Drug Detail:Lantidra (Donislecel-jujn)
Drug Class:
Highlights of Prescribing Information
LANTIDRA (donislecel-jujn) Allogeneic Pancreatic Islet Cellular Suspension for hepatic portal vein infusion
Initial U.S. Approval: 2023
Indications and Usage for Lantidra
LANTIDRA is an allogeneic pancreatic islet cellular therapy indicated for the treatment of adults with Type 1 diabetes who are unable to approach target HbA1c because of current repeated episodes of severe hypoglycemia despite intensive diabetes management and education. Use in conjunction with concomitant immunosuppression. (1)
Lantidra Dosage and Administration
For infusion into the hepatic portal vein only.
- Do not irradiate.
- Do not use leukodepleting filters.
- Do not use if product time exceeds 6-hours post product release or if temperature is not maintained between 15 and 25° C
- The recommended minimum dose is 5,000 equivalent islet number (EIN) per kg patient body weight for initial infusion (transplant) and 4,500 EIN/kg for subsequent infusions (same recipient). (2.1)
- Administer cells through the hepatic portal vein (2.3). The estimated tissue volume should not exceed 10 cc per transplant infusion. (2.1)
Dosage Forms and Strengths
The dosage form is a cellular suspension. Dosage strength depends on the total number of islets packaged for infusion, which is reported on the container label and associated documents. (3)
Contraindications
LANTIDRA is contraindicated in patients for whom immunosuppression is contraindicated. (4)
Warnings and Precautions
- Risks from Concomitant Immunosuppression: Increased risk of severe infections including opportunistic infections, malignancy, and severe anemia. Monitor closely. Administer PCP and CMV prophylaxis. (5.1)
- Procedural Complications: Liver laceration and hemorrhage have occurred. Monitor for bleeding, portal hypertension, and portal vein thrombosis during and immediately following infusion. (5.2)
- Increased Risk of Graft Rejection: Patients with a positive T- and B-cell crossmatch between recipient serum and donor lymphocytes may be at increased risk for graft rejection. (5.3)
- Transmission of Donor-Derived Infections: Monitor for signs of infection following infusion and treat accordingly. (5.4)
- Panel Reactive Antibodies (PRA): Product administration may elevate PRA and negatively impact candidacy for renal transplant. (5.5)
Adverse Reactions/Side Effects
Ninety percent (90%) of subjects had at least one serious adverse reaction. (6.1) The major causes are attributed to:
- Infusion procedure
- liver laceration/hematoma, hemorrhage, and intra-abdominal bleeding (13%)
- elevation of portal pressure (7%)
- Immunosuppression
- Infection (87%)
- Malignancy (37%)
To report SUSPECTED ADVERSE REACTIONS, contact CellTrans at 1-800-500-1617 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
Related/similar drugs
Lantus, Levemir, Tresiba, Basaglar, Novolog, ToujeoFull Prescribing Information
1. Indications and Usage for Lantidra
LANTIDRA is an allogeneic pancreatic islet cellular therapy indicated for the treatment of adults with Type 1 diabetes who are unable to approach target HbA1c because of current repeated episodes of severe hypoglycemia despite intensive diabetes management and education. Use LANTIDRA in conjunction with concomitant immunosuppression.
2. Lantidra Dosage and Administration
For infusion into the hepatic portal vein only.
2.1 Dose
The recommended minimum dose is 5,000 EIN/kg for initial infusion and 4,500 EIN/kg for subsequent infusion in the same recipient. The maximum dose per infusion is dictated by the estimated tissue volume, which should not exceed 10 cc per infusion, and the total EIN present in the infusion bag (up to a maximum of 1 × 106 EIN per bag).
A second infusion may be performed if the patient does not achieve independence from exogenous insulin within one year of infusion or within one year after losing independence from exogenous insulin after a previous infusion. A third infusion may be performed using the same criteria as for the second infusion. There are no data regarding the effectiveness or safety for patients receiving more than three infusions.
2.2 Preparation
- Keep LANTIDRA in the insulated container at 15°C to 25°C no longer than 6 hours from time of product release (See carton label and certificate of analysis). Dispose of any product not used within 6 hours.
- Do not irradiate.
- Select and prepare units under the direction of a medical professional who is experienced in islet infusion (transplantation).
- Use LANTIDRA as supplied and without further dilution.
2.3 Administration
Failure to follow these directions may result in damage and decreased viability of the islets.
Do not administer with leukodepleting filters.
- To optimize viability, administer LANTIDRA as soon as possible after product release.
- Interventional radiologists and surgeons with expertise in islet cell infusion may administer LANTIDRA in an interventional radiology suite or operating suite under controlled aseptic conditions.
- Perform all steps aseptically.
- Use a 5 or 6 French angiographic catheter indicated for the delivery of drugs or other therapeutic fluids for infusion of LANTIDRA.
- Catheter length: 65 cm or less.
- Internal diameter: 0.97mm (0.038 inches) or greater.
- Use only sheaths and introducers in combination with a catheter with the specified dimensions listed above to deliver LANTIDRA.
3. Dosage Forms and Strengths
LANTIDRA is a cellular suspension of allogeneic pancreatic islets (islets of Langerhans) in buffered transplant media containing sodium chloride, dextrose, minerals, amino acids, vitamins, and other compounds supplemented with HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid; 10 mM final concentration) and human serum albumin (0.5% final concentration).
Each infusion uses one lot of LANTIDRA which consists of islets manufactured from the pancreas of a single deceased donor. Each dose of LANTIDRA is provided as two (2) infusion bags connected to each other via sterile connector. One bag contains LANTIDRA up to a maximum of 1 × 106 EIN in 400 ml of transplant media and the second bag (Rinse Bag) contains transplant media used to rinse the LANTIDRA bag and the infusion line.
The dosage strength is represented by the total EIN in a single preparation and varies between product batches. Dosage strength information for an individual batch is provided on the container label and in accompanying documentation (Final Islet Product Certificate of Analysis).
4. Contraindications
Do not administer LANTIDRA to patients who have concomitant diseases or conditions, including pregnancy, that contraindicate the procedure for LANTIDRA infusion or immunosuppression.
5. Warnings and Precautions
5.1 Risks from Concomitant Immunosuppression
Concomitant use of immunosuppression is required to maintain islet cell viability. The use of immunosuppression in patients receiving LANTIDRA increases the risk of serious and potentially fatal adverse reactions. [Adverse Reactions (6.1)]
Patients receiving immunosuppressants are at increased risk of:
- Bacterial, viral, fungal, and parasitic infections, including opportunistic infections.
- Lymphomas and other malignancies, particularly of the skin.
- Severe anemia, sometimes requiring transfusion.
5.2 Procedural Complications
Liver laceration, hemorrhage and intra-abdominal bleeding have occurred with portal administration of LANTIDRA. Manage hemostasis in the catheter track using standard practices following infusion of LANTIDRA to reduce the risk of bleeding. Monitor for bleeding clinically and with laboratory assessments. Blood transfusions have been required.
Elevation in portal blood pressure has occurred during and following intraportal islet infusion [Adverse Reactions (6.1)]. Monitor portal pressure; pause infusion if portal pressure rises above 22 mmHg and do not resume until it falls below 18 mmHg. Terminate infusion if portal pressure remains above 22 mmHg for longer than 10 minutes. [Dosage and Administration (2.3)]
Portal vein branch thrombosis may occur following infusion of LANTIDRA. Repeated intraportal islet infusions are not recommended in patients who have experienced prior portal thrombosis unless the thrombosis was limited to second- or third-order portal vein branches. [Limitations of Use (2.1)]
5.3 Increased Risk of Islet Graft Rejection
Patients with a positive T- and B-cell crossmatch between recipient serum and donor lymphocytes may immediately reject the islet cells. The T- and B-cell crossmatch assay is binary. T- and B-cell both need to be negative.
6. Adverse Reactions/Side Effects
Ninety percent (90%) of subjects had at least one serious adverse reaction. The major causes were attributed to:
- Infusion procedure
- liver laceration/hematoma, hemorrhage, and intra-abdominal bleeding (13%)
- elevation of portal pressure (7%)
- Immunosuppression
- Infection (87%)
- Malignancy (37%)
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of LANTIDRA in subjects with type 1 diabetes and hypoglycemic unawareness was demonstrated in two clinical trials (Study 1, Study 2) involving a total of 30 subjects who received between one and three doses of LANTIDRA. Duration between first and second transplant was one month to 2.8 years and between second and third dose from 3 months to 7.8 years (See Figure 1). Because of the variable duration of follow-up, number of infusions, and interval between infusions, adverse reactions were reported for the total duration for which each subject was followed. [Clinical Studies (14)] Subjects were followed for 0.3 to 14.5 years (mean 3 ± 3.7 years) after the first infusion.
Serious reactions were reported in 27 (90%) of subjects. There were two (7%) deaths; one death from multi-organ failure with sepsis (1.6 years after the first infusion), and one from progressive confusion, global atrophy and micro-ischemic disease (9.7 years after the first infusion). Both subjects were using immunosuppression at the time of the event. Additionally, 8 (27%) subjects experienced at least one life-threatening adverse reaction and 26 (87%) subjects experienced at least one severe reaction before their last follow-up.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of LANTIDRA have not been established in pediatric patients with type 1 diabetes.
8.5 Geriatric Use
The safety and effectiveness of LANTIDRA have not been established in geriatric patients with type 1 diabetes and hypoglycemic unawareness. Clinical studies of LANTIDRA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
11. Lantidra Description
LANTIDRA consists of a suspension of allogeneic pancreatic islets in buffered transplant medium containing sodium chloride, dextrose, minerals, amino acids, vitamins, and other compounds supplemented with HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid; 10 mM final concentration) and human serum albumin (0.5% final concentration).
The active ingredient in LANTIDRA is allogeneic islets of Langerhans derived from a donor pancreas. Islets contain several types of endocrine (hormone-secreting) cells, including β-, α-, pancreatic peptide- (PP-), δ-, and ε-cells.
Each single-donor islet batch consists of two infusion bags connected to each other via a sterile connector. One LANTIDRA bag containing up to a maximum of 1 × 106 EIN in 400 ml of transplant media, and the second Rinse Bag containing 200 ml transplant media used to rinse the LANTIDRA bag and the infusion line.
12. Lantidra - Clinical Pharmacology
12.1 Mechanism of Action
Pancreatic islets regulate blood glucose levels through secretion of multiple hormones in response to increases and decreases in blood glucose. Endocrine cells within pancreatic islets release insulin, glucagon, somatostatin, pancreatic peptide, and ghrelin. Insulin stimulates glucose uptake by peripheral tissues; glucagon mobilizes glucose from the liver into circulation; somatostatin inhibits both α- and β-cell secretions; pancreatic peptide inhibits pancreatic exocrine secretion; and ghrelin inhibits insulin secretion. The primary mechanism of action of LANTIDRA is believed to be secretion of insulin by infused (transplanted) β- cells.
12.2 Pharmacodynamics
The pharmacodynamic effects of LANTIDRA are a result of hormones, especially insulin, that are secreted by the infused (transplanted) islets in response to fluctuations in blood glucose levels.
Basal and stimulated blood glucose were determined at baseline and at 1 year following a subject's last transplant during Study 1 and Study 2 using a mixed meal tolerance test (MMTT). Combined results from these studies are summarized in Table 2. [Clinical Studies (14)]
The pharmacodynamic profile of the allogeneic islet cells is most clearly demonstrated in subjects who are free from the requirement of exogenous insulin.
| Subjects Insulin Independent at time of 1-year MMT | N | Mean (mg/dl) | Std Dev (mg/dl) | Min (mg/dl) | Max (mg/dl) |
|---|---|---|---|---|---|
| Baseline Glucose Basal | 19 | 178 | 76 | 78 | 348 |
| Baseline Glucose 90-min | 19 | 357 | 91 | 122 | 559 |
| 1-year Glucose Basal | 19 | 106 | 17 | 81 | 144 |
| 1-year Glucose 90-min | 19 | 142 | 40 | 65 | 202 |
14. Clinical Studies
The effectiveness of LANTIDRA in subjects with type 1 diabetes and hypoglycemic unawareness was demonstrated in 2 clinical trials (Study 1, Study 2) involving a combined 30 subjects, all of whom received at least one islet infusion and a maximum of 3 infusions. Both trials were prospective, open-label, single-arm studies.
Subject demographics: median age 46.5 (range: 21 – 67) years, 80% female, 100% white, 97% non-Hispanic.
Subjects received a median islet number of 399,178 EIN (range 253,924 EIN to 858,856 EIN) per infusion. Subjects received a median islet dose of 6,570 EIN/kg (range 4,186 EIN/kg to 13,633 EIN/kg) per infusion. Thirty subjects participated in the combined Study 1 and Study 2, with 11 subjects receiving one infusion, 12 subjects receiving two infusions, and 7 subjects receiving three infusions. Of the 19 subjects who received a second infusion, 6 were insulin-independent at the time of their second infusion. Of the 11 subjects who did not receive a second infusion, 4 were insulin-independent, 3 did not have a donor, and 4 were intolerant to immunosuppression or withdrew from the study within 6 months. All 7 subjects who received a third infusion were insulin-dependent. One subject was not able to get a third infusion because of infection. No subject was unable to receive a third infusion because of lack of a donor or intolerance to immunosuppression.
Concomitant study medications were provided as described in Table 3:
| Medication | Study 1 (N=10) | Study 2 (N=20) |
|---|---|---|
| Anakinra; n (%) | 1 (10%) | 0 (0%) |
| Daclizumab; n (%) | 10 (100%) | 5 (24%) |
| Basiliximab; n (%) | 5 (10%) | 19 (95%) |
| Mycophenolate mofetil; n (%) | 6 (60%) | 5 (24%) |
| Etanercept; n (%) | 6 (60%) | 20 (100%) |
| Everolimus; n (%) | 1 (10%) | 2 (10%) |
| Sirolimus; n (%) | 10 (100%) | 20 (100%) |
| Tacrolimus; n (%) | 10 (100%) | 20 (100%) |
| Cyclosporine; n (%) | 1 (10%) | 3 (15%) |
| Anti-thymocyte immunoglobulin; n (%) | 1 (10%) | 4 (20%) |
A glucagon-like peptide-1 (GLP-1) agonist (e.g., exenatide 5 mcg subcutaneously within 60 minutes before infusion), was administered and was supposed to be continued (5 mcg BID), for up to 6 months after transplant. Exenatide was not given to the first 4 subjects in Study 1, and 11 of the remaining 26 subjects used exenatide less than the per protocol 6-months post-transplant because of adverse reactions. Because of the variability of exenatide use in the clinical studies, there are insufficient data to support exenatide use in patients receiving LANTIDRA.
Insulin independence, defined as not requiring exogenous insulin to achieve adequate glycemic control, was also determined. Results are summarized in Table 4.
| Total Duration Insulin Independent (years) | N | Mean | Std Dev | Min | Max |
|---|---|---|---|---|---|
| Study 1 | 10 | 5.1 | 4.2 | 0.2 | 12.8 |
| Study 2 | 20 | 3.2 | 3.1 | 0 | 9.9 |
Five subjects had no days of insulin independence. For the 25 subjects who achieved insulin independence, 4 subjects (13.3%) were insulin independent for less than one year, 12 subjects (36.7%) for 1 to 5 years, and 9 subjects (33.3%) for greater than 5 years. Figure 1 shows the entire experience of the individual subjects.
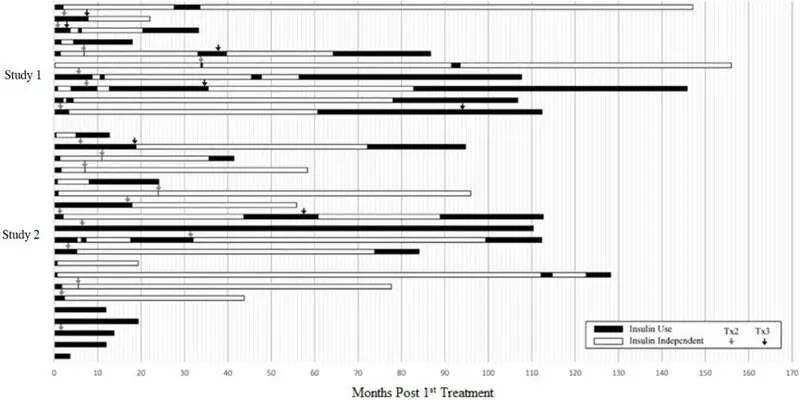
This figure shows the total duration of follow-up for each subject. The period of insulin dependence (use) is denoted in black and the period of insulin independence in white. Time zero (0) is the time of the first infusion. The arrows denote the time of second and third infusions.
16. How is Lantidra supplied
LANTIDRA (NDC 73539-001-01) is supplied as purified allogeneic islets of Langerhans suspended in buffered transplant medium containing sodium chloride, dextrose, minerals, amino acids, vitamins, and other compounds supplemented with HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid; 10 mM final concentration) and human serum albumin (0.5% final concentration)). [Description (11)].
LANTIDRA is contained in one 1000 mL infusion bag filled with a supplied volume of 400 mL, containing not more than 10 cc of estimated packed islet tissue and not more than 1 × 106 EIN. The 1000 mL infusion bag is aseptically connected to a smaller 750 mL Rinse Bag (NDC 73539-002-01) containing 200 mL of supplied volume of transplant media for use in rinsing the 1000 mL bag containing LANTIDRA and infusion line following infusion to assure complete transfer of islets to the patient. Additional product information, including islet number, is included on the Final Islet Product Certificate of Analysis and the container label.
| LANTIDRA
donislecel solution |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CellTrans Inc. (080398821) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CellTrans Inc. | 117774787 | API MANUFACTURE(73539-001) | |