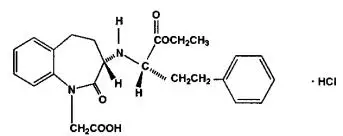
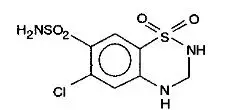
Drug Detail:Lotensin hct (Hydrochlorothiazide and benazepril [ hye-droe-klor-oh-thy-a-zide-and-ben-az-e-pril ])
Drug Class: ACE inhibitors with thiazides
Warnings
Fetal toxicity
Pregnancy
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Lotensin HCT as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Lotensin HCT, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Lotensin HCT for hypotension, oliguria, and hyperkalemia (see PRECAUTIONS, Pediatric Use).
No teratogenic effects of Lotensin were seen in studies of pregnant rats, mice, and rabbits. On a mg/m2 basis, the doses used in these studies were 60 times (in rats), 9 times (in mice), and more than 0.8 times (in rabbits) the maximum recommended human dose (assuming a 50-kg woman). On a mg/kg basis these these multiples are 300 times (in rats), 90 times (in mice), and more than 3 times (in rabbits) the maximum recommended human dose. When hydrochlorothiazide was orally administered without benazepril to pregnant mice and rats during their respective periods of major organogenesis, at doses up to 3000 and 1000 mg/kg/day respectively, there was no evidence of harm to the fetus. Similarly, no teratogenic effects of benazepril were seen in studies of pregnant rats, mice, and rabbits; on a mg/kg basis, the doses used in these studies were 300 times (in rats), 90 times (in mice), and more than 3 times (in rabbits) the maximum recommended human dose.
Thiazides can cross the placenta, and concentrations reached in the umbilical vein approach those in the maternal plasma Hydrochlorothiazide, like other diuretics, can cause placental hypoperfusion. It accumulates in the amniotic fluid, with reported concentrations up to 19 times higher than in umbilical vein plasma. Use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice or thrombocytopenia. Since they do not prevent or alter the course of EPH (Edema, Proteinuria, Hypertension) gestosis (pre-eclampsia), these drugs must not be used to treat hypertension in pregnant women. The use of hydrochlorothiazide for other indications (e.g., heart disease) in pregnancy should be avoided.
Precautions
Drug Interactions
Neprilysin Inhibitors:
Patients taking concomitant neprilysin inhibitors may be at increased risk for angioedema.
Interactions Common for Both Benazepril and Hydrochlorothiazide
Potassium Supplements and Potassium-Sparing Diuretics: Concomitant use with Lotensin HCT may effect potassium levels. Monitor potassium periodically.
mTOR (mammalian target of rapamycin) inhibitors: Patients receiving coadministration of ACE inhibitor and mTOR inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema (see WARNINGS).
Lithium: Renal clearance of lithium is reduced by thiazides and increase the risk of lithium toxicity. Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium. Monitor lithium levels when used concomitantly with Lotensin HCT.
Dual Blockade of the Renin-Angiotensin System (RAS): Dual Blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypertension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on Lotensin HCT and other agents that affect the RAS.
Do not coadminister aliskiren with Lotensin HCT in patients with diabetes. Avoid use of aliskiren with Lotensin HCT in patients with renal impairment (GFR < 60 mL/min).
NSAIDs and Cox-2 selective agents: In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including benazepril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving benazepril and NSAID therapy.
The antihypertensive effect of benazepril and hydrochlorothiazide may be attenuated by NSAIDs.
Benazepril
Benazepril has been used concomitantly with beta-adrenergic-blocking agents, calcium-blocking agents, cimetidine, diuretics, digoxin, hydralazine, and naproxen without evidence of clinically important adverse interactions. Other ACE inhibitors have had less than additive effects with beta-adrenergic blockers, presumably because drugs of both classes lower blood pressure by inhibiting parts of the renin-angiotensin system.
Interaction studies with warfarin and acenocoumarol have failed to identify any clinically important effects of benazepril on the serum concentrations or clinical effects of these anticoagulants.
Gold: Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension)
have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy.
Hydrochlorothiazide
Ion exchange resins: Stagger the dosage of hydrochlorothiazide and ion exchange resins such that hydrochlorothiazide is administered at least 4 hours before or 4 to 6 hours after the administration of resins. Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively.
Digitalis glycosides: Thiazide-induced hypokalemia or hypomagnesemia may predispose the patients to digoxin toxicity
Skeletal muscle relaxants: Possible increased responsiveness to muscle relaxants such as curare derivatives.
Antidiabetic agents: Dosage adjustment of antidiabetic drug may be required.
Antineoplastic agents (e.g., cyclophosphamide, methotrexate): Concomitant use of thiazide diuretics may reduce renal excretion of cytotoxic agents and enhance their myelosuppressive effects.
Drugs that alter gastrointestinal motility: The bioavailability of thiazide-type diuretics may be increased by anticholinergic agents (e.g., atropine, biperiden), apparently due to a decrease in gastrointestinal motility and the stomach emptying rate. Conversely, pro-kinetic drugs may decrease the bioavailability of thiazide diuretics.
Cyclosporin: Concomitant treatment with diuretics may increase the risk of hyperuricaemia and gout-type complications.
Alcohol, barbiturates or narcotics: Concomitant administration of thiazide diuretics with alcohol, barbiturates, or narcotics may potentiate orthostatic hypotension.
Pressor amines: Hydrochlorothiazide may reduce the response to pressor amines such as noradrenaline but the clinical significance of this effect is not sufficient to preclude their use.
Non-clinical safety data
Pediatric Use
Neonates with a history of in utero exposure to Lotensin HCT:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Benazepril, which crosses the placenta, can theoretically be removed from the neonatal circulation by these means; there are occasional reports of benefit from these maneuvers with another ACE inhibitor, but experience is limited.
Safety and effectiveness in pediatric patients have not been established.
Adverse Reactions/Side Effects
Lotensin HCT has been evaluated for safety in over 2500 patients with hypertension; over 500 of these patients were treated for at least 6 months, and over 200 were treated for more than 1 year.
The reported side effects were generally mild and transient, and there was no relationship between side effects and age, sex, race, or duration of therapy. Discontinuation of therapy due to side effects was required in approximately 7% of U.S. patients treated with Lotensin HCT and in 4% of patients treated with placebo.
The most common reasons for discontinuation of therapy with Lotensin HCT in U.S. studies were cough (1.0%; see PRECAUTIONS), “dizziness” (1.0%), headache (0.6%), and fatigue (0.6%).
The side effects considered possibly or probably related to study drug that occurred in U.S. placebo-controlled trials in more than 1% of patients treated with Lotensin HCT are shown in the table below.
| Reactions Possibly or Probably Drug Related
Patients in U.S. Placebo-Controlled Studies |
||||
| LOTENSIN HCT
N = 665 | Placebo
N = 235 |
|||
| N | % | N | % | |
| “Dizziness” | 41 | 6.3 | 8 | 3.4 |
| Fatigue | 34 | 5.2 | 6 | 2.6 |
| Postural Dizziness | 23 | 3.5 | 1 | 0.4 |
| Headache | 20 | 3.1 | 10 | 4.3 |
| Cough | 14 | 2.1 | 3 | 1.3 |
| Hypertonia | 10 | 1.5 | 3 | 1.3 |
| Vertigo | 10 | 1.5 | 2 | 0.9 |
| Nausea | 9 | 1.4 | 2 | 0.9 |
| Impotence | 8 | 1.2 | 0 | 0.0 |
| Somnolence | 8 | 1.2 | 1 | 0.4 |
Other side effects considered possibly or probably related to study drug that occurred in U.S. placebo-controlled trials in 0.3% to 1.0% of patients treated with Lotensin HCT were the following:
Cardiovascular: Palpitations, flushing.
Gastrointestinal: Vomiting, diarrhea, dyspepsia, anorexia, and constipation.
Neurologic and Psychiatric: Insomnia, nervousness, paresthesia, libido decrease, dry mouth, taste perversion, and tinnitus.
Dermatologic: Rash and sweating.
Other: Urinary frequency, arthralgia, myalgia, asthenia, and pain (including chest pain and abdominal pain).
Other adverse experiences reported in 0.3% or more of Lotensin HCT patients in U.S. controlled clinical trials, and rarer events seen in post-marketing experience, were the following; asterisked entries occurred in more than 1% of patients (in some, a causal relationship to Lotensin HCT is uncertain):
Cardiovascular: Syncope, peripheral vascular disorder, and tachycardia.
Body as a Whole: Infection, back pain*, flu syndrome*, fever, chills, and neck pain.
Dermatologic: Photosensitivity and pruritus.
Gastrointestinal: Gastroenteritis, flatulence, and tooth disorder.
Neurologic and Psychiatric: Hypesthesia, abnormal vision, abnormal dreams, and retinal disorder.
Respiratory: Upper respiratory infection*, epistaxis, bronchitis, rhinitis*, sinusitis*, and voice alteration.
Other: Conjunctivitis, arthritis, urinary tract infection, alopecia, and urinary frequency*.
Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of either benazepril or hydrochlorothiazide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure:
Non-melanoma Skin Cancer: Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
Benazepril Stevens-Johnson syndrome, pancreatitis, hemolytic anemia, pemphigus, and thrombocytopenia, eosinophilic pneumonitis
Hydrochlorothiazide
Digestive: Pancreatitis, small bowel angioedema, jaundice (intrahepatic cholestatic), sialadenitis, vomiting, diarrhea, cramping, nausea, gastric irritation, constipation, and anorexia.
Neurologic: Vertigo, lightheadedness, transient blurred vision, headache, paresthesia, xanthopsia, weakness, and restlessness.
Musculoskeletal: Muscle spasm.
Hematologic: Aplastic anemia, agranulocytosis, leukopenia, neutropenia and thrombocytopenia.
Metabolic: Hyperglycemia, glycosuria, and hyperuricemia, pyrexia, asthenia, parathyroid gland changes with hypercalcemia and hypophosphatemia.
Hypersensitivity: Anaphylactoid reactions, necrotizing angiitis, respiratory distress (including pneumonitis and pulmonary edema), purpura, urticaria, rash, and photosensitivity.
Skin: Erythema multiforme including Stevens-Johnson syndrome, and exfoliative dermatitis including toxic epidermal necrolysis.
Lotensin HCT Dosage and Administration
Dose once daily. The dosage may then be increased after 2 to 3 weeks as needed to help achieve blood pressure goals. The maximum recommended dose is 20/25 mg.
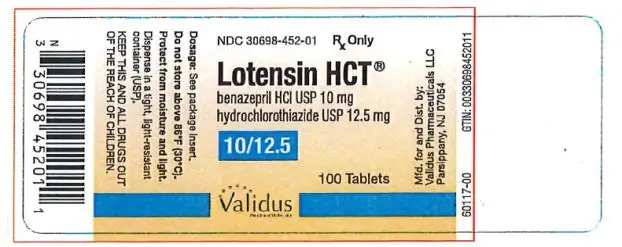
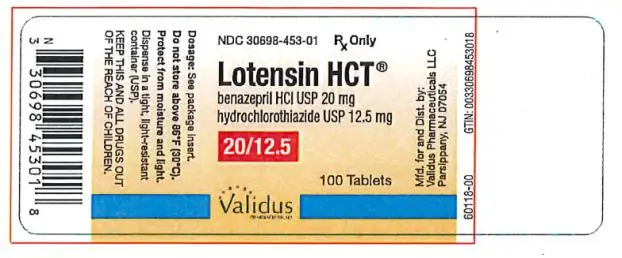
Switch Therapy: A patient whose blood pressure is not adequately controlled with benazepril alone or with hydrochlorothiazide alone may be switched to combination therapy with Lotensin HCT. The usual recommended starting dose is 10/12.5 mg once daily to control blood pressure.
Replacement Therapy: The combination may be substituted for the titrated individual components.
| LOTENSIN HCT
benazepril hydrochloride and hydrochlorothiazide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LOTENSIN HCT
benazepril hydrochloride and hydrochlorothiazide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LOTENSIN HCT
benazepril hydrochloride and hydrochlorothiazide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Validus Pharmaceuticals LLC (801194619) |