Drug Detail:Livtencity (Maribavir)
Drug Class: Miscellaneous antivirals
Highlights of Prescribing Information
LIVTENCITY™ (maribavir) tablets, for oral use
Initial U.S. Approval: 2021
Recent Major Changes
| Dosage and Administration, Administration (2.3) | 9/2022 |
Indications and Usage for Livtencity
LIVTENCITY is a cytomegalovirus (CMV) pUL97 kinase inhibitor indicated for the treatment of adults and pediatric patients (12 years of age and older and weighing at least 35 kg) with post-transplant CMV infection/disease that is refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, cidofovir or foscarnet. (1, 8.4)
Livtencity Dosage and Administration
400 mg (two 200 mg tablets) orally twice daily with or without food. (2.1, 8.4)
Dosage Forms and Strengths
Tablets: 200 mg of maribavir. (3)
Contraindications
None. (4)
Warnings and Precautions
- LIVTENCITY may antagonize the antiviral activity of ganciclovir and valganciclovir. Coadministration is not recommended. (5.1, 7.1)
- Virologic failure can occur during and after treatment with LIVTENCITY. Monitor CMV DNA levels and check for resistance if patient does not respond to treatment. Some maribavir pUL97 resistance-associated substitutions confer cross-resistance to ganciclovir and valganciclovir. (5.2, 12.4, 14.1)
- The concomitant use of LIVTENCITY and certain drugs may result in potentially significant drug interactions, some of which may lead to reduced therapeutic effect of LIVTENCITY or adverse reactions of concomitant drugs. (5.1, 5.3, 7.1, 7.2, 7.3)
- LIVTENCITY has the potential to increase the drug concentrations of immunosuppressant drugs that are CYP3A4 and/or P-gp substrates where minimal concentration changes may lead to serious adverse events (including tacrolimus, cyclosporine, sirolimus and everolimus). Frequently monitor immunosuppressant drug levels throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY and adjust the dose, as needed. (5.3)
Adverse Reactions/Side Effects
The most common adverse events (all grades, >10%) in subjects treated with LIVTENCITY were taste disturbance, nausea, diarrhea, vomiting, and fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Refer to the full prescribing information for important drug interactions with LIVTENCITY. (5.1, 5.3, 7)
- Coadministration with strong CYP3A4 inducers: not recommended. Refer to full prescribing information for dosage modification when coadministered with certain anticonvulsants. (2.2, 7.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
Full Prescribing Information
1. Indications and Usage for Livtencity
LIVTENCITY is indicated for the treatment of adults and pediatric patients (12 years of age and older and weighing at least 35 kg) with post-transplant cytomegalovirus (CMV) infection/disease that is refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, cidofovir or foscarnet [see Use in Specific Populations (8.4) and Clinical Studies (14)].
2. Livtencity Dosage and Administration
2.1 Recommended Dosage
The recommended dosage in adults and pediatric patients (12 years of age and older and weighing at least 35 kg) is 400 mg (two 200 mg tablets) taken orally twice daily with or without food [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3) and Clinical Studies (14)].
2.2 Dosage Adjustment When Coadministered with Anticonvulsants
If LIVTENCITY is coadministered with carbamazepine, increase the dosage of LIVTENCITY to 800 mg (four 200 mg tablets) twice daily [see Drug Interactions (7.3)].
If LIVTENCITY is coadministered with phenytoin or phenobarbital, increase the dosage of LIVTENCITY to 1,200 mg (six 200 mg tablets) twice daily [see Drug Interactions (7.3)].
2.3 Administration
The immediate-release tablets can be taken as whole, dispersed or crushed tablets by mouth, or as dispersed tablets through a nasogastric or orogastric tube (French size 10 or larger). The suspension may be prepared ahead of time and stored at room temperature for up to 8 hours.
Administration of Dispersed Tablets or Crushed Tablets by Mouth
-
Place the appropriate number of tablets for the prescribed dose into a suitable container. If desired, the tablets may be crushed. Add the appropriate volume of drinking water (other liquids have not been tested) to make a suspension (see Table 1 below).
Table 1: Number of Tablets and Volume of Drinking Water Needed to Make a Suspension for Administration of Dispersed or Crushed Tablets by Mouth Recommended Dosage Number of 200 mg Tablets Volume of Drinking Water 400 mg Two 30 mL 800 mg Four 60 mL 1,200 mg Six 90 mL - Swirl the container gently to keep the particles from settling, and administer the suspension before it settles. The mixture will have a bitter taste.
- Rinse the container with 15 mL of drinking water and administer the rinse water.
- Repeat Step 3. Visually confirm that no particles are left in the container. If particles remain, repeat Step 3.
3. Dosage Forms and Strengths
Tablet: 200 mg, blue, oval shaped convex tablet debossed with "SHP" on one side and "620" on the other side.
5. Warnings and Precautions
5.1 Risk of Reduced Antiviral Activity When Coadministered with Ganciclovir and Valganciclovir
LIVTENCITY may antagonize the antiviral activity of ganciclovir and valganciclovir by inhibiting human CMV pUL97 kinase, which is required for activation/phosphorylation of ganciclovir and valganciclovir. Coadministration of LIVTENCITY with ganciclovir or valganciclovir is not recommended [see Drug Interactions (7.1) and Microbiology (12.4)].
5.2 Virologic Failure During Treatment and Relapse Post-Treatment
Virologic failure due to resistance can occur during and after treatment with LIVTENCITY. Virologic relapse during the post-treatment period usually occurred within 4-8 weeks after treatment discontinuation. Some maribavir pUL97 resistance-associated substitutions confer cross-resistance to ganciclovir and valganciclovir. Monitor CMV DNA levels and check for maribavir resistance if the patient is not responding to treatment or relapses [see Microbiology (12.4) and Clinical Studies (14.1)].
5.3 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of LIVTENCITY and certain drugs may result in potentially significant drug interactions, some of which may lead to reduced therapeutic effect of LIVTENCITY or adverse reactions of concomitant drugs [see Drug Interactions (7)].
See Table 4 for steps to prevent or manage these possible or known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during LIVTENCITY therapy; review concomitant medications during LIVTENCITY therapy and monitor for adverse reactions.
Maribavir is primarily metabolized by CYP3A4. Drugs that are strong inducers of CYP3A4 are expected to decrease maribavir plasma concentrations and may result in reduced virologic response; therefore, coadministration of LIVTENCITY with these drugs is not recommended, except for selected anticonvulsants [see Dosage and Administration (2.2) and Drug Interactions (7.3)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of LIVTENCITY was evaluated in one Phase 3 multicenter, randomized, open-label, active-control trial in which 352 adult transplant recipients were randomized, and treated with LIVTENCITY (N=234) or Investigator-Assigned Treatment (IAT) consisting of monotherapy or dual therapy with ganciclovir, valganciclovir, foscarnet, or cidofovir as dosed by the investigator (N=116) for up to 8-weeks following a diagnosis of CMV infection/disease refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, foscarnet or cidofovir. The mean treatment durations (SD) for LIVTENCITY and IAT were 48.6 (± 13.82) and 31.2 (± 16.91) days, respectively. The most common adverse events occurring in more than 10% of subjects receiving LIVTENCITY are outlined in Table 2.
| ADVERSE EVENT | LIVTENCITY N=234 (%) | IAT*
N=116 (%) |
|---|---|---|
|
||
| Taste disturbance† | 46 | 4 |
| Nausea | 21 | 22 |
| Diarrhea | 19 | 21 |
| Vomiting | 14 | 16 |
| Fatigue | 12 | 9 |
Similar proportions of subjects experienced serious adverse events (38% in the LIVTENCITY group and 37% in the IAT group). The most common serious adverse event in both treatment groups occurred in the Infections and Infestations System Organ Class (SOC) (23% in the LIVTENCITY group and 15% in the IAT group) with CMV infection and disease being the most common in both groups.
A higher proportion of subjects in the IAT group discontinued study medication due to an adverse event compared to the LIVTENCITY group (32% in the IAT group vs 13% in the LIVTENCITY group). The most commonly reported causes that led to study drug discontinuation were neutropenia (9%) and acute kidney injury (5%) in the IAT group and dysgeusia, diarrhea, nausea, and recurrence of underlying disease (each reported at 1%) in the LIVTENCITY group.
Taste disturbance occurred in 46% of subjects treated with LIVTENCITY. These events rarely led to discontinuation of LIVTENCITY (1%) and, for 37% of the subjects, these events resolved while on therapy (median duration 43 days; range 7 to 59 days). For the subjects with ongoing taste disturbance after drug discontinuation, resolution occurred in 89%. In subjects with resolution of symptoms after drug discontinuation, the median duration of symptoms off treatment was 6 days (range 2 to 85 days).
7. Drug Interactions
7.1 Reduced Antiviral Activity When Coadministered with Ganciclovir or Valganciclovir
LIVTENCITY is not recommended to be coadministered with valganciclovir/ganciclovir (vGCV/GCV). LIVTENCITY may antagonize the antiviral activity of ganciclovir and valganciclovir by inhibiting human CMV pUL97 kinase, which is required for activation/phosphorylation of ganciclovir and valganciclovir [see Warnings and Precautions (5.1) and Microbiology (12.4)].
7.2 Potential for Other Drugs to Affect LIVTENCITY
Maribavir is a substrate of CYP3A4. Coadministration of LIVTENCITY with strong inducers of CYP3A4 is not recommended, except for selected anticonvulsants [see Dosage and Administration (2.2) and Drug Interactions (7.3)].
7.3 Potential for LIVTENCITY to Affect Other Drugs
Maribavir is a weak inhibitor of CYP3A4, and an inhibitor of P-gp and breast cancer resistance protein (BCRP). Coadministration of LIVTENCITY with drugs that are sensitive substrates of CYP3A, P-gp and BCRP may result in a clinically relevant increase in plasma concentrations of these substrates (see Table 4). Table 4 provides a list of established or potentially clinically significant drug interactions, based on either clinical drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or decrease in efficacy [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
| Concomitant Drug Class: Drug Name | Effect on Concentration | Clinical Comments |
|---|---|---|
| ↓=decrease, ↑=increase. | ||
|
||
| Antiarrhythmics | ||
| Digoxin† | ↑ Digoxin | Use caution when LIVTENCITY and digoxin are coadministered. Monitor serum digoxin concentrations. The dose of digoxin may need to be reduced when coadministered with LIVTENCITY.‡ |
| Anticonvulsants | ||
| Carbamazepine | ↓ Maribavir | A dose adjustment of LIVTENCITY to 800 mg twice daily is recommended when coadministered with carbamazepine. |
| Phenobarbital | ↓ Maribavir | A dose adjustment of LIVTENCITY to 1,200 mg twice daily is recommended when coadministration with phenobarbital. |
| Phenytoin | ↓ Maribavir | A dose adjustment of LIVTENCITY to 1,200 mg twice daily is recommended when coadministration with phenytoin. |
| Antimycobacterials | ||
| Rifabutin | ↓ Maribavir | Coadministration of LIVTENCITY and rifabutin is not recommended due to potential for a decrease in efficacy of LIVTENCITY. |
| Rifampin† | ↓ Maribavir | Coadministration of LIVTENCITY and rifampin is not recommended due to potential for a decrease in efficacy of LIVTENCITY. |
| Herbal Products | ||
| St. John's wort | ↓ Maribavir | Coadministration of LIVTENCITY and St. John's wort is not recommended due to potential for a decrease in efficacy of LIVTENCITY. |
| HMG-CoA Reductase Inhibitors | ||
| Rosuvastatin‡ | ↑ Rosuvastatin | The patient should be closely monitored for rosuvastatin-related events, especially the occurrence of myopathy and rhabdomyolysis.‡ |
| Immunosuppressants | ||
| Cyclosporine | ↑ Cyclosporine | Frequently monitor cyclosporine levels throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY and adjust dose, as needed.‡ |
| Everolimus | ↑ Everolimus | Frequently monitor everolimus levels throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY and adjust dose, as needed.‡ |
| Sirolimus | ↑ Sirolimus | Frequently monitor sirolimus levels throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY and adjust dose, as needed.‡ |
| Tacrolimus† | ↑ Tacrolimus | Frequently monitor tacrolimus levels throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY and adjust dose, as needed.‡ |
7.4 Drugs without Clinically Significant Interactions with LIVTENCITY
No clinically significant interactions were observed in clinical drug-drug interaction studies of LIVTENCITY and ketoconazole, antacid, caffeine, S-warfarin, voriconazole, dextromethorphan, or midazolam [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The recommended dosing regimen in pediatric patients 12 years of age and older and weighing at least 35 kg is the same as that in adults. Use of LIVTENCITY in this age group is based on the following:
- Evidence from controlled studies of LIVTENCITY in adults
- Population pharmacokinetic (PK) modeling and simulation demonstrating that age and body weight had no clinically meaningful effect on plasma exposures of LIVTENCITY
- LIVTENCITY exposure is expected to be similar between adults and children 12 years of age and older and weighing at least 35 kg
- The course of the disease is similar between adults and pediatric patients to allow extrapolation of data in adults to pediatric patients [see Dosage and Administration (2.2), Clinical Pharmacology (12.3) and Clinical Studies (14)]
The safety and effectiveness of LIVTENCITY have not been established in children younger than 12 years of age.
8.5 Geriatric Use
No dosage adjustment is required for patients over 65 years of age based on the results from population pharmacokinetics analysis [see Clinical Pharmacology (12.3)] and efficacy and safety data from the clinical studies. In the clinical Study 303, 54 patients aged 65 years and over were treated with LIVTENCITY. Safety, effectiveness, and pharmacokinetics were consistent between elderly patients (≥65 years) and younger patients (<65 years).
10. Overdosage
There is no known specific antidote for LIVTENCITY. In case of overdose, it is recommended that the patient be monitored for adverse reactions and appropriate symptomatic treatment instituted. Due to the high plasma protein binding of LIVTENCITY, dialysis is unlikely to reduce plasma concentrations of LIVTENCITY significantly.
11. Livtencity Description
LIVTENCITY tablets contain maribavir, a benzimidazole riboside CMV pUL97 protein kinase inhibitor. The chemical name of maribavir is 5,6-Dichloro-N-(1-methylethyl)-1-β-L-ribofuranosyl-1H-benzimidazol-2-amine and the structural formula is:
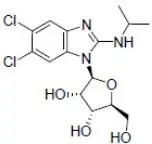
The molecular formula for maribavir is C15H19Cl2N3O4 and its molecular weight is 376.23.
Each 200 mg tablet for oral administration contains 200 mg maribavir and the following inactive ingredients: FD&C Blue #1, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium starch glycolate, titanium dioxide, and talc.
12. Livtencity - Clinical Pharmacology
12.1 Mechanism of Action
LIVTENCITY is an antiviral drug against human CMV [see Microbiology (12.4)].
12.3 Pharmacokinetics
LIVTENCITY pharmacological activity is due to the parent drug. Following oral administration, plasma maribavir exposure (Cmax and AUC) increased approximately dose-proportionally following a single dose of 50 to 1600 mg (0.125 to four times the recommended dose) and multiple doses up to 2400 mg per day (three times the recommended daily dose). Maribavir PK is time-independent. With twice-daily dosing, steady state is reached within 2 days, with mean accumulation ratios of Cmax and AUC ranging from 1.37 to 1.47.
The pharmacokinetic properties of maribavir following administration of LIVTENCITY are displayed in Table 5. The multiple-dose pharmacokinetic parameters are provided in Table 6.
|
|
| Absorption* | |
| Tmax (h), median | 1.0 to 3.0 |
| Distribution | |
| Mean apparent steady-state volume of distribution (Vss, L) | 27.3 |
| % bound to human plasma proteins | 98.0 across the concentration range of 0.05-200 µg/mL |
| Blood-to plasma ratio | 1.37 |
| Elimination | |
| Major route of elimination | Hepatic metabolism |
| Half-life (t1/2) in transplant patients (h), mean | 4.32 |
| Oral clearance (CL/F) in transplant patients (L/h), mean | 2.85 |
| Metabolism | |
| Metabolic pathways† | CYP3A4 (major) and CYP1A2 (minor) |
| Excretion | |
| % of dose excreted as total 14C (unchanged drug) in urine‡ | 61 (<2) |
| % of dose excreted as total 14C (unchanged drug) in feces‡ | 14 (5.7) |
| Geometric Mean (% CV)* | ||
|---|---|---|
| AUC0-tau†
(µg∙h/mL) | Cmax
(µg/mL) | Ctau
(µg/mL) |
| CV=Coefficient of Variation; Cmax=Maximum concentration; AUC0-tau=Area under the time concentration curve over a dosing interval; Ctau=Concentration at the end of a dosing interval. | ||
|
||
| 128 (50.7%) | 17.2 (39.3%) | 4.90 (89.7%) |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in mice and rats administered oral doses up to 150 and 100 mg/kg/day, respectively. Maribavir was not carcinogenic in rats at any dose tested, corresponding to maribavir exposures less than human exposure at the RHD. At 150 mg/kg/day in male mice only, an increased incidence of hemangioma, hemangiosarcoma, and combined hemangioma/hemangiosarcoma was observed across multiple tissues, at exposures less than the human exposure at the RHD. There were no carcinogenic findings in male mice at ≤75 mg/kg/day and female mice at any dose.
14. Clinical Studies
14.1 Treatment of Adults with Post-Transplant CMV Infection/Disease That Is Refractory (with or without Genotypic Resistance) to Ganciclovir, Valganciclovir, Cidofovir, or Foscarnet
LIVTENCITY was evaluated in a Phase 3, multicenter, randomized, open-label, active-controlled superiority trial (NCT02931539, Trial 303) to assess the efficacy and safety of LIVTENCITY compared to Investigator-Assigned Treatment (IAT) (ganciclovir, valganciclovir, foscarnet, or cidofovir) in 352 HSCT or SOT recipients with CMV infections that were refractory to treatment with ganciclovir, valganciclovir, foscarnet, or cidofovir, including CMV infections with or without confirmed resistance to 1 or more of the IATs. Subjects with CMV disease involving the central nervous system, including the retina, were excluded from the study.
Subjects were stratified by transplant type (HSCT or SOT) and screening CMV DNA levels and then randomized in a 2:1 allocation ratio to receive either LIVTENCITY 400 mg twice daily or IAT as dosed by the investigator for up to 8 weeks. After completion of the treatment period, subjects entered a 12-week follow-up phase.
The mean age of trial subjects was 53 years and most subjects were male (61%), white (76%) and not Hispanic or Latino (83%), with similar distributions across the two treatment arms. The most common treatment used in the IAT arm was foscarnet which was administered in 47 (41%) subjects followed by ganciclovir or valganciclovir, each administered in 28 (24%) subjects. Cidofovir was administered in 6 subjects, the combination of foscarnet and valganciclovir in 4 subjects and the combination of foscarnet and ganciclovir in 3 subjects. Baseline disease characteristics are summarized in Table 9 below.
| Characteristic | LIVTENCITY 400 mg Twice Daily N=235 n (%) | IAT N=117 n (%) |
|---|---|---|
| CMV=cytomegalovirus, DNA=deoxyribonucleic acid, HSCT=hematopoietic stem cell transplant, IAT=investigator assigned anti-CMV treatment, N=number of patients, SOT=solid organ transplant. | ||
|
||
| Transplant type | ||
| HSCT | 93 (40) | 48 (41) |
| SOT | 142 (60) | 69 (59) |
| Kidney | 74 (52) | 32 (46) |
| Lung | 40 (28) | 22 (32) |
| Heart | 14 (10) | 9 (13) |
| Other (multiple, liver, pancreas, intestine) | 14 (10) | 6 (9) |
| CMV DNA levels | ||
| Low (<9,100 IU/mL) | 153 (65) | 85 (73) |
| Intermediate (≥9,100 to <91,000 IU/mL) | 68 (29) | 25 (21) |
| High (≥91,000 IU/mL) | 14 (6) | 7 (6) |
| Confirmed symptomatic CMV infection at baseline | ||
| No | 214 (91) | 109 (93) |
| Yes* | 21 (9) | 8 (7) |
| CMV syndrome (SOT only) | 9 (43) | 7 (88) |
| Tissue Invasive disease | 12 (57)* | 1 (13) |
Primary Efficacy Endpoint
The primary efficacy endpoint was confirmed CMV DNA level < LLOQ (i.e;, <137 IU/mL) as assessed by COBAS® AmpliPrep/COBAS® TaqMan® CMV test) at the end of Week 8. The key secondary endpoint was CMV DNA level < LLOQ and CMV infection symptom control at the end of Study Week 8 with maintenance of this treatment effect through Study Week 16.
For the primary endpoint, LIVTENCITY was statistically superior to IAT (56% vs 24%, respectively), as shown in Table 10.
| LIVTENCITY 400 mg Twice Daily N=235 n (%) | IAT N=117 n (%) |
|
|---|---|---|
| CI=confidence interval; CMV=cytomegalovirus; IAT=investigator-assigned anti-CMV treatment; N=number of patients. | ||
|
||
| Primary Endpoint: Confirmed CMV DNA Level < LLOQ at Week 8* | ||
| Responders | 131 (56) | 28 (24) |
| Adjusted difference in proportion of responders (95% CI)† | 33 (23, 43) | |
| p-value: adjusted† | <0.001 | |
The reasons for failure to meet the primary endpoint are summarized in Table 11.
| Outcome at Week 8 | LIVTENCITY N=235 n (%) | IAT N=117 n (%) |
|---|---|---|
| CMV=Cytomegalovirus, IAT=Investigator-assigned anti-CMV Treatment, MBV=maribavir. | ||
| Percentages are based on the number of subjects in the Randomized Set. | ||
|
||
| Responders (Confirmed DNA Level < LLOQ)* | 131 (56) | 28 (24) |
| Non-responders: | 104 (44) | 89 (76) |
| Due to virologic failure†: | 80 (34) | 42 (36) |
| • CMV DNA never < LLOQ | 48 (20) | 35 (30) |
| • CMV DNA breakthrough† | 32 (14) | 7 (6) |
| Due to drug/study discontinuation: | 21 (9) | 44 (38) |
| • Adverse events | 8 (3) | 26 (22) |
| • Deaths | 10 (4) | 3 (3) |
| • Withdrawal of consent | 1 (<1) | 9 (8) |
| • Other reasons‡ | 2 (1) | 6 (5) |
| Due to other reasons but remained on study§ | 3 (1) | 3 (3) |
The treatment effect of LIVTENCITY was consistent across transplant type, age group, and the presence of CMV syndrome/disease at baseline. However, LIVTENCITY was less effective against subjects with increased CMV DNA levels (≥50,000 IU/mL) and subjects with absence of genotypic resistance (see Table 12).
| LIVTENCITY 400 mg Twice Daily N=235 | IAT N=117 |
|||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| Transplant type | ||||
| SOT | 79/142 | 56 | 18/69 | 26 |
| HSCT | 52/93 | 56 | 10/48 | 21 |
| Baseline CMV DNA viral load | ||||
| Low (<9,100 IU/mL) | 95/153 | 62 | 21/85 | 25 |
| Intermediate (≥9,100 to <91,000 IU/mL) | 32/68 | 47 | 5/25 | 20 |
| ≥9,100 to <50,000 IU/mL | 29/59 | 49 | 4/20 | 20 |
| ≥50,000 to <91,000 IU/mL | 3/9 | 33 | 1/5 | 20 |
| High (≥91,000 IU/mL) | 4/14 | 29 | 2/7 | 29 |
| Genotypic resistance to other anti-CMV agents | ||||
| Yes | 76/121 | 63 | 14/69 | 20 |
| No | 42/96 | 44 | 11/34 | 32 |
| CMV syndrome/disease at baseline | ||||
| Yes | 10/21 | 48 | 1/8 | 13 |
| No | 121/214 | 57 | 27/109 | 25 |
| Age Group | ||||
| 18 to 44 years | 28/55 | 51 | 8/32 | 25 |
| 45 to 64 years | 71/126 | 56 | 19/69 | 28 |
| ≥65 years | 32/54 | 59 | 1/16 | 6 |
16. How is Livtencity supplied
Tablet: 200 mg, blue, oval shaped convex tablet debossed with "SHP" on one side and "620" on the other side. They are supplied as follows:
Bottles of 28 tablets with child-resistant caps (NDC 64764-800-28)
Bottles of 56 tablets with child-resistant caps (NDC 64764-800-56)
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Inform patients that LIVTENCITY may interact with other drugs. Advise patients to report to their healthcare provider the use of any other medication [see Warnings and Precautions (5.1 and 5.3), Drug Interactions (7)].
Instructions for Use
LIVTENCITY (liv-TEN-city)
(maribavir)
tablets, for oral use
This Instructions for Use contains information on how to prepare and give a dose of LIVTENCITY tablets by breaking apart (dispersing) or crushing in drinking water and taking by mouth; or dispersing and giving through a Nasogastric (NG) or Orogastric (OG) Tube. Read this Instructions for Use before you prepare or give the first dose of LIVTENCITY, and each time you get a refill. Ask your healthcare provider or pharmacist if you have any questions.
Important information you need to know before preparing a dose of LIVTENCITY:
- You can break apart (disperse) the tablets in drinking water or crush the tablets and mix with drinking water. The tablet will not be completely dispersed in the mixture.
- Do not mix LIVTENCITY with any liquid other than drinking water.
- LIVTENCITY tablets that have been dispersed in drinking water can be given through a Nasogastric (NG) or Orogastric (OG) tube (French size 10 or larger).
- You can prepare the mixture ahead of time and store at room temperature 68°F to 77°F (20°C to 25°C) for up to 8 hours.
Preparing a dose of LIVTENCITY by dispersing or crushing tablets and taking by mouth:
Gather the following supplies:
- small, clean container to place tablets and water in
- drinking water
Step 1: Choose a clean, flat work surface. Place all supplies on the work surface.
Step 2: Wash and dry your hands well.
Step 3: Get the prescribed number of LIVTENCITY tablets needed to prepare the dose.
Step 4: Place the LIVTENCITY tablets into the container.
Note: If you prefer, you can crush the tablets with a spoon before adding water.
Step 5: Add the amount of drinking water needed for your prescribed dose.
| Number of Tablets | Amount of Drinking Water |
|---|---|
| 2 | 30 mL |
| 4 | 60 mL |
| 6 | 90 mL |
Step 6: Swirl the container gently to disperse the tablets in the water and swallow the mixture right away. The mixture will have a bitter taste.
Step 7: Rinse the container with 15 mL of drinking water and swallow the mixture.
Repeat Step 7. Check that no pieces of tablet are left in the container. Repeat Step 7 until no pieces remain.
Preparing and giving a dose of LIVTENCITY through a Nasogastric (NG) or Orogastric (OG) Tube:
Gather the following supplies:
- 50 mL or 60 mL syringe
- drinking water
Step 1: Remove the cap (if capped) and plunger out of a 50 mL or 60 mL syringe. Add 2 tablets into the syringe body and place the plunger back in the syringe.
Note: Only 2 tablets can be given through the NG or OG tube at a time.
Step 2: Withdraw 30 mL of drinking water into the syringe.
Step 3: Hold the syringe with the tip pointing upward. Pull the plunger back so there is some air space in the syringe. If there is a cap, place the cap back on the syringe. Shake the syringe well for about 30 to 45 seconds or until the tablets are completely dispersed. Be careful not to spill the contents of the syringe.
Step 4: Remove the cap (if capped) from the syringe again and attach the syringe to the NG or OG tube and give the mixture right away.
Step 5: Withdraw 15 mL of drinking water into the same syringe and flush through the NG or OG tube.
Repeat Step 5. Check that no pieces of tablet are left in the syringe. Repeat Step 5 until no pieces remain.
Note: If your prescribed dose is more than 2 tablets, Repeat Steps 1 through 5 until you give the full prescribed dose.
Storing LIVTENCITY:
- Store LIVTENCITY at room temperature 68°F to 77°F (20°C to 25°C).
Keep LIVTENCITY and all medicines out of the reach of children.
For more information, go to www.LIVTENCITY.com or call 1-877-TAKEDA-7 (1-877-825-3327).
Distributed by: Takeda Pharmaceuticals America, Inc., Lexington, MA 02421
LIVTENCITY™ and the LIVTENCITY Logo™ are trademarks or registered trademarks of Takeda Pharmaceuticals International AG. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited. ©2023 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
MAR358 R3
Revised: April 2023
| LIVTENCITY
maribavir tablet, coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent CTS, LLC | 962674474 | MANUFACTURE(64764-800) , PACK(64764-800) , LABEL(64764-800) , ANALYSIS(64764-800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CARBOGEN AMCIS AG | 481385565 | API MANUFACTURE(64764-800) , ANALYSIS(64764-800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CARBOGEN AMCIS AG | 480055421 | ANALYSIS(64764-800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CARBOGEN AMCIS AG | 480029695 | ANALYSIS(64764-800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| JETPHARMA SA | 481885861 | PARTICLE SIZE REDUCTION(64764-800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Solvias AG | 480739627 | ANALYSIS(64764-800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Privates Untersuchungsinstitut Heppeler GmbH | 332632009 | ANALYSIS(64764-800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda Ireland Limited | 988980314 | PACK(64764-800) , LABEL(64764-800) , ANALYSIS(64764-800) | |





