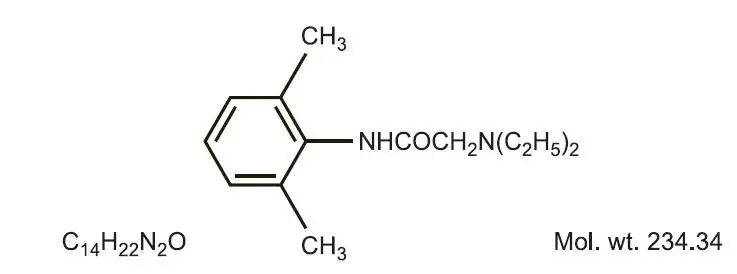
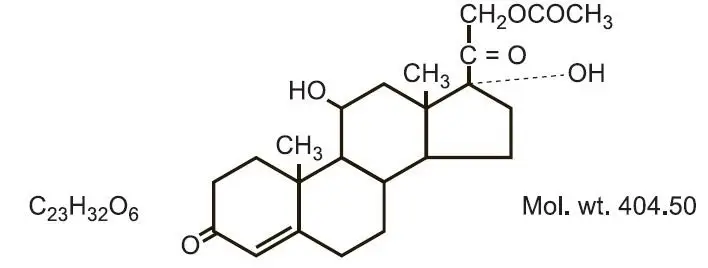
Drug Class:
INACTIVE INGREDIENTS:
aluminum sulfate, calcium acetate, carbomer 980, cetyl alcohol, citric acid, glycerine, methylparaben, mineral oil, polycarbophil, propylene glycol, propylparaben, purified water, sodium citrate, sodium lauryl sulfate, sodium hydroxide, sorbitan stearate, stearic acid, stearyl alcohol, trolamine, urea, and white petrolatum.
Drug Interactions
Patients that are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently exposed to the following oxidizing agents:
| Class | Examples |
| Nitrates/Nitrites | nitroglycerin, nitroprusside, nitric oxide, nitrous oxide |
| Local anesthetics | benzocaine, lidocaine, bupivacaine, mepivacaine, tetracaine, prilocaine, procaine, articaine, ropivacaine |
| Antineoplastic agents | cyclophosphamide, flutamide, rasburicase, ifosfamide, hydroxyurea |
| Antibiotics | dapsone, sulfonamides, nitrofurantoin, para-aminosalicylic acid |
| Antimalarials | chloroquine, primaquine |
| Anticonvulsants | phenytoin, sodium valproate, phenobarbital |
| Other drugs | acetaminophen, metoclopramide, sulfa drugs (i.e., sulfasalazine), quinine |
PATIENT COUNSELING INFORMATION:
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Topical formulations of lidocaine may be absorbed to a greater extent through mucous membranes and abraded, fissured or irritated skin than through intact skin. Product should not be ingested or applied into the mouth, inside of the nose or in the eyes. Product should not be used in the ears. Any situation where lidocaine penetrates beyond the tympanic membrane into the middle ear is contraindicated because of ototoxicity associated with lidocaine observed in animals when instilled in the middle ear. Product should not come into contact with the eye or be applied into the eye because of the risk of severe eye irritation and the loss of eye surface sensation which reduces protective reflexes and can lead to corneal irritation and possibly abrasion. If eye contact occurs, rinse out the eye immediately with saline or water and protect the eye surface until sensation is restored.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on the therapeutic equivalence.
Marketed by:
Seton Pharmaceuticals
Manasquan, NJ 08736
1-800-510-3401
Iss. 01/19
1900009
| LIDOCAINE HYDROCHLORIDE AND HYDROCORTISONE ACETATE
lidocaine hydrochloride and hydrocortisone acetate cream |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Seton Pharmaceuticals (828898002) |