assessed for effects on peri- and post-natal development of rats in a study that involved oral administration to pregnant rats during the period of organogenesis through lactation at dosages up to 1.25 mg/kg/day. These dosages resulted in systemic exposures up to 74 times the exposure associated with the MRHD on an AUC comparison basis. No adverse effects on maternal function or developmental, neurobehavioral, or reproductive performance of offspring were observed.
8.2 Lactation
Risk Summary
There are no data on lactational transfer of KLISYRI to human or animal milk. The effects of KLISYRI on the breastfed infant, or its effects on milk production, are unknown.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for KLISYRI and any potential adverse effects on the breastfed child from tirbanibulin or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of KLISYRI for actinic keratosis in subjects less than 18 years of age have not been established. Actinic keratosis is not a condition generally seen within the pediatric population.
8.5 Geriatric Use
Of the 353 subjects with AK treated with KLISYRI in the 2 controlled Phase 3 trials, 246 (70%) were 65 years of age or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
10. Overdosage
Overdose of KLISYRI could cause an increase in incidence and severity of local skin reactions.
11. Klisyri Ointment Description
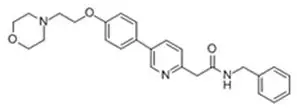
KLISYRI (tirbanibulin) ointment is a microtubule inhibitor for topical use. The chemical name of tirbanibulin is N-benzyl-2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl) acetamide. The molecular weight is 431.4 and the molecular formula is C26H29N3O3. Tirbanibulin’s structural formula is:
Tirbanibulin ointment 1% contains 10 mg tirbanibulin per gram of white to off-white ointment containing mono- and di-glycerides and propylene glycol.
12. Klisyri Ointment - Clinical Pharmacology
12.1 Mechanism of Action
Tirbanibulin is a microtubule inhibitor. The mechanism of action of KLISYRI for the topical treatment of actinic keratosis is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of tirbanibulin in the treatment of actinic keratosis is unknown.
12.3 Pharmacokinetics
Absorption
Following topical treatment of a mean daily dose of 138 mg (range: 54 to 295 mg) of KLISYRI to a 25 cm2 contiguous area of the face or balding scalp, once daily for 5 consecutive days, the steady-state concentration of tirbanibulin was achieved by 72 hours with a mean±SD trough concentration (Ctrough) of 0.11±0.08 ng/mL. On Day 5, systemic exposure to tirbanibulin was low with a mean±SD maximum plasma concentration (Cmax) of 0.34±0.30 ng/mL and 0.18±0.10 ng/mL, and a mean±SD area under the plasma concentration from time zero to 24 hours (AUC24) of 5.0±3.9 h*ng/mL and 3.2±1.9 h*ng/mL, in subjects who received the face and scalp topical treatment, respectively. The median time to reach Cmax (Tmax) was ~7 hours.
Distribution
Plasma protein binding of tirbanibulin is 88% and is independent of concentrations in the range of 0.01 to 10 µg/mL.
Elimination
Metabolism
Following topical treatment with KLISYRI to adult subjects with actinic keratosis, the plasma concentrations of KX2-5036 and KX2-5163, two pharmacologically inactive metabolites, were detectable with the highest plasma concentrations of 0.09 ng/mL and 0.12 ng/mL, respectively.
The in vitro study indicated that incubation of 1 or 10 µM tirbanibulin with human hepatocytes generated KX2-5036, KX-5163 and other unidentified metabolites.
In vitro, tirbanibulin is mainly metabolized by CYP3A4, and to a lesser extent, CYP2C8.
Excretion
Excretion of tirbanibulin has not been fully characterized in humans.
Drug Interactions
Clinical Studies
No clinical studies evaluating the drug interaction potential of KLISYRI have been conducted.
In Vitro Studies
CYP Enzymes: Tirbanibulin and the metabolite KX2-5036 directly or time-dependently inhibited CYP 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4 with an IC50 value of >17 µM. Tirbanibulin up to 1 µM (431.5 ng/mL) and the metabolite KX2-5036 up to 3 µM (1024 ng/mL) did not induce CYP 1A2, 2B6, or 3A4. These findings suggest that KLISYRI has no clinically meaningful effect on the PK of drugs metabolized by CYP 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4.
Drug Transporters: Neither tirbanibulin nor the metabolite KX2-5036 was a substrate of MDR1, BCRP, BSEP, MRP2, MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1 or OCT2. Tirbanibulin and the metabolite KX2-5036 inhibited MATE1, MATE2-K, OATP1B1, OATP1B3, OCT1 and/or OCT2 with an IC50 value of >1 µM. The results suggest that KLISYRI has no clinically meaningful effect on the PK of drugs mediated by MATE1, MATE2-K, OATP1B1, OATP1B3, OCT1 and OCT2.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to evaluate the potential of tirbanibulin to induce carcinogenesis.
Tirbanibulin was negative in an in vitro bacterial reverse mutation (Ames) assay. Tirbanibulin was positive in an in vitro chromosomal aberration assay with Chinese hamster ovary (CHO) cells, an in vitro mouse lymphoma assay with L5178/TK+/- cells, and an in vivo micronucleus assay in rats.
Tirbanibulin was assessed for effects on fertility or reproductive function in rats. Reproductive performance of rats was unaffected by oral doses of tirbanibulin up to 4 mg/kg/day (94 times the MRHD on an AUC comparison basis) in males and 1 mg/kg/day (60 times the MRHD on an AUC comparison basis) in females. However, oral administration of 4 mg/kg/day of tirbanibulin to male rats adversely affected spermatogenesis, including reduced sperm count and motility, and increased observations of morphologically abnormal sperm. No effects on sperm were observed in males treated at 2 mg/kg/day (47 times the MRHD on an AUC comparison basis).
14. Clinical Studies
Actinic Keratosis of the Face or Scalp
Two double-blind, vehicle-controlled clinical trials (NCT03285477 and NCT03285490) were conducted with 702 adult subjects with actinic keratosis on the face or scalp. Subjects were randomized 1:1 to KLISYRI or vehicle. Subjects enrolled had 4 to 8 clinically typical, visible, and discrete AK lesions in a contiguous area of 25 cm2 on the face or scalp. Subjects had an average age of 70 years (range 45 to 96 years), were predominantly Caucasian (99%), male (87%), with Fitzpatrick skin types I or II (72%) and actinic keratosis on the face (68%) or scalp (32%). Treatment groups were comparable across all demographics and baseline characteristics, including AK lesion count and distribution on the face or scalp.
Subjects received 5 consecutive days of once daily treatment with either KLISYRI (353) or vehicle control (349) to the treatment field. Subjects with complete (100%) clearance of AK lesions in the treatment area at Day 57 returned to the clinic for recurrence assessment every 3 months for a total of 12 months post-Day 57.
The primary efficacy endpoint was complete (100%) clearance of AK lesions in the treatment area, defined as the proportion of subjects at Day 57 with no clinically visible AK lesions in the treatment area and the secondary endpoint was partial (≥75%) clearance of AK lesions in the treatment area. Results from both studies are presented below.
|
a. Based on Mantel-Haenszel method |
||||||||
| Study 1 | Study 2 | |||||||
| KLISYRI
N = 175 n/N (%) | Vehicle
N = 176 n/N (%) | Treatment difference (KLISYRI-Vehicle) | 95% Confidence Interval for the Treatment difference | KLISYRI
N = 178 n/N (%) | Vehicle
N = 173 n/N (%) | Treatment difference (KLISYRI-Vehicle) | 95% Confidence Interval for the Treatment difference | |
| All subjects | 77/175
(44%) | 8/176
(5%) | 40%a | (31.6%, 47.5%)a | 97/178
(54%) | 22/173
(13%) | 42%a | (33.1%, 50.7%)a |
| Face | 60/119
(50%) | 7/121
(6%) | 45% | -- | 73/119
(61%) | 16/118
(14%) | 48% | -- |
| Scalp | 17/56
(30%) | 1/55
(2%) | 29% | -- | 24/59
(41%) | 6/55
(11%) | 30% | -- |
|
a. Based on Mantel-Haenszel method |
||||||||
| Study 1 | Study 2 | |||||||
| KLISYRI
N = 175 n/N (%) | Vehicle
N = 176 n/N (%) | Treatment difference (KLISYRI-Vehicle) | 95% Confidence Interval for the Treatment difference | KLISYRI
N = 178 n/N (%) | Vehicle
N = 173 n/N (%) | Treatment difference (KLISYRI-Vehicle) | 95% Confidence Interval for the Treatment difference | |
| All subjects | 119/175
(68%) | 29/176
(16%) | 52%a | (42.9%, 60.3%)a | 136/178
(76%) | 34/173
(20%) | 57% a | (48.3%, 65.4%)a |
| Face | 90/119
(76%) | 23/121
(19%) | 57% | -- | 95/119 (80%) | 26/118
(22%) | 58% | -- |
| Scalp | 29/56
(52%) | 6/55
(11%) | 41% | -- | 41/59
(69%) | 8/55
(15%) | 55% | -- |
Efficacy was consistent across sex and age (<65 and ≥65 years) subgroups.
Subjects who achieved 100% clearance of AK lesions in the treatment area at Day 57 continued to be followed for up to 12 months following Day 57 to determine the recurrence rate. Recurrence was defined as the proportion of subjects with any identified AK lesion (new or previous lesion) in the previously treated area who achieved 100% clearance at Day 57. Of the 174 subjects treated with KLISYRI who were followed, the recurrence rate at 12 months post Day 57 was 73%.
16. How is Klisyri Ointment supplied
KLISYRI is a white to off-white ointment and is supplied in packets containing 250 mg of tirbanibulin ointment 1%. Each packet should be discarded after single use.
NDC 16110-391-05 (5 single-dose packets)
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Discard the packet after a single use.
Ophthalmic Adverse Reactions
Advise patients that KLISYRI is not for ophthalmic use. Advise patients to avoid application around the eyes, and transfer of the drug into the eyes and to the periocular area. If accidental exposure occurs, advise patients to flush eyes with water and seek medical care [see Warnings and Precautions (5.1)].
Local Skin Reactions
Inform patients that treatment with KLISYRI may lead to local skin reactions [see Warnings and Precautions (5.3)].
Important Administration Instructions
Advise patients that KLISYRI is for topical use only. Advise patients to avoid application near and around the eyes, mouth and lips.
Instruct patients to:
- Wash hands well after applying KLISYRI to avoid transfer of the drug into the eyes and to the periocular area after application.
- Avoid washing and touching the treated area for 8 hours after treatment. Following this time, patients may wash the area with a mild soap and water.
- Avoid inadvertent transfer of KLISYRI to other areas, or to another person.
Manufactured for: Almirall, LLC
Malvern, PA. 19355, USA
Revised: 08/2021
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Issued: 08/2021 |
|
Patient Information
KLISYRI (klye si' ree)
|
|
|
Important: KLISYRI is for use on the skin only (topical). Do not use KLISYRI in, around, or near your eyes, mouth or lips. |
|
|
What is KLISYRI? KLISYRI is a prescription medicine used on the skin to treat actinic keratosis on the face or scalp. It is not known if KLISYRI is safe and effective in children less than 18 years of age. |
|
|
Before using KLISYRI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. |
|
|
How should I use KLISYRI?
|
|
|
What are the possible side effects of KLISYRI? KLISYRI may cause serious side effects, including:
The most common side effects of KLISYRI include: itching or pain in the treatment area. These are not all of the possible side effects of KLISYRI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store KLISYRI?
|
|
|
General Information about the safe and effective use of KLISYRI. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use KLISYRI for a condition for which it was not prescribed. Do not give KLISYRI to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about KLISYRI that is written for health professionals. |
|
|
What are the ingredients of KLISYRI? Active ingredient: Tirbanibulin Inactive ingredients: Mono- and di-glycerides and propylene glycol. Manufactured for: Almirall, LLC, Malvern, PA. 19355, USA. For more information, call 1-800-KLISYRI or visit www.KLISYRI.com. |
|
| KLISYRI
tirbanibulin ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Almirall, LLC (605425912) |