Drug Detail:Lucemyra (Lofexidine [ floe-fex-i-deen ])
Drug Class: Antiadrenergic agents, centrally acting
Highlights of Prescribing Information
LUCEMYRA® (lofexidine) tablets, for oral use
Initial U.S. Approval: 2018
Indications and Usage for Lucemyra
LUCEMYRA is a central alpha-2 adrenergic agonist indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults. (1)
Lucemyra Dosage and Administration
- The usual LUCEMYRA dosage is three 0.18 mg tablets taken orally 4 times daily at 5- to 6-hour intervals. LUCEMYRA treatment may be continued for up to 14 days with dosing guided by symptoms. (2.1)
- Discontinue LUCEMYRA with a gradual dose reduction over 2 to 4 days. (2.1)
- Hepatic or Renal Impairment: Dosage adjustments are recommended based on degree of impairment. (2.2, 2.3)
Dosage Forms and Strengths
Tablets: 0.18 mg. (3)
Contraindications
None. (4)
Warnings and Precautions
- Risk of Hypotension, Bradycardia, and Syncope: May cause a decrease in blood pressure, a decrease in pulse, and syncope. Monitor vital signs before dosing and advise patients on how to minimize the risk of these cardiovascular effects and manage symptoms, should they occur. Monitor symptoms related to bradycardia and orthostasis. When using in outpatients, ensure that patients are capable of self-monitoring for signs and symptoms. Avoid use in patients with severe coronary insufficiency, recent myocardial infarction, cerebrovascular disease, or chronic renal failure, as well as in patients with marked bradycardia. (5.1)
- Risk of QT Prolongation: LUCEMYRA prolongs the QT interval. Avoid use in patients with congenital long QT syndrome. Monitor ECG in patients with electrolyte abnormalities, congestive heart failure, bradyarrhythmias, hepatic or renal impairment, or in patients taking other medicinal products that lead to QT prolongation. (5.2)
- Increased Risk of CNS Depression with Concomitant use of CNS Depressant Drugs: LUCEMYRA potentiates the CNS depressant effects of benzodiazepines and may potentiate the CNS depressant effects of alcohol, barbiturates, and other sedating drugs. (5.3)
- Increased Risk of Opioid Overdose after Opioid Discontinuation: Patients who complete opioid discontinuation are at an increased risk of fatal overdose should they resume opioid use. Use in conjunction with a comprehensive management program for treatment of opioid use disorder and inform patients and caregivers of increased risk of overdose. (5.4)
- Risk of Discontinuation Symptoms: Instruct patients not to discontinue therapy without consulting their healthcare provider. When discontinuing therapy, reduce dose gradually. (5.5)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 10% and notably more frequent than placebo) are orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact US WorldMeds at 1-833-LUCEMYRA or FDA at 1-800-FDA-1088 or www.fda.gov/ medwatch
Drug Interactions
- Methadone: Methadone and LUCEMYRA both prolong the QT interval. ECG monitoring is recommended when used concomitantly. (7.1)
- Oral Naltrexone: Concomitant use may reduce efficacy of oral naltrexone. (7.2)
- CYP2D6 Inhibitors: Concomitant use of paroxetine resulted in increased plasma levels of LUCEMYRA. Monitor for symptoms of orthostasis and bradycardia with concomitant use of a CYP2D6 inhibitor. (7.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2020
Related/similar drugs
methadone, chlorpromazine, Thorazine, Dolophine, lofexidine, MethadoseFull Prescribing Information
1. Indications and Usage for Lucemyra
LUCEMYRA is indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults.
2. Lucemyra Dosage and Administration
2.1 Dosing Information
The usual LUCEMYRA starting dosage is three 0.18 mg tablets taken orally 4 times daily during the period of peak withdrawal symptoms (generally the first 5 to 7 days following last use of opioid) with dosing guided by symptoms and side effects. There should be 5 to 6 hours between each dose. The total daily dosage of LUCEMYRA should not exceed 2.88 mg (16 tablets) and no single dose should exceed 0.72 mg (4 tablets).
LUCEMYRA treatment may be continued for up to 14 days with dosing guided by symptoms.
Discontinue LUCEMYRA with a gradual dose reduction over a 2- to 4-day period to mitigate LUCEMYRA withdrawal symptoms (e.g., reducing by 1 tablet per dose every 1 to 2 days) [see Warnings & Precautions (5.5)]. The LUCEMYRA dose should be reduced, held, or discontinued for individuals who demonstrate a greater sensitivity to LUCEMYRA side effects [see Warnings and Precautions (5.1), Adverse Reactions (6.1)]. Lower doses may be appropriate as opioid withdrawal symptoms wane.
LUCEMYRA can be administered in the presence or absence of food.
2.2 Dosage Recommendations for Patients with Hepatic Impairment
Recommended dosage adjustments based on the degree of hepatic impairment are shown in Table 1. [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
| Mild Impairment | Moderate Impairment | Severe Impairment | |
|---|---|---|---|
| Child-Pugh score | 5-6 | 7-9 | > 9 |
| Recommended dose | 3 tablets 4 times daily (2.16 mg per day) | 2 tablets 4 times daily (1.44 mg per day) | 1 tablet 4 times daily (0.72 mg per day) |
2.3 Dosage Recommendations for Patients with Renal Impairment
Recommended dosage adjustments based on the degree of renal impairment are shown in Table 2. LUCEMYRA may be administered without regard to the timing of dialysis [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
| Moderate Impairment | Severe Impairment, End-Stage Renal Disease, or on Dialysis | |
|---|---|---|
| Estimated GFR, mL/min/1.73 m2 | 30-89.9 | < 30 |
| Recommended dose | 2 tablets 4 times daily (1.44 mg per day) | 1 tablet 4 times daily (0.72 mg per day) |
3. Dosage Forms and Strengths
LUCEMYRA is available as round, peach-colored, film-coated tablets, imprinted with "LFX" on one side and "18" on the other side. Each tablet contains 0.18 mg lofexidine (equivalent to 0.2 mg of lofexidine hydrochloride).
5. Warnings and Precautions
5.1 Risk of Hypotension, Bradycardia, and Syncope
LUCEMYRA can cause a decrease in blood pressure, a decrease in pulse, and syncope [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)]. Monitor vital signs before dosing. Monitor symptoms related to bradycardia and orthostasis.
Patients being given LUCEMYRA in an outpatient setting should be capable of and instructed on self-monitoring for hypotension, orthostasis, bradycardia, and associated symptoms. If clinically significant or symptomatic hypotension and/or bradycardia occur, the next dose of LUCEMYRA should be reduced in amount, delayed, or skipped.
Inform patients that LUCEMYRA may cause hypotension and that patients moving from a supine to an upright position may be at increased risk for hypotension and orthostatic effects. Instruct patients to stay hydrated, on how to recognize symptoms of low blood pressure, and on how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position). Instruct outpatients to withhold LUCEMYRA doses when experiencing symptoms of hypotension or bradycardia and to contact their healthcare provider for guidance on how to adjust dosing.
Avoid using LUCEMYRA in patients with severe coronary insufficiency, recent myocardial infarction, cerebrovascular disease, chronic renal failure, and in patients with marked bradycardia.
Avoid using LUCEMYRA in combination with medications that decrease pulse or blood pressure to avoid the risk of excessive bradycardia and hypotension.
5.2 Risk of QT Prolongation
LUCEMYRA prolongs the QT interval.
Avoid using LUCEMYRA in patients with congenital long QT syndrome.
Monitor ECG in patients with congestive heart failure, bradyarrhythmias, hepatic impairment, renal impairment, or patients taking other medicinal products that lead to QT prolongation (e.g., methadone). In patients with electrolyte abnormalities (e.g., hypokalemia or hypomagnesemia), correct these abnormalities first, and monitor ECG upon initiation of LUCEMYRA [see Dosing and Administration (2.1), Adverse Reactions (6.1), Special Populations (8.6, 8.7), Clinical Pharmacology (12.2)].
5.3 Increased Risk of Central Nervous System Depression with Concomitant use of CNS Depressant Drugs
LUCEMYRA potentiates the CNS depressive effects of benzodiazepines and can also be expected to potentiate the CNS depressive effects of alcohol, barbiturates, and other sedating drugs. Advise patients to inform their healthcare provider of other medications they are taking, including alcohol.
Advise patients using LUCEMYRA in an outpatient setting that, until they learn how they respond to LUCEMYRA, they should be careful or avoid doing activities such as driving or operating heavy machinery.
5.4 Increased Risk of Opioid Overdose after Opioid Discontinuation
LUCEMYRA is not a treatment for opioid use disorder. Patients who complete opioid discontinuation are likely to have a reduced tolerance to opioids and are at increased risk of fatal overdose should they resume opioid use. Use LUCEMYRA in patients with opioid use disorder only in conjunction with a comprehensive management program for the treatment of opioid use disorder and inform patients and caregivers of this increased risk of overdose.
5.5 Risk of Discontinuation Symptoms
Stopping LUCEMYRA abruptly can cause a marked rise in blood pressure. Symptoms including diarrhea, insomnia, anxiety, chills, hyperhidrosis, and extremity pain have also been observed with LUCEMYRA discontinuation. Instruct patients not to discontinue therapy without consulting their healthcare provider. When discontinuing therapy with LUCEMYRA, gradually reduce the dose [see Dosing and Administration (2.1)].
Symptoms related to discontinuation can be managed by administration of the previous LUCEMYRA dose and subsequent taper.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in labeling:
- Hypotension, Bradycardia, and Syncope [see Warnings and Precautions (5.1)]
- QT Prolongation [see Warnings and Precautions (5.2)]
- Central Nervous System Depression [see Warnings and Precautions (5.3)]
- Opioid Overdose [see Warnings and Precautions (5.4)]
- Discontinuation Symptoms [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to adverse reaction rates observed for another drug and may not reflect the rates observed in practice.
The safety of LUCEMYRA was supported by three randomized, double-blind, placebo-controlled clinical trials, an open-label study, and clinical pharmacology studies with concomitant administration of either methadone, buprenorphine, or naltrexone.
The three randomized, double-blind, placebo-controlled clinical trials enrolled 935 subjects dependent on short-acting opioids undergoing abrupt opioid withdrawal. Patients were monitored before each dose in an inpatient setting.
Table 3 presents the incidence, rounded to the nearest percent, of adverse events that occurred in at least 10% of subjects treated with LUCEMYRA and for which the incidence in patients treated with LUCEMYRA was greater than the incidence in subjects treated with placebo in a study that tested two doses of LUCEMYRA, 2.16 mg per day and 2.88 mg per day, and placebo. The overall safety profile in the combined dataset was similar.
Orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth were notably more common in subjects treated with LUCEMYRA than subjects treated with placebo.
| Adverse Reaction | LUCEMYRA 2.16 mg* (%) N=229 | LUCEMYRA 2.88 mg* (%) N=222 | Placebo (%) N=151 |
|---|---|---|---|
|
|||
| Insomnia | 51 | 55 | 48 |
| Orthostatic Hypotension | 29 | 42 | 5 |
| Bradycardia | 24 | 32 | 5 |
| Hypotension | 30 | 30 | 1 |
| Dizziness | 19 | 23 | 3 |
| Somnolence | 11 | 13 | 5 |
| Sedation | 13 | 12 | 5 |
| Dry Mouth | 10 | 11 | 0 |
Other notable adverse reactions associated with the use of LUCEMYRA but reported in <10% of patients in the LUCEMYRA group included:
- Syncope: 0.9%, 1.4% and 0% for LUCEMYRA 2.16 mg/day and 2.88 mg/day and placebo, respectively
- Tinnitus: 0.9%, 3.2% and 0% for LUCEMYRA 2.16 mg/day and 2.88 mg/day and placebo, respectively
6.2 Postmarketing Experience
Lofexidine is marketed in other countries for relief of opioid withdrawal symptoms. The following events have been identified during postmarketing use of lofexidine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Since lofexidine's initial market introduction in 1992, the most frequently reported postmarketing adverse event with lofexidine has been hypotension [see Warnings and Precautions (5.1)]. There has been one report of QT prolongation, bradycardia, torsades de pointes, and cardiac arrest with successful resuscitation in a patient who received lofexidine, and three reports of clinically significant QT prolongation in subjects concurrently receiving methadone with lofexidine.
7. Drug Interactions
7.1 Methadone
LUCEMYRA and methadone both prolong the QT interval. ECG monitoring is recommended in patients receiving methadone and LUCEMYRA concomitantly [see Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
7.2 Oral Naltrexone
Coadministration of LUCEMYRA and oral naltrexone resulted in statistically significant differences in the steady-state pharmacokinetics of naltrexone. It is possible that oral naltrexone efficacy may be reduced if used concomitantly within 2 hours of LUCEMYRA. This interaction is not expected if naltrexone is administered by non-oral routes [see Clinical Pharmacology (12.3)].
7.3 CNS Depressant Drugs
LUCEMYRA potentiates the CNS depressant effects of benzodiazepines and may potentiate the CNS depressant effects of alcohol, barbiturates, and other sedating drugs. Advise patients to inform their healthcare provider of other medications they are taking, including alcohol [see Warnings and Precautions (5.3)].
8. Use In Specific Populations
8.1 Pregnancy
Data
Animal Data
Increased incidence of resorptions, decreased number of implantations, and a concomitant reduction in the number of fetuses were observed when pregnant rabbits were orally administered lofexidine hydrochloride during organogenesis (from gestation day [GD] 7 to 19) at a daily dose of 5.0 mg/kg/day (approximately 0.08 times the maximum recommended human dose [MRHD] of 2.88 mg lofexidine base on an AUC basis). Maternal toxicity evidenced by increased mortality was noted at the highest tested dose of 15 mg/kg/day (approximately 0.4 times the MRHD on an AUC basis).
Decreased implantations per dam and decreased mean fetal weights were noted in a study in which pregnant rats were treated with oral lofexidine hydrochloride during organogenesis (from GD 7 to 16) at a daily dose of 3.0 mg/kg/day (approximately 0.9 times the MRHD on an AUC basis). This dose was associated with maternal toxicity (decreased body weight gain and mortality). No malformations or evidence of developmental toxicity were evident at 1.0 mg/kg/day (approximately 0.2 times the MRHD on an AUC basis).
A dose-dependent increase in pup mortality was noted in all doses of lofexidine hydrochloride administered orally to pregnant rats from GD 6 through lactation at an exposure less than the human exposure based on AUC comparisons. Doses higher than 1.0 mg/kg/day (approximately 0.2 times the MRHD on an AUC basis) resulted in incidences of total litter loss and maternal toxicity (piloerection and decreased body weight gain). At the highest dose tested of 2.0 mg/kg/day (approximately 0.6 times the MRHD on an AUC basis), increased stillbirths as well as decreased viability and lactation indices were reported. Surviving offspring exhibited lower body weights, developmental delays, and increased delays in auditory startle at doses of 1.0 mg/kg/ day or higher. Sexual maturation was delayed in male offspring (preputial separation) at 2.0 mg/kg/day and in female offspring (vaginal opening) at 1.0 mg/kg/day or higher.
8.3 Females and Males of Reproductive Potential
In animal studies that included some fertility endpoints, lofexidine decreased breeding rate and increased resorptions at exposures below human exposures. The impact of lofexidine on male fertility has not been adequately characterized in animal studies [see Impairment of Fertility (13.1)].
8.4 Pediatric Use
The safety and effectiveness of LUCEMYRA have not been established in pediatric patients.
8.5 Geriatric Use
No studies have been performed to characterize the pharmacokinetics of LUCEMYRA or to establish its safety and effectiveness in geriatric patients. Caution should be exercised when LUCEMYRA is administered to patients over 65 years of age. Dosing adjustments similar to those recommended in patients with renal impairment should be considered [see Dosage and Administration (2.3), Use in Specific Populations (8.7)].
8.6 Hepatic Impairment
Hepatic impairment slows the elimination of LUCEMYRA but exhibits less effect on the peak plasma concentration than on AUC values following a single dose. Dosage adjustments are recommended based on the degree of hepatic impairment. [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
Clinically relevant QT prolongation may occur in subjects with hepatic impairment [see Warnings and Precautions (5.2), Clinical Pharmacology (12.2)].
8.7 Renal Impairment
Renal impairment slows the elimination of LUCEMYRA but exhibits less effect on the peak plasma concentration than on AUC values following a single dose. Dosage adjustments are recommended based on the degree of renal impairment [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
Only a negligible fraction of the LUCEMYRA dose is removed during a typical dialysis session, so no additional dose needs to be administered after a dialysis session; LUCEMYRA may be administered without regard to the timing of dialysis [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
Clinically relevant QT prolongation may occur in subjects with renal impairment [see Warnings and Precautions (5.2), Clinical Pharmacology (12.2)].
8.8 CYP2D6 Poor Metabolizers
Although the pharmacokinetics of LUCEMYRA have not been systematically evaluated in patients who do not express the drug metabolizing enzyme CYP2D6, it is likely that the exposure to LUCEMYRA would be increased similarly to taking strong CYP2D6 inhibitors (approximately 28%). Monitor adverse events such as orthostatic hypotension and bradycardia in known CYP2D6 poor metabolizers. Approximately 8% of Caucasians and 3 to 8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers (PM) [see Clinical Pharmacology (12.3)].
10. Overdosage
Overdose with LUCEMYRA may manifest as hypotension, bradycardia, and sedation. In the event of acute overdose, perform gastric lavage where appropriate. Dialysis will not remove a substantial portion of the drug. Initiate general symptomatic and supportive measures in cases of overdosage.
11. Lucemyra Description
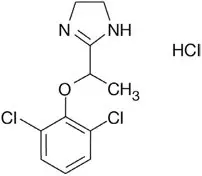
LUCEMYRA tablets contain lofexidine, a central alpha-2 adrenergic agonist, as the hydrochloride salt. Lofexidine hydrochloride is chemically designated as 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5 dihydro-1H- imidazole monohydrochloride with a molecular formula of C11H12Cl2N2O∙HCl. Its molecular weight is 295.6 g/mole and its structural formula is:
Lofexidine hydrochloride is a white to off-white crystalline powder freely soluble in water, methanol, and ethanol. It is slightly soluble in chloroform and practically insoluble in n-hexane and benzene.
LUCEMYRA is available as round, convex-shaped, peach-colored, film-coated tablets for oral administration. Each tablet contains 0.18 lofexidine, equivalent to 0.2 mg of lofexidine hydrochloride, and the following inactive ingredients: 92.6 mg lactose, 12.3 mg citric acid, 1.1 mg povidone, 5.7 mg microcrystalline cellulose, 1.4 mg calcium stearate, 0.7 mg sodium lauryl sulphate, and Opadry OY S 9480 (contains indigo carmine and sunset yellow).
12. Lucemyra - Clinical Pharmacology
12.1 Mechanism of Action
Lofexidine is a central alpha-2 adrenergic agonist that binds to receptors on adrenergic neurons. This reduces the release of norepinephrine and decreases sympathetic tone.
12.2 Pharmacodynamics
14. Clinical Studies
Two randomized, double-blind, placebo-controlled trials supported the efficacy of LUCEMYRA.
Study 1, NCT01863186
Study 1 was a 2-part efficacy, safety, and dose-response study conducted in the United States in patients meeting DSM-IV criteria for opioid dependence who were physically dependent on short-acting opioids (e.g., heroin, hydrocodone, oxycodone). The first part of the study was an inpatient, randomized, double-blind, placebo-controlled design consisting of 7 days of inpatient treatment (Days 1 – 7) with LUCEMYRA 2.16 mg total daily dose (0.54 mg 4 times daily) (n=229), LUCEMYRA 2.88 mg total daily dose (0.72 mg 4 times daily) (n=222), or matching placebo (n=151). Patients also had access to a variety of support medications for withdrawal symptoms (guaifenesin, antacids, dioctyl sodium sulfosuccinate, psyllium hydrocolloid suspension, bismuth sulfate, acetaminophen, and zolpidem). The second part of the study (Days 8 – 14) was an open-label design where all patients who successfully completed Days 1 – 7 were eligible to receive open-label treatment with variable dose LUCEMYRA treatment (as determined by the investigator, but not to exceed 2.88 mg total daily dose) for up to an additional 7 days (Days 8 – 14) in either an inpatient or outpatient setting as determined by the investigator and the patient. No patient received LUCEMYRA for more than 14 days.
The two endpoints to support efficacy were the mean Short Opiate Withdrawal Scale of Gossop (SOWS-Gossop) total score on Days 1 – 7 of treatment and the proportion of patients who completed 7 days of treatment. The SOWS-Gossop, a patient-reported outcome (PRO) instrument, evaluates the following opioid withdrawal symptoms: feeling sick, stomach cramps, muscle spasms/twitching, feeling of coldness, heart pounding, muscular tension, aches and pains, yawning, runny eyes and insomnia/problems sleeping. For each opioid withdrawal symptom, patients are asked to rate their symptom severity using four response options (none, mild, moderate, and severe). The SOWS-Gossop total score ranges from 0 to 30, where a higher score indicates greater withdrawal symptom severity. The SOWS-Gossop was administered at baseline and once daily 3.5 hours after the first morning dose on Days 1 – 7.
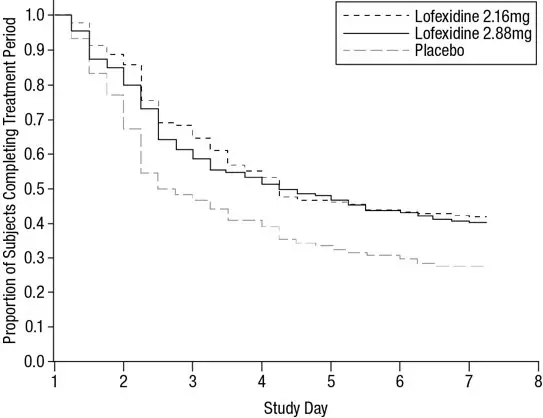
Of the randomized and treated patients, 28% of placebo patients, 41% of LUCEMYRA 2.16 mg and 40% of LUCEMYRA 2.88 mg patients completed 7 days of treatment. The difference in proportion in both LUCEMYRA groups was significant compared to placebo. See Figure 1. Patients in the placebo group were more likely to drop out of the study prematurely due to lack of efficacy than patients treated with LUCEMYRA.
Figure 1: Completion of Treatment Period for Study 1
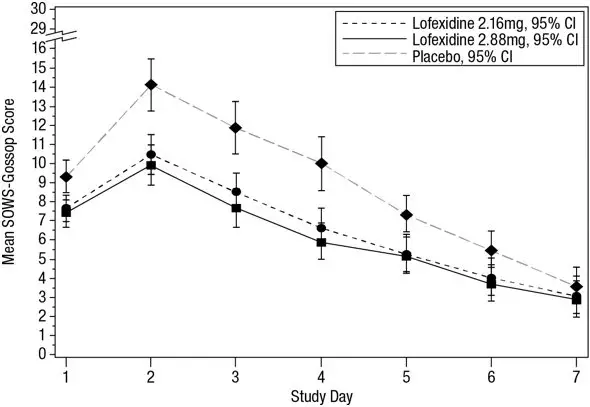
The mean SOWS-Gossop scores for Days 1 – 7 were 8.8, 6.5, and 6.1 for placebo, LUCEMYRA 2.16 mg and LUCEMYRA 2.88 mg, respectively. Results are shown in Figure 2. The mean difference between LUCEMYRA 2.16 mg and placebo was -2.3 with a 95% CI of (-3.4, -1.2). The mean difference between LUCEMYRA 2.88 mg and placebo was -2.7 with a 95% CI of (-3.9, -1.6). They were both significant. Symptoms assessed on the SOWS-Gossop were recorded as absent or mild for almost all patients remaining to the end of the assessment period.
Figure 2: Mean SOWS-Gossop Scores for Days 1 – 7 in Study 1
Study 2, NCT00235729
Study 2 was an inpatient, randomized, multicenter, double-blind, placebo-controlled study carried out in the United States in patients meeting DSM-IV criteria for opioid dependence who were physically dependent on short-acting opioids (e.g., heroin, hydrocodone, oxycodone). Patients were treated with LUCEMYRA tablets (2.88 mg/day [0.72 mg 4 times daily]) or matching placebo for 5 days (Days 1 – 5). Patients also had access to a variety of support medications for withdrawal symptoms (guaifenesin, antacids, dioctyl sodium sulfosuccinate, psyllium hydrocolloid suspension, bismuth sulfate, acetaminophen, and zolpidem). All patients then received placebo on Days 6 and 7 and were discharged on Day 8.
The two endpoints to support efficacy were the mean SOWS-Gossop total score on Days 1 – 5 of treatment and the proportion of patients who completed 5 days of treatment. The SOWS-Gossop was administered at baseline and once daily 3.5 hours after the first morning dose on Days 1 – 5.
A total of 264 patients were randomized into the study. Of these, 134 patients were randomized to LUCEMYRA 2.88 mg/day and 130 patients to placebo.
Of the randomized and treated patients, 33% of placebo patients and 49% of LUCEMYRA patients completed 5 days of treatment. The difference in proportion between the two groups was significant. See Figure 3. Patients in the placebo group were more likely to drop out of the study prematurely due to lack of efficacy than patients treated with LUCEMYRA.
Figure 3: Completion of Treatment Period in Study 2
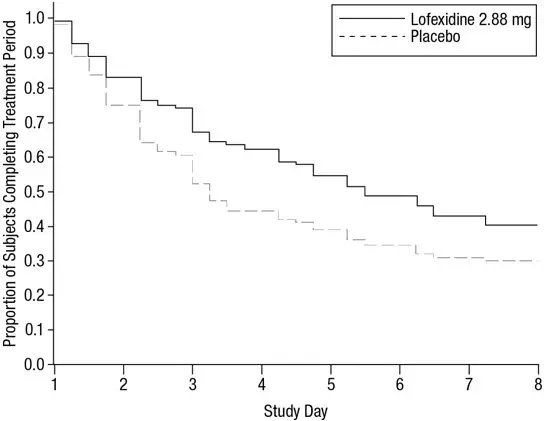
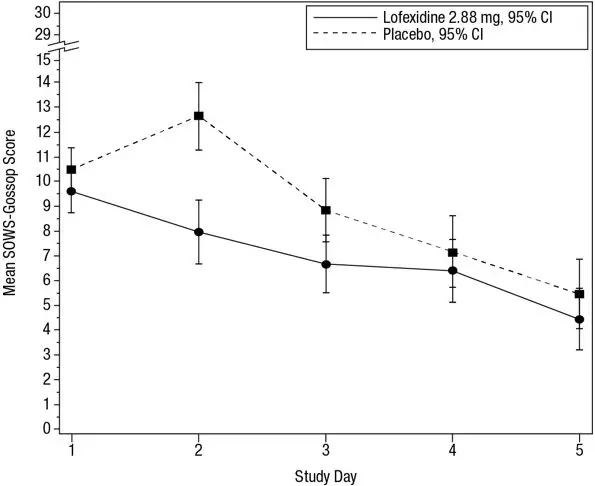
The mean SOWS-Gossop scores for Days 1 – 5 were 8.9 and 7.0 for placebo and LUCEMYRA 2.88 mg, respectively. Results are shown in Figure 4. The mean difference was -1.9 with a 95% CI of (-3.2, -0.6) and was statistically significant.
Figure 4: Mean SOWS-Gossop Scores for Days 1 – 5 in Study 2
17. Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Patient Information).
LUCEMYRA may mitigate, but not completely prevent, the symptoms associated with opioid withdrawal syndrome, which may include feeling sick, stomach cramps, muscle spasms or twitching, feeling of cold, heart pounding, muscular tension, aches and pains, yawning, runny eyes and sleep problems (insomnia). Patients should be advised that withdrawal will not be easy. Additional supportive measures should be clearly advised, as needed.
| This Patient Information has been approved by the U.S. Food and Drug Administration. 360-10020.01 | Issued: 09/2020 | |
| PATIENT INFORMATION
LUCEMYRA® (LEW-sem-EER-uh) (lofexidine) tablets |
||
| What is the most important information I should know about LUCEMYRA and discontinuing opioid drugs? | ||
| LUCEMYRA can cause serious side effects, including low blood pressure (hypotension), slow heart rate (bradycardia), and fainting. | ||
| If you have any of the following signs or symptoms, tell your healthcare provider right away: | ||
|
|
|
| If you take LUCEMYRA at home and have any of these signs and symptoms, do not take your next dose of LUCEMYRA until you have talked to your healthcare provider. You should avoid becoming dehydrated or overheated during treatment with LUCEMYRA, which may increase your risk of low blood pressure and fainting. You should also be careful not to stand up too suddenly from lying down or sitting. | ||
| When your treatment is complete you will need to stop taking LUCEMYRA gradually or your blood pressure could increase. For more information about side effects, see "What are the possible side effects of LUCEMYRA?" | ||
| Increased risk of opioid overdose. After a period of time of not using opioid drugs, you can become more sensitive to the effects of opioids if you start using opioids again. This may increase your risk of overdose and death. | ||
| What is LUCEMYRA? | ||
| LUCEMYRA is a non-opioid prescription medicine used in adults to help with the symptoms of opioid withdrawal that may happen when you stop taking an opioid suddenly. | ||
| LUCEMYRA will not completely prevent the symptoms of opioid withdrawal, which may include feeling sick, stomach cramps, muscle spasms or twitching, feeling of cold, heart pounding, muscular tension, aches and pains, yawning, runny eyes and sleep problems (insomnia). | ||
| LUCEMYRA is not a treatment for opioid use disorder. If you have been diagnosed with opioid use disorder (opioid addiction), your healthcare provider may prescribe LUCEMYRA as part of a complete treatment program for your opioid use disorder (opioid addiction). | ||
| It is not known if LUCEMYRA is safe and effective in children. | ||
Before taking LUCEMYRA, tell your healthcare provider about all of your medical conditions, including if you:
|
||
| Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements, and any medications you may take for the individual symptoms of opioid withdrawal (such as pain relievers or medications for upset stomach). | ||
| Especially tell your healthcare provider if you take benzodiazepines, barbiturates, tranquilizers, or sleeping pills. Taking LUCEMYRA with these medicines can cause serious side effects. Ask your healthcare provider or pharmacist if you are not sure if you are taking any of these medicines. | ||
How should I take LUCEMYRA?
|
||
| What should I avoid while taking LUCEMYRA? | ||
| Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how LUCEMYRA affects you. | ||
| What are the possible side effects of LUCEMYRA? | ||
| The most common side effects of LUCEMYRA include: | ||
|
|
|
| These are not all the possible side effects of LUCEMYRA. | ||
| Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to US WorldMeds at 1-833-LUCEMYRA. | ||
How should I store LUCEMYRA?
|
||
| Keep LUCEMYRA and all medicines out of the reach of children. | ||
| General information about the safe and effective use of LUCEMYRA. | ||
| Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LUCEMYRA for a condition for which it was not prescribed. Do not give LUCEMYRA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LUCEMYRA that is written for health professionals. | ||
| What are the ingredients of LUCEMYRA? | ||
| Active ingredient: lofexidine. | ||
| Inactive ingredients: lactose, citric acid, povidone, microcrystalline cellulose, calcium stearate, sodium lauryl sulphate, and Opadry OY S 9480 (contains indigo carmine and sunset yellow). | ||
| Distributed by: USWM, LLC, 4441 Springdale Road, Louisville, KY 40241 | ||
| Under License from Britannia Pharmaceuticals Limited. USWM, LLC is the exclusive licensee and distributor of LUCEMYRA® in the United States and Its territories. ©2020. LUCEMYRA® is a registered trademark of USWM, LLC. For more information, go to www.LUCEMYRA.com or call 1-833-LUCEMYRA |
||
| LUCEMYRA
lofexidine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - USWM, LLC (117542566) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Pharma Solutions, LLC | 829672745 | MANUFACTURE(78670-050) , ANALYSIS(78670-050) , PACK(78670-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Acceleration Laboratory Services, Inc | 187562629 | ANALYSIS(78670-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| UFAG Laboratoriien AG | 486383151 | ANALYSIS(78670-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Helsinn Birex Pharmaceuticals LTD | 985084409 | PACK(78670-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apace Packaging LLC | 361961142 | PACK(78670-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| A+ Secure Packaging, LLC | 963589036 | LABEL(78670-050) , PACK(78670-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Helsinn Advanced Synthesis SA | 481296960 | API MANUFACTURE(78670-050) , ANALYSIS(78670-050) | |