Drug Detail:Barium sulfate (oral/rectal) (Barium sulfate (oral/rectal) [ ber-ee-um-sul-fate ])
Drug Class: Non-iodinated contrast media
Highlights of Prescribing Information
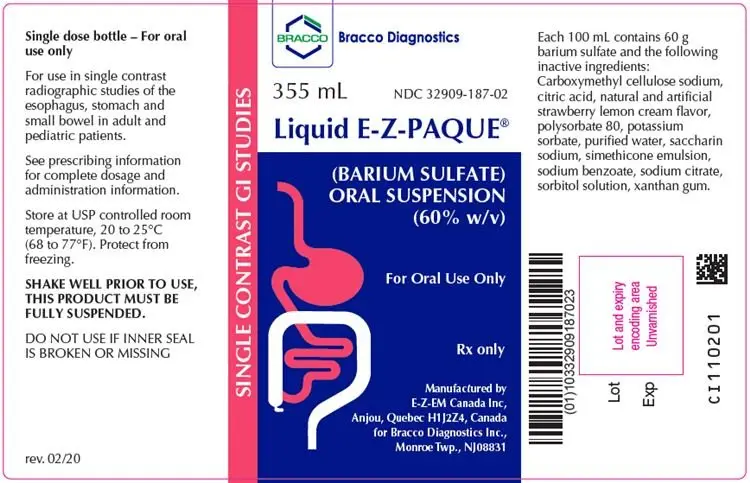
Liquid E-Z-PAQUE (barium sulfate) oral suspension
Initial U.S. Approval: 2016
Indications and Usage for Liquid E-Z Paque
Liquid E-Z-PAQUE is a radiographic contrast agent indicated for use in single contrast radiographic examinations of the esophagus, stomach, and small bowel to visualize the gastrointestinal (GI) tract in adult and pediatric patients (1)
Liquid E-Z Paque Dosage and Administration
- Adults: Recommended oral dose is 150 mL to 750 mL (87g to 435g of barium sulfate, respectively) (2.1)
- Pediatric patients: adjust dose based on relative GI volume (2.1)
Dosage Forms and Strengths
- Oral suspension 213 g barium sulfate (60% w/v) (3)
Contraindications
- Known or suspected perforation of the GI tract (4)
- Known obstruction of the GI tract (4)
- Conditions associated with high risk of GI perforation or aspiration (4)
- Known severe hypersensitivity to barium sulfate or any of the excipients of Liquid E-Z-PAQUE (4)
Warnings and Precautions
- Hypersensitivity reactions: Emergency equipment and trained personnel should be immediately available (5.1)
- Intra-abdominal barium leakage: May occur in conditions such as GI fistula, ulcer, inflammatory bowel disease, appendicitis or diverticulitis, severe stenosis or obstructing lesions of the GI tract (5.2)
- Delayed GI transit and obstruction: Patients should maintain adequate hydration in days following a barium sulfate procedure to avoid obstruction or impaction (5.3)
- Aspiration pneumonitis: Patients with history of food aspiration or with swallowing disorders are at increased risk (5.4)
Adverse Reactions/Side Effects
Common adverse reactions include nausea, vomiting, diarrhea and abdominal cramping (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bracco Diagnostics Inc at 1-800-257-5181 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2017
Related/similar drugs
barium sulfate, DaTscan, Volumen, iopromide, iodixanolFull Prescribing Information
Liquid E-Z Paque Dosage and Administration
2.1 Recommended Dosage
- Adults: 150 to 750 mL (87 g to 435 g of barium sulfate, respectively). Volumes closer to 150 mL are recommended for examination of the esophagus and stomach and volumes up to 750 mL are recommended for examination of the small bowel
- Pediatric Patients: Adjust dose based on GI volume
- For examinations of the upper GI tract, administer a volume sufficient to fully distend the esophagus or stomach.
- For small bowel examinations:
- Age birth to less than 2 years: 30 mL to 75 mL
- Age 2 years to less than 17 years: 75 mL to 480 mL
2.2 Administration Instructions
- For oral use only
- Shake bottle vigorously for 30 seconds prior to oral administration to fully suspend product
- Administer undiluted
- Ensure patients have nothing by mouth for the following
time period prior to the examination:
- Neonates and Infants < 3 months 2 hours
- Infants 3-12 months 3 hours
- > 12 months of age 4 hours
- Discard any unused suspension
- Encourage patients to maintain hydration following the barium sulfate procedure
Contraindications
Liquid E-Z-PAQUE is contraindicated in patients with the following conditions:
- known or suspected perforation of the GI tract
- known obstruction of the GI tract
- high risk of GI perforation such as those with a recent GI perforation, acute GI hemorrhage or ischemia, toxic megacolon, severe ileus, post GI surgery or biopsy, acute GI injury or burn, or recent radiotherapy to the pelvis
- high risk of aspiration such as those with prior aspiration, tracheo-esophageal fistula, or obtundation
- known severe hypersensitivity to barium sulfate or any of the excipients of Liquid E-Z-PAQUE
Warnings and Precautions
Adverse Reactions/Side Effects
- Nausea, vomiting, diarrhea and abdominal cramping
- Serious adverse reactions and fatalities include aspiration pneumonitis, barium sulfate impaction, intestinal perforation with consequent peritonitis and granuloma formation, vasovagal and syncopal episodes
Patient Counseling Information
After administration advise patients to:
- Maintain adequate hydration
- Seek medical attention for worsening of constipation or slow gastrointestinal passage
- Seek medical attention for any delayed onset of hypersensitivity, such as: rash, urticaria, or respiratory difficulty
| E-Z-PAQUE
barium sulfate suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| E-Z-PAQUE
barium sulfate suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - E-Z-EM Canada Inc (204211163) |
| Registrant - BRACCO DIAGNOSTICS INC (849234661) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| E-Z-EM Canada Inc | 204211163 | PACK(32909-186, 32909-187) , MANUFACTURE(32909-186, 32909-187) , LABEL(32909-187, 32909-186) , ANALYSIS(32909-187, 32909-186) | |