Drug Detail:Lemtrada (Alemtuzumab [ al-em-tooz-ue-mab ])
Drug Class: CD52 monoclonal antibodies
Highlights of Prescribing Information
LEMTRADA® (alemtuzumab) injection, for intravenous use
Initial U.S. Approval: 2001
WARNING: AUTOIMMUNITY, INFUSION REACTIONS, STROKE, AND MALIGNANCIES
See full prescribing information for complete boxed warning.
- LEMTRADA causes serious, sometimes fatal, autoimmune conditions such as immune thrombocytopenia and anti-glomerular basement membrane disease. Monitor complete blood counts with differential, serum creatinine levels, and urinalysis with urine cell counts monthly until 48 months after the last dose. (5.1)
- LEMTRADA causes serious and life-threatening infusion reactions. LEMTRADA must be administered in a setting with appropriate equipment and personnel to manage anaphylaxis or serious infusion reactions. Monitor patients for two hours after each infusion. Make patients aware that serious infusion reactions can also occur after the 2-hour monitoring period. (5.2)
- Serious and life-threatening stroke has been reported within 3 days of LEMTRADA administration. Instruct patients to seek immediate medical attention if symptoms of stroke occur. (5.3)
- LEMTRADA may cause an increased risk of malignancies, including thyroid cancer, melanoma, and lymphoproliferative disorders. Perform baseline and yearly skin exams. (5.4)
- LEMTRADA is available only through a restricted distribution program. (5.5)
Indications and Usage for Lemtrada
- LEMTRADA is a CD52-directed cytolytic monoclonal antibody indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, the use of LEMTRADA should generally be reserved for patients who have had an inadequate response to two or more drugs indicated for the treatment of MS [see Warnings and Precautions (5)]. (1)
Limitations of Use:
LEMTRADA is not recommended for use in patients with clinically isolated syndrome (CIS) because of its safety profile [see Warnings and Precautions (5)]. (1)
Lemtrada Dosage and Administration
- Baseline laboratory tests are required prior to treatment. (2.1)
- Administer LEMTRADA by intravenous infusion over 4 hours for 2 or more treatment courses:
Initial treatment of 2 courses:- First course: 12 mg/day on 5 consecutive days. (2.3)
- Second course: 12 mg/day on 3 consecutive days 12 months after first treatment course. (2.3)
- Premedicate with corticosteroids prior to LEMTRADA infusion for the first 3 days of each treatment course. (2.2)
- Administer antiviral agents for herpetic prophylaxis starting on the first day of LEMTRADA dosing and continuing for a minimum of two months after completion of LEMTRADA dosing or until CD4+ lymphocyte count is more than 200 cells per microliter, whichever occurs later. (2.2)
- Must be diluted prior to administration. (2.4)
Dosage Forms and Strengths
Injection: 12 mg/1.2 mL (10 mg/mL) in a single-dose vial. (3)
Contraindications
- Known hypersensitivity or anaphylactic reactions to alemtuzumab or any of the excipients in LEMTRADA (4)
- Infection with Human Immunodeficiency Virus (4)
- Active infection (4)
Warnings and Precautions
- Immune Thrombocytopenia: Obtain complete blood counts (CBCs) with differential prior to initiation of treatment and at monthly intervals thereafter until 48 months after the last infusion. (5.6)
- Glomerular Nephropathies: Obtain serum creatinine levels, urinalysis with cell counts and urine protein to creatinine ratio prior to initiation of treatment. Monitor serum creatinine levels and urinalysis with cell counts at monthly intervals thereafter until 48 months after the last infusion. (5.7)
- Thyroid Disorders: Obtain thyroid function tests prior to initiation of treatment and every 3 months until 48 months after the last infusion. (5.8)
- Other Autoimmune Cytopenias: Monitor CBCs monthly until 48 months after the last infusion. (2.6, 5.9)
- Autoimmune Hepatitis: If signs of hepatic dysfunction occur, promptly measure serum transaminases and total bilirubin and interrupt or discontinue treatment. (5.10)
- Hemophagocytic Lymphohistiocytosis: Consider this diagnosis and evaluate patients immediately if they develop signs or symptoms of systemic inflammation. Discontinue LEMTRADA if an alternative etiology is not established. (5.11)
- Adult Onset Still's Disease (AOSD): If a patient develops AOSD, they require prompt evaluation and treatment. (5.12)
- Thrombotic Thrombocytopenic Purpura (TTP): Evaluate patients immediately if they develop clinical symptoms or laboratory findings consistent with TTP. Discontinue LEMTRADA if TTP is confirmed or if an alternative etiology is not established. (5.13)
- Autoimmune Encephalitis (AIE): Evaluate patients if they develop signs and symptoms suggestive of AIE, such as subacute onset of memory impairment, altered mental status, psychiatric symptoms, neurological findings, and seizures. (5.14)
- Acquired Hemophilia A: Obtain a coagulopathy panel including aPTT in patients who present with signs such as spontaneous subcutaneous hematomas, extensive bruising, hematuria, epistaxis, or gastrointestinal or other types of bleeding. (5.15)
- Infections: Administration is contraindicated in patients with active infection. Do not administer live viral vaccines following a course of LEMTRADA. (4, 5.16)
- Progressive Multifocal Leukoencephalopathy (PML): Withhold LEMTRADA at the first sign or symptom suggestive of PML. (5.17)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥10% and > interferon beta-1a): rash, headache, pyrexia, nasopharyngitis, nausea, urinary tract infection, fatigue, insomnia, upper respiratory tract infection, herpes viral infection, urticaria, pruritus, thyroid gland disorders, fungal infection, arthralgia, pain in extremity, back pain, diarrhea, sinusitis, oropharyngeal pain, paresthesia, dizziness, abdominal pain, flushing, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-800-745-4447, option 2 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pregnancy: May cause fetal harm. (8.1)
Women of childbearing potential should use effective contraception during and for 4 months after a course of treatment with LEMTRADA. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2023
Full Prescribing Information
WARNING: AUTOIMMUNITY, INFUSION REACTIONS, STROKE, AND MALIGNANCIES
- LEMTRADA causes serious, sometimes fatal, autoimmune conditions such as immune thrombocytopenia and anti-glomerular basement membrane disease. Monitor complete blood counts with differential, serum creatinine levels, and urinalysis with urine cell counts before starting treatment and then at monthly intervals until 48 months after the last dose of LEMTRADA [see Warnings and Precautions (5.1)].
- LEMTRADA causes serious and life-threatening infusion reactions. LEMTRADA must be administered in a setting with appropriate equipment and personnel to manage anaphylaxis or serious infusion reactions. Monitor patients for two hours after each infusion. Make patients aware that serious infusion reactions can also occur after the 2-hour monitoring period [see Warnings and Precautions (5.2)].
- Serious and life-threatening stroke (including ischemic and hemorrhagic stroke) has been reported within 3 days of LEMTRADA administration. Instruct patients to seek immediate medical attention if symptoms of stroke occur [see Warnings and Precautions (5.3)].
- LEMTRADA may cause an increased risk of malignancies, including thyroid cancer, melanoma, and lymphoproliferative disorders. Perform baseline and yearly skin exams [see Warnings and Precautions (5.4)].
- Because of the risk of autoimmunity, infusion reactions, and malignancies, LEMTRADA is available only through restricted distribution under a Risk Evaluation Mitigation Strategy (REMS) Program. Call 1-855-676-6326 to enroll in the LEMTRADA REMS program [see Warnings and Precautions (5.5)].
1. Indications and Usage for Lemtrada
LEMTRADA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, the use of LEMTRADA should generally be reserved for patients who have had an inadequate response to two or more drugs indicated for the treatment of MS [see Warnings and Precautions (5)].
2. Lemtrada Dosage and Administration
2.1 Testing and Procedures Prior to Treatment
Baseline laboratory tests are required prior to treatment with LEMTRADA [see Dosage and Administration (2.6)]. In addition, prior to starting treatment with LEMTRADA [see Warnings and Precautions (5.15)]:
- complete any necessary immunizations at least 6 weeks prior to treatment.
- determine whether patients have a history of varicella or have been vaccinated for varicella zoster virus (VZV). If not, test the patient for antibodies to VZV and consider vaccination for those who are antibody-negative. Postpone treatment with LEMTRADA until 6 weeks after VZV vaccination.
- perform tuberculosis screening according to local guidelines.
- instruct patients to avoid potential sources of Listeria monocytogenes.
2.3 Recommended Dosage
- The recommended dosage of LEMTRADA is 12 mg/day administered by intravenous infusion for 2 treatment courses: First Treatment Course: 12 mg/day on 5 consecutive days (60 mg total dose).
- Second Treatment Course: 12 mg/day on 3 consecutive days (36 mg total dose) administered 12 months after the first treatment course.
Following the second treatment course, subsequent treatment courses of 12 mg per day on 3 consecutive days (36 mg total dose) may be administered, as needed, at least 12 months after the last dose of any prior treatment courses.
2.4 Preparation Instructions
Follow the steps below to prepare the diluted solution of LEMTRADA for intravenous infusion:
- Inspect LEMTRADA visually for particulate matter and discoloration prior to administration. Do not use if particulate matter is present or the solution is discolored. Do not freeze or shake vials prior to use.
- Withdraw 1.2 mL of LEMTRADA from the vial into a syringe using aseptic technique and inject into a 100 mL bag of sterile 0.9% Sodium Chloride, USP or 5% Dextrose in Water, USP.
- Gently invert the bag to mix the solution. Ensure the sterility of the prepared solution because it contains no antimicrobial preservatives. Each vial is for single use only.
Prior to administration, protect diluted LEMTRADA solution from light and store for as long as 8 hours either at room temperature 15°C to 25°C (59°F to 77°F) or keep refrigerated at conditions 2°C to 8°C (36°F to 46°F).
2.5 Infusion Instructions
Infuse LEMTRADA over 4 hours starting within 8 hours after dilution. Extend the duration of the infusion if clinically indicated.
Administer LEMTRADA in a setting in which equipment and personnel to appropriately manage anaphylaxis, serious infusion reactions, myocardial ischemia, myocardial infarction, and cerebrovascular and respiratory adverse reactions are available [see Warnings and Precautions (5.2)].
Do not add or simultaneously infuse other drug substances through the same intravenous line. Do not administer as an intravenous push or bolus.
Obtain a baseline ECG. Monitor vital signs before the infusion and periodically during the infusion. Provide appropriate symptomatic treatment for infusion reactions as needed. Consider immediate discontinuation of the intravenous infusion if severe infusion reactions occur.
Observe patients for infusion reactions during and for at least 2 hours after each LEMTRADA infusion. Consider longer periods of observation if clinically indicated. Inform patients that they should report symptoms that occur during and after each infusion because they may indicate a need for prompt medical intervention [see Warnings and Precautions (5.2)].
2.6 Laboratory Testing and Monitoring to Assess Safety
Measure the urine protein to creatinine ratio prior to initiation of treatment. Conduct the following laboratory tests at baseline and at periodic intervals until 48 months after the last treatment course of LEMTRADA in order to monitor for early signs of potentially serious adverse effects:
- Complete blood count (CBC) with differential (prior to treatment initiation and at monthly intervals thereafter)
- Serum creatinine levels (prior to treatment initiation and at monthly intervals thereafter)
- Urinalysis with urine cell counts (prior to treatment initiation and at monthly intervals thereafter)
- A test of thyroid function, such as thyroid stimulating hormone (TSH) level (prior to treatment initiation and every 3 months thereafter)
- Serum transaminases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) and total bilirubin levels (prior to treatment initiation and periodically thereafter)
Conduct baseline and yearly skin exams to monitor for melanoma [see Warnings and Precautions (5.4)].
3. Dosage Forms and Strengths
Injection: 12 mg/1.2 mL (10 mg/mL) in a single-dose vial. LEMTRADA is a clear and colorless to slightly yellow solution that requires dilution prior to intravenous infusion.
4. Contraindications
LEMTRADA is contraindicated in patients:
- with known hypersensitivity or anaphylactic reactions to alemtuzumab or any of the excipients in LEMTRADA
- who are infected with human immunodeficiency virus (HIV) because LEMTRADA causes prolonged reductions of CD4+ lymphocyte counts
- with active infection
5. Warnings and Precautions
5.1 Autoimmunity
Treatment with LEMTRADA can result in the formation of autoantibodies and increase the risk of serious autoimmune mediated conditions, which may be life threatening.
In clinical studies (controlled and open-label extension), LEMTRADA-treated patients experienced thyroid disorders (36.8%), immune thrombocytopenia (2%), and glomerular nephropathies (0.3%) [see Warnings and Precautions (5.6, 5.7, 5.8)]. Vitiligo and autoimmune hemolytic anemia occurred in 0.3% of patients. Autoimmune pancytopenia [see Warnings and Precautions (5.9)], undifferentiated connective tissue disorders, and type 1 diabetes each occurred in 0.2% of patients. Rheumatoid arthritis, retinal pigment epitheliopathy, and acquired hemophilia A (anti-Factor VIII antibodies) [see Warnings and Precautions (5.15)] occurred in 0.1% of patients. During postmarketing use, cases of vasculitis, autoimmune hepatitis [see Warnings and Precautions (5.10)], Guillain-Barré syndrome [see Adverse Reactions (6.5)], thrombotic thrombocytopenic purpura [see Warnings and Precautions (5.13)], and autoimmune encephalitis [see Warnings and Precautions (5.14)] have been reported.
Chronic inflammatory demyelinating polyradiculoneuropathy has been reported in the treatment of patients with B-cell chronic lymphocytic leukemia (B-CLL), as well as other autoimmune disorders, generally at higher and more frequent doses than recommended in MS. An oncology patient treated with alemtuzumab had fatal transfusion-associated graft-versus-host disease.
Autoantibodies may be transferred from the mother to the fetus during pregnancy. A case of transplacental transfer of anti-thyrotropin receptor antibodies resulting in neonatal Graves' disease occurred after alemtuzumab treatment in the mother [see Use in Specific Populations (8.1)].
LEMTRADA may increase the risk of other autoimmune conditions because of the broad range of autoantibody formation with LEMTRADA.
Measure the urine protein to creatinine ratio prior to initiation of treatment. Monitor complete blood counts with differential, serum creatinine levels, and urinalysis with urine cell counts before starting treatment and then at monthly intervals until 48 months after the last dose of LEMTRADA to allow for early detection and treatment of autoimmune adverse reactions [see Dosage and Administration (2.6)]. After 48 months, testing should be performed based on clinical findings suggestive of autoimmunity.
LEMTRADA is available only through a restricted program under a REMS [see Warnings and Precautions (5.5)].
5.2 Infusion Reactions
LEMTRADA causes cytokine release syndrome resulting in infusion reactions, some of which may be serious and life threatening. In clinical studies, 92% of LEMTRADA-treated patients experienced infusion reactions. In some patients, infusion reactions were reported more than 24 hours after LEMTRADA infusion. Serious reactions occurred in 3% of patients and included anaphylaxis in 2 patients (including anaphylactic shock), angioedema, bronchospasm, hypotension, chest pain, bradycardia, tachycardia (including atrial fibrillation), transient neurologic symptoms, hypertension, headache, pyrexia, and rash. Other infusion reactions included nausea, urticaria, pruritus, insomnia, chills, flushing, fatigue, dyspnea, pulmonary infiltrates, dysgeusia, dyspepsia, dizziness, and pain. In clinical studies, 0.6% of patients with infusion reactions received epinephrine or atropine.
During postmarketing use, cases of pulmonary alveolar hemorrhage, myocardial ischemia, myocardial infarction, stroke (including ischemic and hemorrhagic stroke), and cervicocephalic (e.g., vertebral, carotid) arterial dissection have been reported. Reactions may occur following any of the doses during the treatment course. In the majority of cases, time to onset was within 1 to 3 days of LEMTRADA infusion. Patients should be informed about the signs and symptoms and advised to seek immediate medical attention if any of these symptoms occur. Cases of severe (including fatal) neutropenia have been reported within 2 months of LEMTRADA infusion; some cases resolved with receiving granulocyte-colony stimulating factor treatment. Mild to moderate decreases in platelet counts, starting at the time of alemtuzumab infusion and often resolving without treatment, have been reported. Other serious and sometimes fatal infusion reactions (e.g., hypoxia, syncope, acute respiratory distress syndrome, respiratory arrest, myocardial infarction, acute cardiac insufficiency, cardiac arrest) have been reported in the treatment of patients with B-CLL, as well as other disorders, generally at higher and more frequent doses than recommended in MS.
Premedicate patients with corticosteroids immediately prior to LEMTRADA infusion for the first 3 days of each treatment course. In clinical studies, patients received 1,000 mg of methylprednisolone for the first 3 days of each LEMTRADA treatment course. Consider pretreatment with antihistamines and/or antipyretics prior to LEMTRADA administration. Infusion reactions may occur despite pretreatment.
Consider additional monitoring in patients with medical conditions which predispose them to cardiovascular or pulmonary compromise. Physicians should alert patients that an infusion reaction could occur within 48 hours of infusion.
LEMTRADA can only be administered in certified healthcare settings that have on-site access to equipment and personnel trained to manage infusion reactions (including anaphylaxis, cerebrovascular, cardiac and respiratory emergencies) [see Dosage and Administration (2.5)].
LEMTRADA is available only through a restricted program under a REMS [see Warnings and Precautions (5.5)].
5.5 LEMTRADA REMS Program
LEMTRADA is available only through a restricted program under a REMS called the LEMTRADA REMS Program because of the risks of autoimmunity, infusion reactions, and malignancies [see Warnings and Precautions (5.1, 5.2, 5.4)].
Notable requirements of the LEMTRADA REMS Program include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- Patients must enroll in the program and comply with ongoing monitoring requirements [see Dosage and Administration (2.6)].
- Pharmacies must be certified with the program and must only dispense to certified healthcare facilities that are authorized to receive LEMTRADA.
- Healthcare facilities must enroll in the program and verify that patients are authorized before infusing LEMTRADA. Healthcare facilities must have on-site access to equipment and personnel trained to manage infusion reactions.
Further information, including a list of qualified healthcare facilities, is available at 1-855-676-6326.
5.6 Immune Thrombocytopenia
Immune thrombocytopenia (ITP) occurred in 2% of LEMTRADA-treated patients in MS clinical studies (controlled and open-label extension).
In a controlled clinical study in patients with MS, one LEMTRADA-treated patient developed ITP that went unrecognized prior to the implementation of monthly blood monitoring requirements, and died from intracerebral hemorrhage. Nadir platelet counts ≤20,000 cells per microliter as a result of ITP occurred in 2% of all LEMTRADA-treated patients in clinical studies in MS. Anti-platelet antibodies did not precede ITP onset. ITP has been diagnosed more than 3 years after the last LEMTRADA dose.
Symptoms of ITP include easy bruising, petechiae, spontaneous mucocutaneous bleeding (e.g., epistaxis, hemoptysis), and heavier than normal or irregular menstrual bleeding. Hemoptysis may also be indicative of anti-glomerular basement membrane (GBM) disease [see Warnings and Precautions (5.7)], and an appropriate differential diagnosis has to be undertaken. Remind the patient to remain vigilant for symptoms they may experience and to seek immediate medical help if they have any concerns.
Obtain complete blood counts (CBCs) with differential prior to initiation of treatment and at monthly intervals thereafter until 48 months after the last infusion [see Dosage and Administration (2.6)]. After this period of time, testing should be performed based on clinical findings suggestive of ITP. If ITP is suspected, a complete blood count should be obtained immediately. If ITP onset is confirmed, promptly initiate appropriate medical intervention.
5.7 Glomerular Nephropathies Including Anti-glomerular Basement Membrane Disease
Glomerular nephropathies occurred in 0.3% of LEMTRADA-treated patients in MS clinical studies. There were 3 cases of membranous glomerulonephritis and 2 cases of anti-glomerular basement membrane (anti-GBM) disease.
In postmarketing cases, some LEMTRADA-treated patients with anti-GBM disease developed end-stage renal disease requiring dialysis or renal transplantation. Urgent evaluation and treatment are required because early treatment can improve the preservation of renal function. Anti-GBM disease can be life-threatening if left untreated. Alveolar hemorrhage, manifested as hemoptysis, is a common component of anti-GBM disease and has been reported in postmarketing cases. Cases of anti-GBM disease have been diagnosed up to 40 months after the last dose of LEMTRADA.
Symptoms of nephropathy may include edema, hematuria, change in urine color, decreased urine output, fatigue, dyspnea, and hemoptysis. Patients and caregivers should be instructed to seek medical advice if they have concerns.
Obtain serum creatinine levels, urinalysis with cell counts, and urine protein to creatinine ratio prior to initiation of treatment. Obtain serum creatinine levels and urinalysis with cell counts at monthly intervals thereafter until 48 months after the last infusion. After this period of time, testing should be performed based on clinical findings suggestive of nephropathies.
For urine dipstick results of 1+ protein or greater, measure the urine protein to creatinine ratio. For urine protein to creatinine ratio greater than 200 mg/g, increase in serum creatinine greater than 30%, or unexplained hematuria, perform further evaluation for nephropathies. Increased serum creatinine with hematuria or signs of pulmonary involvement of anti-GBM disease (e.g., hemoptysis, exertional dyspnea) warrant immediate evaluation. Early detection and treatment of nephropathies may decrease the risk of poor outcomes.
5.8 Thyroid Disorders
Thyroid endocrine disorders, including autoimmune thyroid disorders, occurred in 36.8% of LEMTRADA-treated patients in MS clinical studies (controlled and open-label extension). Newly diagnosed thyroid disorders occurred throughout the uncontrolled clinical study follow-up period, more than 7 years after the first LEMTRADA dose. Autoimmune thyroid disorders included Graves' disease, hyperthyroidism, hypothyroidism, autoimmune thyroiditis, and goiter. Graves' ophthalmopathy with decreased vision, eye pain, and exophthalmos occurred in 2% of LEMTRADA-treated patients. Seven patients required surgical orbital decompression. Serious thyroid events occurred in about 5.2% of LEMTRADA-treated patients in clinical studies and included cardiac and psychiatric events associated with thyroid disease. Of all LEMTRADA-treated patients, 3.8% underwent thyroidectomy.
Thyroid disease poses special risks in women who are pregnant [see Use in Specific Populations (8.1)].
Obtain thyroid function tests, such as TSH levels, prior to initiation of treatment and every 3 months thereafter until 48 months after the last infusion. Continue to test thyroid function after 48 months if clinically indicated or in case of pregnancy.
In patients with ongoing thyroid disorder, LEMTRADA should be administered only if the potential benefit justifies the potential risks.
5.9 Other Autoimmune Cytopenias
Autoimmune cytopenias such as neutropenia (0.1%), hemolytic anemia (0.3%), and pancytopenia (0.2%) occurred in LEMTRADA-treated patients in MS clinical studies (controlled and open-label extension). In cases of autoimmune hemolytic anemia, patients tested positive for direct antiglobulin antibodies, and nadir hemoglobin levels ranged from 2.9–8.6 g/dL. Symptoms of autoimmune hemolytic anemia include weakness, chest pain, jaundice, dark urine, and tachycardia. One LEMTRADA-treated patient with autoimmune pancytopenia died from sepsis.
During postmarketing use, additional autoimmune cytopenias, including fatal autoimmune hemolytic anemia and aplastic anemia, have been reported in the treatment of patients with B-CLL, as well as other disorders, generally at higher and more frequent doses of alemtuzumab than recommended in MS.
Use CBC results to monitor for cytopenias. Prompt medical intervention is indicated if a cytopenia is confirmed.
5.10 Autoimmune Hepatitis
Autoimmune hepatitis causing clinically significant liver injury, including acute liver failure requiring transplant, has been reported in patients treated with LEMTRADA in the postmarketing setting. If a patient develops clinical signs, including unexplained liver enzyme elevations or symptoms suggestive of hepatic dysfunction (e.g., unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine), promptly measure serum transaminases and total bilirubin and interrupt or discontinue treatment with LEMTRADA, as appropriate.
Prior to starting treatment with LEMTRADA, obtain serum transaminases (ALT and AST) and total bilirubin levels. Obtain transaminase levels and total bilirubin levels at periodic intervals until 48 months after the last dose.
5.11 Hemophagocytic Lymphohistiocytosis
Hemophagocytic lymphohistiocytosis (HLH) has occurred in patients taking LEMTRADA. HLH is a life-threatening syndrome of pathologic immune activation characterized by clinical signs and symptoms of extreme systemic inflammation. It is associated with high mortality rates if not recognized early and treated. Common findings include fever, hepatosplenomegaly, rash, lymphadenopathy, neurologic symptoms (e.g., mental status changes, ataxia, or seizures), cytopenias, high serum ferritin, hypertriglyceridemia, and liver function and coagulation abnormalities. Hemophagocytosis may be seen on histologic examination of bone marrow, spleen, or lymph nodes. In cases of HLH reported with LEMTRADA, most patients presented with fever, elevated ferritin, transaminitis, hypertriglyceridemia, and all patients required hospitalization. Although the small number of cases limits the ability to draw conclusions pertaining to mean or range of latency for HLH, symptoms have been reported to occur within approximately thirteen months to thirty-three months following the initiation of treatment. Patients who develop early manifestations of pathologic immune activation should be evaluated immediately, and a diagnosis of HLH should be considered. LEMTRADA should be discontinued if an alternate etiology for the signs or symptoms cannot be established.
5.12 Adult Onset Still's Disease (AOSD)
During postmarketing use, Adult Onset Still's Disease (AOSD) has been reported in patients treated with LEMTRADA. AOSD is a rare inflammatory condition that requires urgent evaluation and treatment. Patients with AOSD may have a combination of the following signs and symptoms: fever, arthritis, rash, and leukocytosis in the absence of infections, malignancies, and other rheumatic conditions. Patients with manifestations of AOSD should be evaluated immediately and LEMTRADA should be discontinued if an alternate etiology for the signs or symptoms cannot be established.
5.13 Thrombotic Thrombocytopenic Purpura (TTP)
TTP has been reported in patients treated with LEMTRADA. TTP is characterized by thrombocytopenia, microangiopathic hemolytic anemia, neurological sequelae, fever, and renal impairment. TTP is associated with high morbidity and mortality rates if not recognized and treated early. LEMTRADA should be discontinued if TTP is confirmed or an alternate etiology for the signs or symptoms cannot be established.
5.14 Autoimmune Encephalitis (AIE)
During postmarketing use, cases of AIE have been reported in patients treated with LEMTRADA. AIE can present with a variety of clinical manifestations, including subacute onset of memory impairment, altered mental status, psychiatric symptoms, neurological findings, and seizures. LEMTRADA should be discontinued if AIE is confirmed by the presence of neural autoantibodies or an alternate etiology cannot be established.
5.15 Acquired Hemophilia A
Cases of acquired hemophilia A (anti-Factor VIII antibodies) have been reported in both the clinical trial and postmarketing settings. Patients typically present with spontaneous subcutaneous hematomas and extensive bruising, although hematuria, epistaxis, gastrointestinal, or other types of bleeding may occur. Obtain a coagulopathy panel including aPTT in patients who present with such symptoms. Patients should be informed about the signs and symptoms of acquired hemophilia A and advised to seek immediate medical attention if any of these symptoms occur.
5.16 Infections
Infections occurred in 71% of LEMTRADA-treated patients compared to 53% of patients treated with interferon beta-1a in controlled clinical studies in MS up to 2 years in duration. Infections that occurred more often in LEMTRADA-treated patients than interferon beta-1a patients included nasopharyngitis, urinary tract infection, upper respiratory tract infection, sinusitis, herpetic infections, influenza, and bronchitis. Serious infections occurred in 3% of patients treated with LEMTRADA as compared to 1% of patients treated with interferon beta-1a. Serious infections in the LEMTRADA group included: appendicitis, gastroenteritis, pneumonia, herpes zoster, and tooth infection.
Do not administer live viral vaccines following a course of LEMTRADA. Patients treated with LEMTRADA have altered immunity and may be at increased risk of infection following administration of live viral vaccines.
LEMTRADA administration is contraindicated in patients with active infection [see Contraindications (4)].
Concomitant use of LEMTRADA with antineoplastic or immunosuppressive therapies could increase the risk of immunosuppression.
5.17 Progressive Multifocal Leukoencephalopathy (PML)
Progressive multifocal leukoencephalopathy (PML) has occurred in a patient with MS treated with LEMTRADA. PML is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. PML was diagnosed two months after the second course of LEMTRADA. The patient had previously received multiple MS therapies, but had not received other drugs for treatment of MS for more than one year. The patient had no other identified systemic medical conditions resulting in compromised immune system function and had not previously been treated with natalizumab, which has a known association with PML. The patient was not taking any immunosuppressive or immunomodulatory medications concomitantly. After the diagnosis of PML, the patient developed immune reconstitution inflammatory syndrome (IRIS). The patient's condition improved, but mild residual neurologic sequelae remained at last follow-up.
At the first sign or symptom suggestive of PML, withhold LEMTRADA and perform an appropriate diagnostic evaluation. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
MRI findings may be apparent before clinical signs or symptoms. Cases of PML, diagnosed based on MRI findings and the detection of JCV DNA in the cerebrospinal fluid in the absence of clinical signs or symptoms specific to PML, have been reported in patients treated with other MS medications associated with PML. Many of these patients subsequently became symptomatic with PML. Therefore, monitoring with MRI for signs that may be consistent with PML may be useful, and any suspicious findings should lead to further investigation to allow for an early diagnosis of PML, if present. Following discontinuation of another MS medication associated with PML, lower PML-related mortality and morbidity have been reported in patients who were initially asymptomatic at diagnosis compared to patients who had characteristic clinical signs and symptoms at diagnosis. It is not known whether these differences are due to early detection and discontinuation of MS treatment or due to differences in disease in these patients.
5.18 Acute Acalculous Cholecystitis
LEMTRADA may increase the risk of acute acalculous cholecystitis. In controlled clinical studies, 0.2% of LEMTRADA-treated MS patients developed acute acalculous cholecystitis, compared to 0% of patients treated with interferon beta-1a. During postmarketing use, additional cases of acute acalculous cholecystitis have been reported in LEMTRADA-treated patients. Time to onset of symptoms ranged from less than 24 hours to 2 months after LEMTRADA infusion. Typical risk or predisposing factors such as concurrent critical illness was often not reported. Abnormal ultrasound or computed tomography was used to support the diagnosis of acute acalculous cholecystitis in some cases. Some patients were treated conservatively with antibiotics and recovered without surgical intervention, whereas others underwent cholecystectomy.
Symptoms of acute acalculous cholecystitis include abdominal pain, abdominal tenderness, fever, nausea, and vomiting. Leukocytosis and abnormal liver enzymes are also commonly observed. Acute acalculous cholecystitis is a condition that is associated with high morbidity and mortality rates if not diagnosed early and treated. If acute acalculous cholecystitis is suspected, evaluate and treat promptly.
5.19 Pneumonitis
In clinical studies, 6 of 1217 (0.5%) LEMTRADA-treated patients had pneumonitis of varying severity. Cases of hypersensitivity pneumonitis and pneumonitis with fibrosis occurred in clinical studies. Patients should be advised to report symptoms of pneumonitis, which may include shortness of breath, cough, wheezing, chest pain or tightness, and hemoptysis.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below and elsewhere in the labeling:
- Autoimmunity [see Boxed Warning and Warnings and Precautions (5.1)]
- Infusion Reactions [see Boxed Warning and Warnings and Precautions (5.2)]
- Stroke and Cervicocephalic Arterial Dissection [see Warnings and Precautions (5.3)]
- Malignancies [see Warnings and Precautions (5.4)]
- Immune Thrombocytopenia [see Warnings and Precautions (5.6)]
- Glomerular Nephropathies Including Anti-glomerular Basement Membrane Disease [see Warnings and Precautions (5.7)]
- Thyroid Disorders [see Warnings and Precautions (5.8)]
- Other Autoimmune Cytopenias [see Warnings and Precautions (5.9)]
- Autoimmune Hepatitis [see Warnings and Precautions (5.10)]
- Hemophagocytic Lymphohistiocytosis [see Warnings and Precautions (5.11)]
- Adult Onset Still's Disease [see Warnings and Precautions (5.12)]
- Thrombotic Thrombocytopenic Purpura (TTP) [see Warnings and Precautions (5.13)]
- Autoimmune Encephalitis (AIE) [see Warnings and Precautions (5.14)]
- Acquired Hemophilia A [see Warnings and Precautions (5.15)]
- Infections [see Warnings and Precautions (5.16)]
- Progressive Multifocal Leukoencephalopathy (PML) [see Warnings and Precautions (5.17)]
- Acute Acalculous Cholecystitis [see Warnings and Precautions (5.18)]
- Pneumonitis [see Warnings and Precautions (5.19)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical trials (Study 1 and Study 2), a total of 811 patients with relapsing forms of MS received LEMTRADA. The population was 18 to 55 years of age, 65% were female, and 92% were Caucasian. A total of 811 patients received 1 course of therapy, and 789 patients received a second course of therapy at 12 months. The overall follow-up in the controlled trials was equivalent to 1622 patient years.
In MS clinical studies (controlled and open-label extension), overall, a total of 1217 patients received LEMTRADA. Approximately 60% of patients received a total of 2 treatment courses and approximately 24% of patients received a total of 3 treatment courses; others received a total of 4 or more treatment courses, although data beyond 3 treatment courses are limited. The overall follow-up was 6858 person-years. Patients had a median of 6 years of follow-up from the first LEMTRADA dose, with approximately 14% having at least 7 years of follow-up.
6.2 Lymphopenia
Nearly all (99.9%) patients treated with LEMTRADA in MS clinical trials experienced lymphopenia. The lowest lymphocyte counts occurred approximately by 1 month after each course of treatment. The mean lymphocyte count at 1 month after LEMTRADA treatment was 0.25 × 109 L (range 0.02–2.30 × 109 L) and 0.32 (0.02–1.81 × 109 L) for treatment courses 1 and 2, respectively. Total lymphocyte counts increased to reach the lower limit of normal in approximately 40% of patients by 6 months after each LEMTRADA treatment course and approximately 80% of patients by 12 months after each course [see Clinical Pharmacology (12.2)].
6.3 Suicidal Behavior or Ideation
In clinical studies, 0.6% of patients in both the LEMTRADA and interferon beta-1a groups had events of attempted suicide or suicidal ideation. There were no completed suicides in either clinical study treatment group. Suicidal behavior or ideation occurred in patients with or without a history of a psychiatric or thyroid disorder. Advise patients to report immediately any symptoms of depression or suicidal ideation to the prescribing physician.
6.4 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The incidence of antibodies is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including inhibitory antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to LEMTRADA with the incidence of antibodies to other products may be misleading.
Using an enzyme-linked immunosorbent assay (ELISA) and a competitive binding assay, anti-alemtuzumab binding antibodies were detected in 62%, 67%, and 29% of LEMTRADA-treated patients, at months 1, 3, and 12 (Course 1) as well as 83%, 83%, and 75% of LEMTRADA-treated patients at months 13, 15, and 24 (Course 2). Samples that tested positive for binding antibodies were further evaluated for evidence of in vitro inhibition using a flow cytometry assay. Neutralizing antibodies were detected in 87%, 46%, and 5% of positive binding antibody patients at months 1, 3, and 12 (Course 1) as well as 94%, 88%, and 42% of positive binding antibody patients at months 13, 15, and 24 (Course 2). Anti-alemtuzumab antibodies were associated with decreased alemtuzumab concentration during Course 2, but not Course 1. Through 2 treatment courses, there was no evidence from clinical trials that the presence of binding or inhibitory anti-alemtuzumab antibodies had a significant effect on clinical outcomes, total lymphocyte count, or adverse events. High titer anti-alemtuzumab antibodies, which were observed in 13 patients, were associated with incomplete lymphocyte depletion following a third or fourth treatment course, but there was no clear effect of anti-alemtuzumab antibodies on the clinical efficacy or safety profile of LEMTRADA.
6.5 Postmarketing Experience
The following adverse reactions have been identified during post approval use of alemtuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
8. Use In Specific Populations
8.1 Pregnancy
Data
Animal data
When LEMTRADA was administered to pregnant huCD52 transgenic mice during organogenesis (gestation days [GD] 6–10 or GD 11–15) at doses of 3 or 10 mg/kg IV, no teratogenic effects were observed. However, there was an increase in embryolethality (increased postimplantation loss and the number of dams with all fetuses dead or resorbed) in pregnant animals dosed during GD 11–15. In a separate study in pregnant huCD52 transgenic mice, administration of LEMTRADA during organogenesis (GD 6–10 or GD 11–15) at doses of 3 or 10 mg/kg IV, decreases in B- and T-lymphocyte populations were observed in the offspring at both doses tested.
In pregnant huCD52 transgenic mice administered LEMTRADA at doses of 3 or 10 mg/kg/day IV throughout gestation and lactation, there was an increase in pup deaths during the lactation period at 10 mg/kg. Decreases in T- and B-lymphocyte populations and in antibody response were observed in offspring at both doses tested.
8.2 Lactation
Data
Animal data
Alemtuzumab was detected in the milk of lactating huCD52 transgenic mice following intravenous administration of LEMTRADA at a dose of 10 mg/kg on postpartum days 8–12. Serum levels of alemtuzumab were similar in lactating mice and offspring on postpartum Day 13 and were associated with evidence of pharmacological activity (decrease in lymphocyte counts) in the offspring.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients less than 17 years of age have not been established. Use of LEMTRADA is not recommended in pediatric patients due to the risks of autoimmunity, infusion reactions, and stroke, and because it may increase the risk of malignancies (thyroid, melanoma, lymphoproliferative disorders, and lymphoma) [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4)].
10. Overdosage
Two MS patients experienced serious reactions (headache, rash, and either hypotension or sinus tachycardia) after a single accidental infusion up to 60 mg of LEMTRADA. Doses of LEMTRADA greater than those recommended may increase the intensity and/or duration of infusion reactions or its immune effects. There is no known antidote for alemtuzumab overdosage.
11. Lemtrada Description
Alemtuzumab is a recombinant humanized IgG1 kappa monoclonal antibody directed against the cell surface glycoprotein, CD52. Alemtuzumab has an approximate molecular weight of 150 kD. Alemtuzumab is produced in mammalian cell (Chinese hamster ovary) suspension culture in a nutrient medium containing neomycin. Neomycin is not detectable in the final product.
LEMTRADA (alemtuzumab) injection is a sterile, clear and colorless to slightly yellow, solution (pH 7.2 ± 0.2) for intravenous infusion.
Each 1 mL of solution contains 10 mg alemtuzumab, dibasic sodium phosphate (1.15 mg), disodium edetate dihydrate (0.0187 mg), polysorbate 80 (0.1 mg), potassium chloride (0.2 mg), potassium dihydrogen phosphate (0.2 mg), sodium chloride (8 mg), and Water for Injection, USP.
12. Lemtrada - Clinical Pharmacology
12.1 Mechanism of Action
The precise mechanism by which alemtuzumab exerts its therapeutic effects in multiple sclerosis is unknown but is presumed to involve binding to CD52, a cell surface antigen present on T and B lymphocytes, and on natural killer cells, monocytes, and macrophages. Following cell surface binding to T and B lymphocytes, alemtuzumab results in antibody-dependent cellular cytolysis and complement-mediated lysis.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess the carcinogenic or genotoxic potential of LEMTRADA have not been conducted.
When LEMTRADA (3 or 10 mg/kg IV) was administered to huCD52 transgenic male mice on 5 consecutive days prior to cohabitation with untreated wild-type females, no effect on fertility or reproductive performance was observed. However, adverse effects on sperm parameters (including abnormal morphology [detached/no head] and reduced total count and motility) were observed at both doses tested.
When LEMTRADA (3 or 10 mg/kg IV) was administered to huCD52 transgenic female mice for 5 consecutive days prior to cohabitation with untreated wild-type males, there was a decrease in the average number of corpora lutea and implantation sites and an increase in postimplantation loss, resulting in fewer viable embryos at the higher dose tested.
14. Clinical Studies
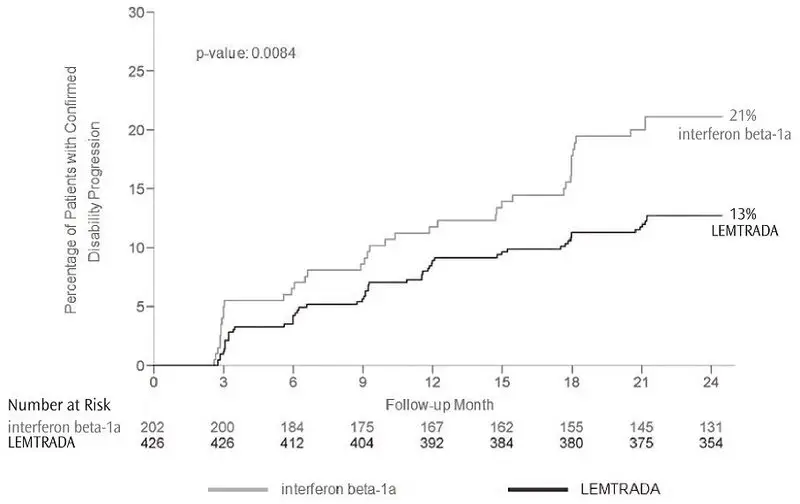
The efficacy of LEMTRADA was demonstrated in two studies (Study 1 and 2) that evaluated LEMTRADA 12 mg in patients with relapsing-remitting multiple sclerosis (RRMS). LEMTRADA was administered by intravenous infusion once daily over a 5-day course, followed one year later by intravenous infusion once daily over a 3-day course. Both studies included patients who had experienced at least 2 relapses during the 2 years prior to trial entry and at least 1 relapse during the year prior to trial entry. Neurological examinations were performed every 12 weeks and at the time of suspected relapse. Magnetic resonance imaging (MRI) evaluations were performed annually.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: October 2022 | ||
|
MEDICATION GUIDE
|
|||
|
Read this Medication Guide before you start receiving LEMTRADA and before you begin each treatment course. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. |
|||
|
What is the most important information I should know about LEMTRADA? LEMTRADA can cause serious side effects, including:
Side effects may happen while you receive LEMTRADA and for 4 years after you stop receiving LEMTRADA. Your healthcare provider will order blood and urine tests before you receive, while you are receiving, and every month for 4 years after you receive your last LEMTRADA infusion. You may need to continue these blood and urine tests after 4 years if you have any autoimmune signs or symptoms. The blood and urine tests will help your healthcare provider watch for signs and symptoms of serious autoimmune problems. It is important to have your blood and urine tested, even if you are feeling well and do not have any symptoms from LEMTRADA and your multiple sclerosis. This may help your healthcare provider find any problems early.
You will receive your infusion at a healthcare facility with equipment and staff trained to manage infusion reactions. You will be watched while you receive and for at least 2 hours after you receive LEMTRADA. It is important that you stay at the infusion center for at least 2 hours after your infusion is finished or longer if your healthcare provider decides you need to stay longer. If a serious infusion reaction happens while you are receiving LEMTRADA, your infusion may be stopped. Tell your healthcare provider right away if you have any of the following symptoms of a serious infusion reaction during the infusion, and after you have left the healthcare facility:
To lower your chances of getting a serious infusion reaction, your healthcare provider will give you a medicine called corticosteroids before your first 3 infusions of a treatment course. You may also be given other medicines before or after the infusion to try to reduce your chances of these reactions or to treat them after they happen.
|
|||
|
|
||
|
|||
|
|
||
|
You should have your skin checked before you start receiving LEMTRADA and each year while you are receiving treatment to monitor symptoms of skin cancer. Because of your risk of autoimmunity, infusion reactions, and the risk of some kinds of cancers, LEMTRADA is only available through a restricted program called the LEMTRADA Risk Evaluation and Mitigation Strategy (REMS) Program. Call 1-855-676-6326 to enroll in the LEMTRADA REMS Program.
|
|||
|
What is LEMTRADA? LEMTRADA is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Since treatment with LEMTRADA can increase your risk of getting certain conditions and diseases, LEMTRADA is generally prescribed for people who have tried 2 or more MS medicines that have not worked well enough. LEMTRADA is not recommended for use in patients with clinically isolated syndrome (CIS). It is not known if LEMTRADA is safe and effective for use in children under 17 years of age. |
|||
|
Who should not receive LEMTRADA? Do not receive LEMTRADA if you:
|
|||
|
What should I tell my healthcare provider before receiving LEMTRADA? Before receiving LEMTRADA, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEMTRADA and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take medicines that increase your chance of getting infections, including medicines used to treat cancer or to control your immune system. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|||
|
How will I receive LEMTRADA?
|
|||
|
What are the possible side effects of LEMTRADA? LEMTRADA may cause serious side effects including:
|
|||
|
|
||
|
|||
|
|
||
|
|||
|
|
||
|
Your healthcare provider will do blood tests to check for cytopenias. Call your healthcare provider right away if you have symptoms listed above.
Call your healthcare provider right away if you have symptoms of a serious infection, such as fever or swollen glands. You may need to go to the hospital for treatment if you get a serious infection. It is important to tell the healthcare providers that you have received LEMTRADA. Talk to your healthcare provider before you get vaccinations after receiving LEMTRADA. Certain vaccinations may increase your chances of getting infections.
|
|||
|
|
||
|
The most common side effects of LEMTRADA include: |
|||
|
|
||
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of LEMTRADA. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088. |
|||
|
General information about the safe and effective use of LEMTRADA. This Medication Guide summarizes the most important information about LEMTRADA. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about LEMTRADA that is written for health professionals. For more information, go to www.LemtradaREMS.com or call Genzyme at 1-855-676-6326. |
|||
|
What are the ingredients in LEMTRADA? Active ingredient: alemtuzumab Inactive ingredients: dibasic sodium phosphate, disodium edetate dihydrate, polysorbate 80, potassium chloride, potassium dihydrogen phosphate, sodium chloride, and Water for Injection, USP. |
|||
|
Manufactured and distributed by: LEMTRADA and CAMPATH are registered trademarks of Genzyme Corporation. |
|||
| LEMTRADA
alemtuzumab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genzyme Corporation (025322157) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH & Co. KG | 340700520 | ANALYSIS(58468-0200) , API MANUFACTURE(58468-0200) , MANUFACTURE(58468-0200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi-Aventis Deutschland GmbH | 313218430 | ANALYSIS(58468-0200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EUROAPI UK LIMITED | 229522842 | PACK(58468-0200) , LABEL(58468-0200) | |