Drug Detail:Lo loestrin fe (Ethinyl estradiol and norethindrone acetate)
Drug Class: Contraceptives Sex hormone combinations
Highlights of Prescribing Information
Lo Loestrin® Fe (norethindrone acetate and ethinyl estradiol tablets, ethinyl estradiol tablets and ferrous fumarate tablets)
Initial U.S. Approval: 1968
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
See Full Prescribing Information for complete boxed warning.
-
Women over 35 years old who smoke should not use Lo Loestrin Fe (4)
- Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use (4)
Recent Major Changes
| Warnings and Precautions (5.2) | 04/2022 |
Indications and Usage for Lo loestrin Fe
- Lo Loestrin Fe is a combination of norethindrone acetate, a progestin, and ethinyl estradiol, an estrogen, indicated for use by women to prevent pregnancy (1)
- The efficacy of Lo Loestrin Fe in women with a body mass index of > 35 kg/m2 has not been evaluated (1, 8.8)
Lo loestrin Fe Dosage and Administration
- Take one tablet by mouth at the same time every day for 28 days (2.1)
- Take tablets in the order directed on the blister pack (2.1)
Dosage Forms and Strengths
Lo Loestrin Fe consists of 28 tablets in the following order (3):
- 24 blue tablets (active), each containing 1 mg norethindrone acetate and 10 mcg ethinyl estradiol
- 2 white tablets (active), each containing 10 mcg ethinyl estradiol
- 2 brown tablets (non-hormonal placebo), each containing 75 mg ferrous fumarate. The ferrous fumarate tablets do not serve any therapeutic purpose
Contraindications
- A high risk of arterial or venous thrombotic diseases (4)
- Breast cancer (4)
- Liver tumors or liver disease (4)
- Undiagnosed abnormal uterine bleeding (4)
- Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir (4)
Warnings and Precautions
- Vascular risks: Stop Lo Loestrin Fe if a thrombotic event occurs. Stop Lo Loestrin Fe at least 4 weeks before through 2 weeks after major surgery. Start Lo Loestrin Fe no earlier than 4 weeks after delivery, in women who are not breastfeeding (5.1)
- Liver disease: Discontinue Lo Loestrin Fe if jaundice occurs (5.3)
- High blood pressure: Do not prescribe Lo Loestrin Fe for women with uncontrolled hypertension or hypertension with vascular disease. (5.5)
- Carbohydrate and lipid metabolic effects: Monitor prediabetic and diabetic women taking Lo Loestrin Fe. Consider an alternative contraceptive method for women with uncontrolled dyslipidemia (5.7)
- Headache: Evaluate significant change in headaches and discontinue Lo Loestrin Fe if indicated (5.8)
- Uterine bleeding: Evaluate irregular bleeding or amenorrhea (5.9)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 2 percent) are nausea/vomiting (7 percent), headache (7 percent), bleeding irregularities (5 percent), dysmenorrhea (4 percent), weight change (4 percent), breast tenderness (4 percent), acne (3 percent), abdominal pain (3 percent), anxiety (2 percent) and depression (2 percent) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Drugs or herbal products that induce certain enzymes, including CYP3A4, may decrease the effectiveness of COCs or increase breakthrough bleeding. Counsel patients to use a back-up method or alternative method of contraception when enzyme inducers are used with COCs (7.1)
-------------------USE IN SPECIFIC POPULATIONS---------------------
- Lactation: Not recommended; Lo Loestrin Fe can decrease milk production (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2022
Full Prescribing Information
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs should not be used by women who are over 35 years of age and smoke [see Contraindications (4)].
1. Indications and Usage for Lo loestrin Fe
Lo Loestrin® Fe is indicated for use by women to prevent pregnancy [see Clinical Studies (14)].
The efficacy of Lo Loestrin Fe in women with a body mass index (BMI) of > 35 kg/m2 has not been evaluated.
2. Lo loestrin Fe Dosage and Administration
2.1 How to Take Lo Loestrin Fe
To achieve maximum contraceptive effectiveness, Lo Loestrin Fe must be taken exactly as directed. Take one tablet by mouth at the same time every day. Tablets must be taken in the order directed on the blister pack. Tablets should not be skipped or taken at intervals exceeding 24 hours. Lo Loestrin Fe tablets may be administered without regard to meals [see Clinical Pharmacology (12.3)].
2.2 How to Start Lo Loestrin Fe
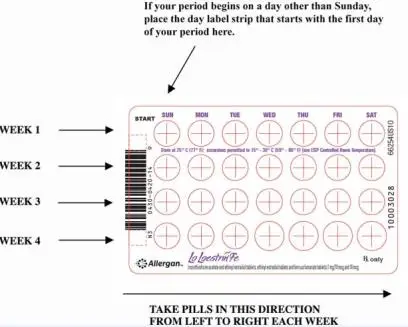
Instruct the patient to begin taking Lo Loestrin Fe on Day 1 of her menstrual cycle (that is, the first day of her menstrual bleeding). One blue tablet should be taken daily for 24 consecutive days, followed by one white tablet daily for 2 consecutive days, followed by one brown tablet daily for 2 consecutive days. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days if she starts taking Lo Loestrin Fe other than on the first day of her menstrual cycle.
For postpartum women who do not breastfeed or after a second trimester abortion, Lo Loestrin Fe may be started no earlier than 4 weeks postpartum. Recommend use of a non-hormonal back-up method for the first 7 days. When COCs are used during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered [see Warnings and Precautions (5.1)]. The possibility of ovulation and conception before starting COCs should also be considered.
Lo Loestrin Fe may be initiated immediately after a first-trimester abortion or miscarriage; if the patient starts Lo Loestrin Fe immediately, additional contraceptive measures are not needed.
2.4 Missed Doses
Table 1: Instructions for Missed Lo Loestrin Fe Tablets in a Monthly Dosing Regimen
| Take the tablet as soon as possible, even if two tablets are taken in one day. Continue taking one tablet a day until the pack is finished. |
| Take the two missed tablets as soon as possible, and the next two tablets the next day. Continue taking the remaining tablets, one tablet a day until the pack is finished. Use additional non-hormonal contraception (such as condoms and spermicide) for 7 consecutive days after missing tablets. |
| Throw out the rest of the pack and start a new pack the same day. A withdrawal bleed may not occur. Use additional non-hormonal contraception (such as condoms and spermicide) for 7 consecutive days after missing tablets. |
| Throw out the rest of the pack and start a new pack that same day. A withdrawal bleeding may not occur. Use additional non-hormonal contraception (such as condoms and spermicide) for 7 consecutive days after missing tablets. |
| Throw out the tablet you missed. Start a new pack on the same day a new pack is usually started. |
3. Dosage Forms and Strengths
Lo Loestrin Fe (norethindrone acetate and ethinyl estradiol tablets, ethinyl estradiol tablets and ferrous fumarate tablets) is available in blister packs.
Each blister pack (28 tablets) contains in the following order:
- 24 blue, round (active) tablets imprinted with “WC” on one side and “421” on the other and each containing 1 mg norethindrone acetate and 10 mcg ethinyl estradiol.
- 2 white, hexagonal (active) tablets imprinted with “WC” on one side and “422” on the other and each containing 10 mcg ethinyl estradiol.
- 2 brown, round (non-hormonal placebo) tablets imprinted with “WC” on one side and “624” on the other and each containing 75 mg ferrous fumarate. The ferrous fumarate tablets do not serve any therapeutic purpose.
4. Contraindications
Lo Loestrin Fe is contraindicated in females who are known to have or develop the following conditions:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
• Smoke, if over age 35 [see Boxed Warning and Warnings and Precautions (5.1)]
• Have deep vein thrombosis or pulmonary embolism, now or in the past [see Warnings and Precautions (5.1)]
• Have cerebrovascular disease [see Warnings and Precautions (5.1)]
• Have coronary artery disease [see Warnings and Precautions (5.1)]
• Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1)]
• Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1)]
• Have uncontrolled hypertension [see Warnings and Precautions (5.5)]
• Have diabetes mellitus with vascular disease [see Warnings and Precautions (5.7)]
• Have headaches with focal neurological symptoms or have migraine headaches with or without aura if over age 35 [see Warnings and Precautions (5.8)]
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive [see Warnings and Precautions (5.2)]
- Liver tumors, benign or malignant, or liver disease [see Warnings and Precautions (5.3)]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.9)]
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see Warnings and Precautions (5.4)].
5. Warnings and Precautions
5.1 Thrombotic and Other Vascular Events
Stop Lo Loestrin Fe if an arterial or deep venous thrombotic event occurs. Although use of COCs increases the risk of venous thromboembolism, pregnancy increases the risk of venous thromboembolism as much or more than the use of COCs. The risk of venous thromboembolism in women using COCs is 3 to 9 per 10,000 woman-years. The risk is highest during the first year of use of a COC. Use of COCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. The risk of thromboembolic disease due to oral contraceptives gradually disappears after COC use is discontinued.
If feasible, stop Lo Loestrin Fe at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start Lo Loestrin Fe no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
COCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest in older (> 35 years of age), hypertensive women who also smoke. COCs also increase the risk for stroke in women with underlying risk factors.
Oral contraceptives must be used with caution in women with cardiovascular disease risk factors.
Stop Lo Loestrin Fe if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
5.2 Malignant Neoplasms
Breast Cancer
Lo Loestrin Fe is contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive [see Contraindications (4)].
Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use [see Adverse Reactions (6.2)].
Cervical Cancer
Some studies suggest that COCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which these findings may be due to differences in sexual behavior and other factors.
5.3 Liver Disease
Discontinue Lo Loestrin Fe if jaundice develops. Steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded.
Hepatic adenomas are associated with COC use. An estimate of the attributable risk is 3.3 cases per 100,000 COC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (>8 years) COC users. However, the attributable risk of liver cancers in COC users is less than one case per million users.
Oral contraceptive-related cholestasis may occur in women with a history of pregnancy-related cholestasis. Women with a history of COC-related cholestasis may have the condition recur with subsequent COC use.
5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as COCs. Discontinue Lo Loestrin Fe prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications (4)]. Lo Loestrin Fe can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
5.5 High Blood Pressure
For women with well-controlled hypertension, monitor blood pressure and stop Lo Loestrin Fe if blood pressure rises significantly. Women with uncontrolled hypertension or hypertension with vascular disease should not use COCs.
An increase in blood pressure has been reported in women taking COCs, and this increase is more likely in older women with extended duration of use. The incidence of hypertension increases with increasing concentrations of progestin.
5.7 Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who are taking Lo Loestrin Fe. COCs may decrease glucose tolerance in a dose-related fashion.
Consider alternative contraception for women with uncontrolled dyslipidemias. A small proportion of women will have adverse lipid changes while on COCs.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
5.8 Headache
If a woman taking Lo Loestrin Fe develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue Lo Loestrin Fe if indicated.
An increase in frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation of the COC.
5.9 Bleeding Irregularities and Amenorrhea
Unscheduled (breakthrough or intracyclic) bleeding and spotting sometimes occur in patients on COCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different COC.
The clinical trial that evaluated the efficacy of Lo Loestrin Fe also assessed unscheduled bleeding and/or spotting. The participants in this 12-month clinical trial (N = 1,582 who had at least one post-treatment evaluation) completed over 15,000 cycles of exposure.
A total of 1,257 women (85.9 percent) experienced unscheduled bleeding and/or spotting at some time during Cycles 2 to 13 of this study. The incidence of unscheduled bleeding and/or spotting was highest during Cycle 2 (53 percent) and lowest at Cycle 13 (36 percent). Among these women, the mean number of days of unscheduled bleeding and/or spotting during a 28-day cycle ranged from 1.8 to 3.2 days.
Scheduled (withdrawal) bleeding and/or spotting remained fairly constant over the one year study, with an average of less than 2 days per cycle.
Women who are not pregnant and use Lo Loestrin Fe may experience amenorrhea (absence of scheduled and unscheduled bleeding/spotting). In the clinical trial with Lo Loestrin Fe, the incidence of amenorrhea increased from 32 percent in Cycle 1 to 49 percent by Cycle 13. If scheduled (withdrawal) bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
Some women may experience amenorrhea or oligomenorrhea after stopping COCs, especially when such a condition was preexistent.
6. Adverse Reactions/Side Effects
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- Serious cardiovascular events and smoking [see Boxed Warning and Warnings and Precautions (5.1)]
- Vascular events [see Warnings and Precautions (5.1)]
- Liver disease [see Warnings and Precautions (5.3)]
Adverse reactions commonly reported by COC users are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
6.2 Postmarketing Experience
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 1).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 1). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
Figure 1.
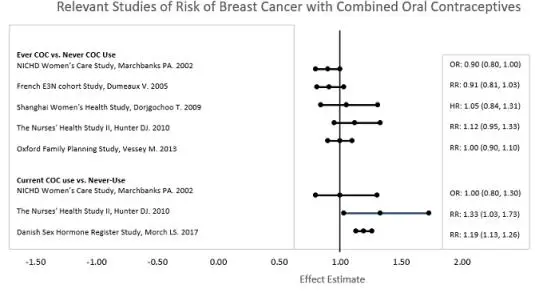
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
7. Drug Interactions
No drug-drug interaction studies were conducted with Lo Loestrin Fe.
7.1 Changes in Contraceptive Effectiveness Associated with Co-Administration of Other Products
If a woman on hormonal contraceptives takes a drug or herbal product that induces enzymes, including CYP3A4, that metabolize contraceptive hormones, counsel her to use additional contraception or a different method of contraception. Drugs or herbal products that induce such enzymes may decrease the plasma concentrations of contraceptive hormones, and may decrease the effectiveness of hormonal contraceptives or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include:
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John’s wort
- topiramate
HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant changes (increase or decrease) in the plasma levels of the estrogen and progestin have been noted in some cases of co-administration of HIV protease inhibitors or of non-nucleoside reverse transcriptase inhibitors.
Antibiotics: There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There is no use for contraception in pregnancy; therefore, Lo Loestrin Fe should be discontinued during pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb reduction defects) following exposure to combined hormonal contraceptives (CHCs) before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
Data
Human Data
Epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to CHCs before conception or during early pregnancy.
8.2 Lactation
Risk Summary
Contraceptive hormones and/or metabolites are present in human milk. CHCs can reduce milk production in breastfeeding females. This reduction can occur at any time but is less likely to occur once breastfeeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breastfeeding. [See also Dosage and Administration (2.2).] The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Lo Loestrin Fe and any potential adverse effects on the breastfed child from Lo Loestrin Fe or from the underlying maternal condition.
8.6 Renal Impairment
The pharmacokinetics of Lo Loestrin Fe has not been studied in subjects with renal impairment.
8.7 Hepatic Impairment
No studies have been conducted to evaluate the effect of hepatic impairment on the disposition of Lo Loestrin Fe. However, steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded [see Contraindications (4) and Warnings and Precautions (5.3)].
12. Lo loestrin Fe - Clinical Pharmacology
12.3 Pharmacokinetics
Absorption
Norethindrone acetate is deacetylated to norethindrone after oral administration, and the disposition of norethindrone acetate is indistinguishable from that of orally administered norethindrone. Norethindrone acetate and ethinyl estradiol are absorbed from Lo Loestrin Fe, with maximum plasma concentrations of norethindrone and ethinyl estradiol generally occurring 1 to 2 hours postdose. Both are subject to first-pass metabolism after oral dosing, resulting in an absolute bioavailability of approximately 64 percent for norethindrone and 55 percent for ethinyl estradiol.
The rate of norethindrone and ethinyl estradiol absorption from Lo Loestrin Fe tablets containing the combination of 1 mg norethindrone acetate and 10 mcg ethinyl estradiol is slower than that from a norethindrone suspension/ethinyl estradiol solution, but the extent of absorption is equivalent.
Ethinyl estradiol bioavailability from Lo Loestrin Fe tablets containing 10 mcg ethinyl estradiol alone is equivalent to that from an ethinyl estradiol solution.
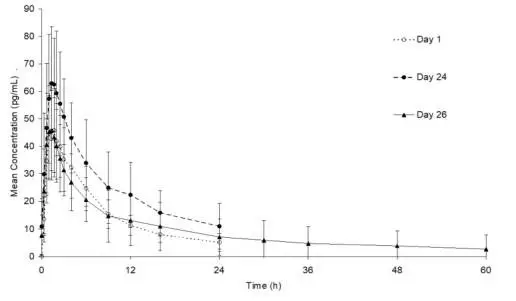
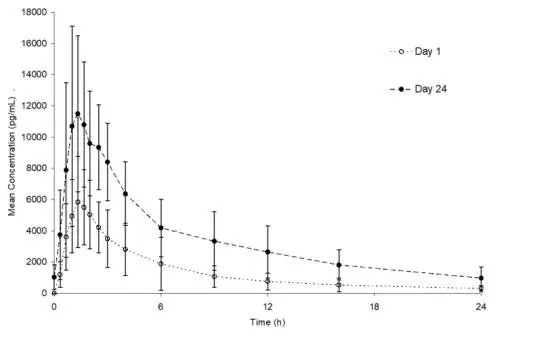
The plasma norethindrone and ethinyl estradiol pharmacokinetic profiles and serum sex hormone binding globulin (SHBG) concentrations following multiple-dose administration of Lo Loestrin Fe were characterized in 15 healthy female volunteers. The mean plasma concentrations are shown below (Figures 2 and 3), and pharmacokinetic parameters are found in Table 2.
Ethinyl estradiol and norethindrone Cmax values increase by a factor of 1.4 and 1.9, respectively, following 24 days administration of Lo Loestrin Fe combination tablets as compared to single-dose administration. Ethinyl estradiol and norethindrone AUC0–24h values increase by a factor of 1.6 and 2.5, respectively, following 24 days administration of Lo Loestrin Fe combination tablets as compared to single-dose administration. Norethindrone concentrations more than double by Day 24 due to both accumulation and increased SHBG concentration. Steady state with respect to ethinyl estradiol and norethindrone is reached by Day 5 and Day 13, respectively.
Figure 2. Mean (± SD) plasma ethinyl estradiol concentration versus time profiles following single- and multiple-dose oral administration of Lo Loestrin Fe to healthy female volunteers (n = 15)
Figure 3. Mean (± SD) plasma norethindrone concentration versus time profiles following single- and multiple-dose oral administration of Lo Loestrin Fe to healthy female volunteers (n = 15)
Table 2. Summary of Norethindrone (NE) and Ethinyl Estradiol (EE) Pharmacokinetic Parameter Values Following Oral Administration of Lo Loestrin Fe to Healthy Female Volunteers (n = 15)
| Regimen | Study Day | Arithmetic Meana (Percent CV) by Pharmacokinetic Parameter | |||||
| Analyte | Cmax | tmax | AUC0-24h | Cmin | Cavg | ||
| Single Dose Lo Loestrin Fe combination tabletc | 1 | NE | 7360 (21) | 1.7 (1.3-6.0) | 33280 (33) | -- | -- |
| EE | 50.9 (27) | 1.3 (1.0-6.0) | 389.9 (27) | -- | -- | ||
| SHBG | -- | -- | -- | 54.8 (33)b | -- | ||
| Multiple Dose Lo Loestrin Fe combination tabletc x 24 days | 24 | NE | 13900 (34) | 1.3 (0.7-3.0) | 84160 (41) | 917 (84) | 3510 (41) |
| EE | 71.3 (33) | 1.3 (0.3–2.0) | 621.3 (41) | 10.0 (92) | 25.9 (41) | ||
| SHBG | -- | -- | -- | 109 (38) | -- | ||
| Multiple Dose Lo Loestrin Fe combination tabletc x 24 days and ethinyl estradiol alone tabletd x 2 days | 26 | EE | 49.9 (34) | 1.3 (0.7–3.0) | 403.6 (50) | -- | -- |
| Cmax = Maximum plasma concentration (pg/mL); tmax = Time of Cmax (h); AUC0-24h = Area under plasma concentration versus time curve from 0 to 24 hours (pg·h/mL); Cmin = Minimum plasma concentration (pg/mL); Cavg = Average plasma concentration = AUC0-24h/24 (pg/mL) Percent CV = Coefficient of Variation (percent); SHBG = Sex hormone binding globulin (nmol/L) aThe median (range) is reported for tmax bThe Cmin concentration reported for SHBG is the pre-dose concentration cLo Loestrin Fe combination tablets contain 1 mg norethindrone acetate and 10 mcg ethinyl estradiol dLo Loestrin Fe ethinyl estradiol alone tablets contain 10 mcg ethinyl estradiol |
|||||||
Food Effect:
Lo Loestrin Fe tablets may be administered without regard to meals.
Administration of food with a single-dose of a Lo Loestrin Fe combination tablet did not affect the maximum concentration of norethindrone and increased the extent of absorption by 24 percent; it decreased the maximum concentration of ethinyl estradiol by 23 percent and did not affect the extent of absorption.
Administration of food with a single-dose of a Lo Loestrin Fe ethinyl estradiol alone tablet decreased the maximum concentration of ethinyl estradiol by 31 percent and did not affect the extent of absorption.
Distribution
Volume of distribution of norethindrone and ethinyl estradiol ranges from 2 to 4 L/kg. Plasma protein binding of both steroids is extensive (>95 percent); norethindrone binds to both albumin and SHBG, whereas ethinyl estradiol binds only to albumin. Although ethinyl estradiol does not bind to SHBG, it induces SHBG synthesis.
Metabolism
Norethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation. The majority of metabolites in the circulation are sulfates, with glucuronides accounting for most of the urinary metabolites. A small amount of norethindrone acetate is metabolically converted to ethinyl estradiol.
Ethinyl estradiol is also extensively metabolized, both by oxidation and by conjugation with sulfate and glucuronide. Sulfates are the major circulating conjugates of ethinyl estradiol and glucuronides predominate in urine. The primary oxidative metabolite is 2-hydroxy ethinyl estradiol, formed by the CYP3A4 isoform of cytochrome P450. Part of the first-pass metabolism of ethinyl estradiol is believed to occur in gastrointestinal mucosa. Ethinyl estradiol may undergo enterohepatic circulation.
Excretion
Norethindrone and ethinyl estradiol are excreted in both urine and feces, primarily as metabolites. Plasma clearance values for norethindrone and ethinyl estradiol are similar (approximately 0.4 L/hr/kg). Elimination half-lives of norethindrone and ethinyl estradiol following administration of 1 mg norethindrone acetate/10 mcg ethinyl estradiol tablets are approximately 10 hours and 16 hours, respectively.
Specific populations
The pharmacokinetics of Lo Loestrin Fe in presence of renal or hepatic impairment has not been evaluated [see Use in Specific Populations (8.6) and (8.7)].
14. Clinical Studies
In a one year (thirteen 28-day cycles) multicenter open-label clinical trial, 1,270 women 18 to 35 years of age, were studied to assess the efficacy of Lo Loestrin Fe, completing the equivalent of 12,482 28-day evaluable cycles of exposure. The racial demographic of all enrolled women was: Caucasian (74.9 percent), African-American (11.8 percent), Hispanic (9.8 percent), Asian (1.3 percent), and Other (2.2 percent). Women with body mass index (BMI) greater than 35 kg/m2 were excluded from the study. The weight range for those women treated was 89 to 260 lbs., with a mean weight of 150 lbs. Among the women in the trial, 51 percent had not used hormonal contraception immediately prior to enrolling in this study. Of treated women, 13.7 percent were lost to follow-up, 10.7 percent discontinued due to an adverse event, and 8.9 percent discontinued by withdrawing their consent.
The pregnancy rate (Pearl Index [PI]) in women 18 to 35 years of age was 2.92 pregnancies per 100 women-years of use (95 percent confidence interval 1.94 – 4.21), based on 28 pregnancies that occurred after the onset of treatment and extending through the 7 days following the last dose of Lo Loestrin Fe. Cycles in which conception did not occur, but which included the use of backup contraception, were not included in the calculation of the PI. The PI includes women who did not take the drug correctly.
| LO LOESTRIN FE
norethindrone acetate and ethinyl estradiol, ethinyl estradiol and ferrous fumarate kit |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |




![The chemical name of ethinyl estradiol is [19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol, (17)-].](https://cdn.themeditary.com/images/2023/09/01/lo-loestrin-fe-02.webp)
![The chemical name of norethindrone acetate is [19-Norpregn-4-en-20-yn-3-one, 17-(acetyloxy)-, (17)-].](https://cdn.themeditary.com/images/2023/09/01/lo-loestrin-fe-03.webp)