Drug Detail:Low-ogestrel (Ethinyl estradiol and norgestrel [ eth-in-il-ess-tra-dye-ol-and-nor-jess-trel ])
Drug Class: Contraceptives
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age and smoke [see Contraindications].
Contraindications
Low-Ogestrel is contraindicated in females who are known to have or develop the following conditions:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- Smoke, if over age 35
- Have deep-vein thrombosis or pulmonary embolism, now or in the past
- Have inherited or acquired coagulopathies
- Have cerebrovascular disease
- Have coronary artery disease
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease or atrial fibrillation)
- Have uncontrolled hypertension
- Have diabetes mellitus with vascular disease
- Headaches with focal neurological symptoms or migraine headaches with aura
- Women over age 35 with any migraine headaches
- Liver tumors, benign or malignant, or liver disease
- Undiagnosed abnormal uterine bleeding
- Pregnancy, because there is no reason to use COCs during pregnancy
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive
- Hypersensitivity to any of the components of Low-Ogestrel
Women who are receiving Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT Elevations (see Warnings, Risk of liver enzyme elevations with concomitant hepatitis c treatment).
Warnings
2. Liver Disease
Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications such as COCs. Discontinue Low-Ogestrel prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications]. Low-Ogestrel can be restarted approximately 2 weeks following completion of treatment with the combination drug regimen.
Precautions
4. Drug Interactions
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
Effects of Other Drugs on Combined Oral Contraceptives
Adverse Reactions/Side Effects
An increased risk of the following serious adverse reactions (see Warnings section for additional information) has been associated with the use of oral contraceptives:
- Serious cardiovascular events and stroke [see Boxed Warning]
- Vascular events
- Liver disease
Adverse reactions commonly reported by COC users are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
Postmarketing Experience
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 1).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 1). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
Figure 1: Risk of Breast Cancer with Combined Oral Contraceptive Use
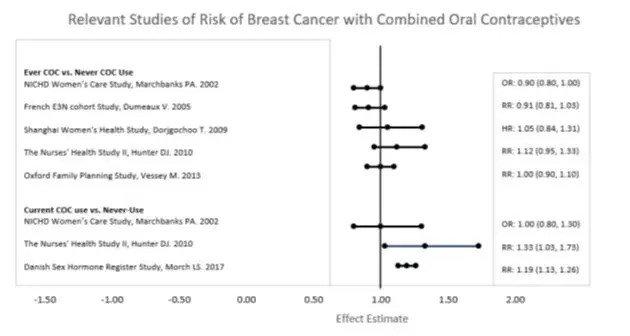
RR = relative risk; OR = odds ratio; HR = hazard ratio. "ever COC" are females with current or past COC use; "never COC use" are females that never used COCs.
The following additional adverse drug reactions have been reported from worldwide postmarketing experience with Low-Ogestrel. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Arterial Events: Arterial thromboembolism, Myocardial infarction, Cerebral hemorrhage
Eye Disorder: Optic neuritis, which may lead to partial or complete loss of vision, Intolerance to contact lenses, Change (steepening) in corneal curvature
Gastrointestinal Disorders: Colitis, Nausea, Pancreatitis
Hepatobiliary Disorders: Gallbladder disease, Cholestatic jaundice, Budd-Chiari syndrome
Immune System Disorders: Anaphylactic/anaphylactoid reactions, including urticaria, angioedema, and severe reactions with respiratory and circulatory symptoms
Metabolism and Nutrition Disorders: Carbohydrate and lipid effects, Porphyria, exacerbation of Porphyria
Neoplasms, Benign, Malignant, and Unspecified: Carcinoma of the reproductive organs and breasts, Hepatic neoplasia (including hepatic adenomas or benign liver tumors)
Psychiatric Disorders: Mood changes
Reproductive System and Breast Disorders: Temporary infertility after discontinuation of treatment, Changes in libido, Vaginitis, including candidiasis; Breast secretion
Skin and Subcutaneous Tissue Disorders: Melasma/chloasma, which may persist; Erythema multiforme, Erythema nodosum, Hemorrhagic eruption, Hirsutism
Vascular Events: Venous thrombosis, Pulmonary embolism, Cerebral thrombosis, Mesenteric thrombosis, Retinal vascular thrombosis
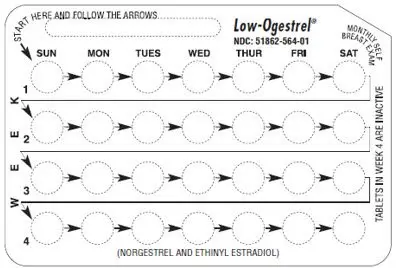
Low-Ogestrel Dosage and Administration
To achieve maximum contraceptive effectiveness, Low-Ogestrel (norgestrel and ethinyl estradiol tablets) must be taken exactly as directed and at intervals not exceeding 24 hours. The dosage of Low-Ogestrel is one white tablet daily for 21 consecutive days, followed by one peach inert tablet daily for 7 consecutive days, according to prescribed schedule. It is recommended that Low-Ogestrel tablets be taken by mouth at the same time each day.
| LOW-OGESTREL
norgestrel and ethinyl estradiol kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mayne Pharma Inc. (867220261) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Inc. | 240769596 | ANALYSIS(51862-564) , LABEL(51862-564) , MANUFACTURE(51862-564) , PACK(51862-564) | |