Drug Detail:Lumakras (Sotorasib)
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
LUMAKRAS® (sotorasib) tablets, for oral use
Initial U.S. Approval: 2021
Recent Major Changes
| Dosage and Administration (2.2, 2.3) | 01/2023 |
Indications and Usage for Lumakras
LUMAKRAS is an inhibitor of the RAS GTPase family indicated for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy. (1)
This indication is approved under accelerated approval based on overall response rate (ORR) and duration of response (DOR). Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
Lumakras Dosage and Administration
- Recommended dosage: 960 mg orally once daily. (2.2)
- Swallow tablets whole with or without food. (2.2)
Dosage Forms and Strengths
Tablets: 320 mg, 120 mg. (3)
Contraindications
None. (4)
Warnings and Precautions
- Hepatotoxicity: Monitor liver function tests every 3 weeks for the first 3 months of treatment then once monthly as clinically indicated. Withhold, reduce dose, or permanently discontinue LUMAKRAS based on the severity. (2.3, 5.1)
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor for new or worsening pulmonary symptoms. Immediately withhold LUMAKRAS for suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified. (2.3, 5.2)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 20%) were diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity, and cough. The most common laboratory abnormalities (≥ 25%) were decreased lymphocytes, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, decreased calcium, increased alkaline phosphatase, increased urine protein, and decreased sodium. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Inc. at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Acid-Reducing Agents: Avoid coadministration with proton pump inhibitors (PPIs) and H2 receptor antagonists. If an acid-reducing agent cannot be avoided, administer LUMAKRAS 4 hours before or 10 hours after a local antacid. (2.4, 7.1)
- Strong CYP3A4 Inducers: Avoid coadministration with strong CYP3A4 inducers. (7.1)
- CYP3A4 Substrates: Avoid coadministration with CYP3A4 substrates for which minimal concentration changes may lead to therapeutic failures of the substrate. If coadministration cannot be avoided, adjust the substrate dosage in accordance to its Prescribing Information. (7.2)
- P-gp substrates: Avoid coadministration with P-gp substrates for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the substrate dosage in accordance to its Prescribing Information. (7.2)
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
Related/similar drugs
Opdivo, Retevmo, Rybrevant, methotrexate, Keytruda, Avastin, pembrolizumabFull Prescribing Information
1. Indications and Usage for Lumakras
LUMAKRAS is indicated for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test [see Dosage and Administration (2.1)], who have received at least one prior systemic therapy.
This indication is approved under accelerated approval based on overall response rate (ORR) and duration of response (DOR) [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
2. Lumakras Dosage and Administration
2.1 Patient Selection
Select patients for treatment of locally advanced or metastatic NSCLC with LUMAKRAS based on the presence of KRAS G12C mutation in tumor or plasma specimens [see Clinical Studies (14)]. If no mutation is detected in a plasma specimen, test tumor tissue.
Information on FDA-approved tests for the detection of KRAS G12C mutations is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage and Administration
The recommended dosage of LUMAKRAS is 960 mg (three 320 mg tablets or eight 120 mg tablets) orally once daily until disease progression or unacceptable toxicity.
Take the daily dose of LUMAKRAS at the same time each day with or without food [see Clinical Pharmacology (12.3)]. Swallow tablets whole. Do not chew, crush or split tablets. If a dose of LUMAKRAS is missed by more than 6 hours, take the next dose as prescribed the next day. Do not take 2 doses at the same time to make up for the missed dose.
If vomiting occurs after taking LUMAKRAS, do not take an additional dose. Take the next dose as prescribed the next day.
2.3 Dosage Modifications for Adverse Reactions
LUMAKRAS dose reduction levels are summarized in Table 1. Dosage modifications for adverse reactions are provided in Table 2.
If adverse reactions occur, a maximum of two dose reductions are permitted. Discontinue LUMAKRAS if patients are unable to tolerate the minimum dose of 240 mg once daily.
| Dose Reduction Level | Dose |
|---|---|
| First dose reduction | 480 mg (four 120 mg tablets) once daily |
| Second dose reduction | 240 mg (two 120 mg tablets) once daily |
| Adverse Reaction | Severity* | Dosage Modification |
|---|---|---|
| ALT = alanine aminotransferase; AST = aspartate aminotransferase; ULN = upper limit of normal | ||
|
||
| Hepatotoxicity [see Warnings and Precautions (5.1)] | Grade 2 AST or ALT with symptoms or Grade 3 to 4 AST or ALT |
|
| AST or ALT > 3 × ULN with total bilirubin > 2 × ULN in the absence of alternative causes |
|
|
| Interstitial Lung Disease (ILD)/ pneumonitis [see Warnings and Precautions (5.2)] | Any Grade |
|
| Nausea or vomiting despite appropriate supportive care (including anti-emetic therapy) [see Adverse Reactions (6.1)] | Grade 3 to 4 |
|
| Diarrhea despite appropriate supportive care (including anti-diarrheal therapy) [see Adverse Reactions (6.1)] | Grade 3 to 4 |
|
| Other adverse reactions [see Adverse Reactions (6.1)] | Grade 3 to 4 |
|
2.4 Coadministration of LUMAKRAS with Acid-Reducing Agents
Avoid coadministration of proton pump inhibitors (PPIs) and H2 receptor antagonists with LUMAKRAS. If treatment with an acid-reducing agent cannot be avoided, take LUMAKRAS 4 hours before or 10 hours after administration of a local antacid [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Tablets: 320 mg, beige, oval shaped, immediate release, film coated, debossed with "AMG" on one side and "320" on the opposite side.
Tablets: 120 mg, yellow, oblong-shaped, immediate release, film-coated, debossed with "AMG" on one side and "120" on the opposite side.
5. Warnings and Precautions
5.1 Hepatotoxicity
LUMAKRAS can cause hepatotoxicity, which may lead to drug-induced liver injury and hepatitis. Among 357 patients who received LUMAKRAS in CodeBreaK 100 [see Adverse Reactions (6.1)], hepatotoxicity occurred in 1.7% (all grades) and 1.4% (Grade 3). A total of 18% of patients who received LUMAKRAS had increased alanine aminotransferase (ALT)/increased aspartate aminotransferase (AST); 6% were Grade 3 and 0.6% were Grade 4. The median time to first onset of increased ALT/AST was 9 weeks (range: 0.3 to 42). Increased ALT/AST leading to dose interruption or reduction occurred in 7% of patients. LUMAKRAS was discontinued due to increased ALT/AST in 2.0% of patients. In addition to dose interruption or reduction, 5% of patients received corticosteroids for the treatment of hepatotoxicity.
Monitor liver function tests (ALT, AST, and total bilirubin) prior to the start of LUMAKRAS, every 3 weeks for the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop transaminase and/or bilirubin elevations. Withhold, dose reduce or permanently discontinue LUMAKRAS based on severity of adverse reaction [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
5.2 Interstitial Lung Disease (ILD)/Pneumonitis
LUMAKRAS can cause ILD/pneumonitis that can be fatal. Among 357 patients who received LUMAKRAS in CodeBreaK 100 [see Adverse Reactions (6.1)], ILD/pneumonitis occurred in 0.8% of patients, all cases were Grade 3 or 4 at onset, and 1 case was fatal. The median time to first onset for ILD/pneumonitis was 2 weeks (range: 2 to 18 weeks). LUMAKRAS was discontinued due to ILD/pneumonitis in 0.6% of patients. Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, fever). Immediately withhold LUMAKRAS in patients with suspected ILD/pneumonitis and permanently discontinue LUMAKRAS if no other potential causes of ILD/pneumonitis are identified [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Hepatotoxicity [see Warnings and Precautions (5.1)]
- Interstitial Lung Disease (ILD)/Pneumonitis [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflect exposure to LUMAKRAS as a single agent at 960 mg orally once daily in 357 patients with NSCLC and other solid tumors with KRAS G12C mutation enrolled in CodeBreaK 100, 28% were exposed for 6 months or longer and 3% were exposed for greater than one year.
Non-Small Cell Lung Cancer
The safety of LUMAKRAS was evaluated in a subset of patients with KRAS G12C-mutated locally advanced or metastatic NSCLC in CodeBreaK 100 [see Clinical Studies (14)]. Patients received LUMAKRAS 960 mg orally once daily until disease progression or unacceptable toxicity (n = 204). Among patients who received LUMAKRAS, 39% were exposed for 6 months or longer and 3% were exposed for greater than one year.
The median age of patients who received LUMAKRAS was 66 years (range: 37 to 86); 55% female; 80% White, 15% Asian, and 3% Black.
Serious adverse reactions occurred in 50% of patients treated with LUMAKRAS. Serious adverse reactions in ≥ 2% of patients were pneumonia (8%), hepatotoxicity (3.4%), and diarrhea (2%). Fatal adverse reactions occurred in 3.4% of patients who received LUMAKRAS due to respiratory failure (0.8%), pneumonitis (0.4%), cardiac arrest (0.4%), cardiac failure (0.4%), gastric ulcer (0.4%), and pneumonia (0.4%).
Permanent discontinuation of LUMAKRAS due to an adverse reaction occurred in 9% of patients. Adverse reactions resulting in permanent discontinuation of LUMAKRAS in ≥ 2% of patients included hepatotoxicity (4.9%).
Dosage interruptions of LUMAKRAS due to an adverse reaction occurred in 34% of patients. Adverse reactions which required dosage interruption in ≥ 2% of patients were hepatotoxicity (11%), diarrhea (8%), musculoskeletal pain (3.9%), nausea (2.9%), and pneumonia (2.5%).
Dose reductions of LUMAKRAS due to an adverse reaction occurred in 5% of patients. Adverse reactions which required dose reductions in ≥ 2% of patients included increased ALT (2.9%) and increased AST (2.5%).
The most common adverse reactions (≥ 20%) were diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity, and cough. The most common laboratory abnormalities (≥ 25%) were decreased lymphocytes, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, decreased calcium, increased alkaline phosphatase, increased urine protein, and decreased sodium.
Table 3 summarizes the common adverse reactions observed in CodeBreaK 100.
| Adverse Reaction | LUMAKRAS N = 204 |
|
|---|---|---|
| All Grades (%) | Grades 3 to 4 (%) |
|
|
||
| Gastrointestinal disorders | ||
| Diarrhea | 42 | 5 |
| Nausea | 26 | 1 |
| Vomiting | 17 | 1.5 |
| Constipation | 16 | 0.5 |
| Abdominal pain† | 15 | 1.0 |
| Hepatobiliary disorders | ||
| Hepatotoxicity‡ | 25 | 12 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Cough§ | 20 | 1.5 |
| Dyspnea¶ | 16 | 2.9 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain# | 35 | 8 |
| Arthralgia | 12 | 1.0 |
| General disorders and administration site conditions | ||
| FatigueÞ | 26 | 2.0 |
| Edemaß | 15 | 0 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 13 | 1.0 |
| Infections and infestations | ||
| Pneumoniaà | 12 | 7 |
| Skin and subcutaneous tissue disorders | ||
| Rashè | 12 | 0 |
Table 4 summarizes the selected laboratory adverse reactions observed in CodeBreaK 100.
| Laboratory Abnormalities | LUMAKRAS N = 204* |
|
|---|---|---|
| Grades 1 to 4 (%) | Grades 3 to 4 (%) |
|
|
||
| Chemistry | ||
| Increased aspartate aminotransferase | 39 | 9 |
| Increased alanine aminotransferase | 38 | 11 |
| Decreased calcium | 35 | 0 |
| Increased alkaline phosphatase | 33 | 2.5 |
| Increased urine protein | 29 | 3.9 |
| Decreased sodium | 28 | 1.0 |
| Decreased albumin | 22 | 0.5 |
| Hematology | ||
| Decreased lymphocytes | 48 | 2 |
| Decreased hemoglobin | 43 | 0.5 |
| Increased activated partial thromboplastin time | 23 | 1.5 |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of LUMAKRAS have not been established in pediatric patients.
8.5 Geriatric Use
Of the 357 patients with any tumor type who received LUMAKRAS 960 mg orally once daily in CodeBreaK 100, 46% were 65 and over, and 10% were 75 and over. No overall differences in safety or effectiveness were observed between older patients and younger patients.
8.6 Hepatic Impairment
No dosage modification is recommended in patients with mild to moderate hepatic impairment (Child Pugh A or B).
The effect of severe hepatic impairment (Child-Pugh C) on the safety of LUMAKRAS is unknown. Monitor for sotorasib adverse reactions in patients with hepatic impairment more frequently since these patients may be at increased risk for adverse reactions including hepatotoxicity [see Clinical Pharmacology (12.3)].
11. Lumakras Description
Sotorasib is an inhibitor of the RAS GTPase family. The molecular formula is C30H30F2N6O3, and the molecular weight is 560.6 g/mol. The chemical name of sotorasib is 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-(1M)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]-4-[(2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl]pyrido[2,3-d]pyrimidin-2(1H)-one.
The chemical structure of sotorasib is shown below:
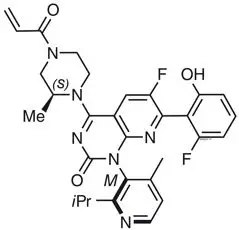
Sotorasib has pKa values of 8.06 and 4.56. The solubility of sotorasib in the aqueous media decreases over the range pH 1.2 to 6.8 from 1.3 mg/mL to 0.03 mg/mL.
LUMAKRAS is supplied as film-coated tablets for oral use containing 320 mg or 120 mg of sotorasib. Inactive ingredients in the tablet core are microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate. The film coating material consists of polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, iron oxide yellow and iron oxide red (320 mg tablet only).
12. Lumakras - Clinical Pharmacology
12.1 Mechanism of Action
Sotorasib is an inhibitor of KRASG12C, a tumor-restricted, mutant-oncogenic form of the RAS GTPase, KRAS. Sotorasib forms an irreversible, covalent bond with the unique cysteine of KRASG12C, locking the protein in an inactive state that prevents downstream signaling without affecting wild-type KRAS. Sotorasib blocked KRAS signaling, inhibited cell growth, and promoted apoptosis only in KRAS G12C tumor cell lines. Sotorasib inhibited KRASG12C in vitro and in vivo with minimal detectable off-target activity. In mouse tumor xenograft models, sotorasib-treatment led to tumor regressions and prolonged survival, and was associated with anti-tumor immunity in KRAS G12C models.
12.2 Pharmacodynamics
Sotorasib exposure-response relationships and the time course of the pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The pharmacokinetics of sotorasib have been characterized in healthy subjects and in patients with KRAS G12C-mutated solid tumors, including NSCLC. Sotorasib exhibited non-linear, time-dependent, pharmacokinetics over the dose range of 180 mg to 960 mg (0.19 to 1 time the approved recommended dosage) once daily with similar systemic exposure (i.e., AUC0-24h and Cmax) across doses at steady state. Sotorasib systemic exposure was comparable between film-coated tablets and film-coated tablets predispersed in water administered under fasted conditions. Sotorasib plasma concentrations reached steady state within 22 days. No accumulation was observed after repeat LUMAKRAS dosages with a mean accumulation ratio of 0.56 (coefficient of variation (CV): 59%).
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with sotorasib.
Sotorasib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay and was not genotoxic in the in vivo rat micronucleus and comet assays.
Fertility/early embryonic development studies were not conducted with sotorasib. There were no adverse effects on female or male reproductive organs in general toxicology studies conducted in dogs and rats.
13.2 Animal Toxicology and/or Pharmacology
In rats, renal toxicity including minimal to marked histologic tubular degeneration/necrosis and increased kidney weight, urea nitrogen, creatinine, and urinary biomarkers of renal tubular injury were present at doses resulting in exposures approximately ≥ 0.5 times the human AUC at the clinical dose of 960 mg. Increases in cysteine S-conjugate β-lyase pathway metabolism in the rat kidney compared to human may make rats more susceptible to renal toxicity due to local formation of a putative sulfur-containing metabolite than humans.
In the 3-month toxicology study in dogs, sotorasib induced findings in the liver (centrilobular hepatocellular hypertrophy), pituitary gland (hypertrophy of basophils), and thyroid gland (marked follicular cell atrophy, moderate to marked colloid depletion, and follicular cell hypertrophy) at exposures approximately 0.4 times the human exposure based on AUC at the clinical dose of 960 mg. These findings may be due to an adaptive response to hepatocellular enzyme induction and subsequent reduced thyroid hormone levels (i.e., secondary hypothyroidism). Although thyroid levels were not measured in dogs, induction of uridine diphosphate glucuronosyltransferase known to be involved in thyroid hormone metabolism was confirmed in the in vitro dog hepatocyte assay.
14. Clinical Studies
The efficacy of LUMAKRAS was demonstrated in a subset of patients enrolled in a single-arm, open-label, multicenter trial (CodeBreaK 100 [NCT03600883]). Eligible patients were required to have locally advanced or metastatic KRAS G12C-mutated NSCLC with disease progression after receiving an immune checkpoint inhibitor and/or platinum-based chemotherapy, an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1, and at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
All patients were required to have prospectively identified KRAS G12C-mutated NSCLC in tumor tissue samples by using the QIAGEN therascreen® KRAS RGQ PCR Kit performed in a central laboratory. Of 126 total enrolled subjects, 2 (2%) were unevaluable for efficacy analysis due to the absence of radiographically measurable lesions at baseline. Of the 124 patients with KRAS G12C mutations confirmed in tumor tissue, plasma samples from 112 patients were tested retrospectively using the Guardant360® CDx. 78/112 patients (70%) had KRAS G12C mutation identified in plasma specimen, 31/112 patients (28%) did not have KRAS G12C mutation identified in plasma specimen and 3/112 (2%) were unevaluable due to Guardant360® CDx test failure.
A total of 124 patients had at least one measurable lesion at baseline assessed by Blinded Independent Central Review (BICR) according to RECIST v1.1 and were treated with LUMAKRAS 960 mg once daily until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) as evaluated by BICR according to RECIST v1.1.
The baseline demographic and disease characteristics of the study population were: median age 64 years (range: 37 to 80) with 48% ≥ 65 years and 8% ≥ 75 years; 50% Female; 82% White, 15% Asian, 2% Black; 70% ECOG PS 1; 96% had stage IV disease; 99% with non-squamous histology; 81% former smokers, 12% current smokers, 5% never smokers. All patients received at least 1 prior line of systemic therapy for metastatic NSCLC; 43% received only 1 prior line of therapy, 35% received 2 prior lines of therapy, 23% received 3 prior lines of therapy; 91% received prior anti-PD-1/PD-L1 immunotherapy, 90% received prior platinum-based chemotherapy, 81% received both platinum-based chemotherapy and anti-PD-1/PD-L1. The sites of known extra-thoracic metastasis included 48% bone, 21% brain, and 21% liver.
Efficacy results are summarized in Table 5.
| Efficacy Parameter | LUMAKRAS N = 124 |
|---|---|
| CI = confidence interval | |
|
|
| Objective Response Rate (95% CI)* | 36 (28, 45) |
| Complete response rate, % | 2 |
| Partial response rate, % | 35 |
| Duration of Response* | |
| Median†, months (range) | 10.0 (1.3+, 11.1) |
| Patients with duration ≥ 6 months‡, % | 58% |
| LUMAKRAS
sotorasib tablet, coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LUMAKRAS
sotorasib tablet, coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amgen Inc (039976196) |
| Registrant - Amgen, Inc (039976196) |