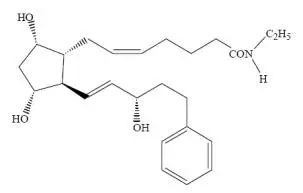
Drug Detail:Lumigan (Bimatoprost ophthalmic [ bih-mat-o-prost ])
Drug Class: Ophthalmic glaucoma agents
Highlights of Prescribing Information
LUMIGAN® (bimatoprost ophthalmic solution) 0.01%, for topical ophthalmic use
Initial U.S. Approval: 2001
Indications and Usage for Lumigan
LUMIGAN® 0.01% is a prostaglandin analog indicated for the reduction of elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension. (1)
Lumigan Dosage and Administration
One drop in the affected eye(s) once daily in the evening. (2)
Dosage Forms and Strengths
Ophthalmic solution containing 0.1 mg/mL of bimatoprost. (3)
Contraindications
Hypersensitivity. (4)
Warnings and Precautions
-
Pigmentation: Pigmentation of the iris, periorbital tissue (eyelid) and eyelashes can occur. Iris pigmentation is likely to be permanent. (5.1)
- Eyelash Changes: Gradual change to eyelashes including increased length, thickness and number of lashes. Usually reversible. (5.2)
Adverse Reactions/Side Effects
Most common adverse reaction (31%) is conjunctival hyperemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Use in pediatric patients below the age of 16 years is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2022
Full Prescribing Information
1. Indications and Usage for Lumigan
LUMIGAN® (bimatoprost ophthalmic solution) 0.01% is indicated for the reduction of elevated intraocular pressure in patients with open angle glaucoma or ocular hypertension.
2. Lumigan Dosage and Administration
The recommended dosage is one drop in the affected eye(s) once daily in the evening. LUMIGAN® (bimatoprost ophthalmic solution) 0.01% should not be administered more than once daily since it has been shown that more frequent administration of prostaglandin analogs may decrease the intraocular pressure lowering effect.
Reduction of the intraocular pressure starts approximately 4 hours after the first administration with maximum effect reached within approximately 8 to 12 hours.
LUMIGAN® 0.01% may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
4. Contraindications
LUMIGAN® 0.01% is contraindicated in patients with hypersensitivity to bimatoprost or to any of the ingredients [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Pigmentation
Bimatoprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid) and eyelashes. Pigmentation is expected to increase as long as bimatoprost is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of bimatoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive
treatment should be informed of the possibility of increased pigmentation. The long term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with LUMIGAN® (bimatoprost ophthalmic solution) 0.01% can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
5.2 Eyelash Changes
LUMIGAN® 0.01% may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, thickness, and number of lashes. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Intraocular Inflammation
Prostaglandin analogs, including bimatoprost, have been reported to cause intraocular inflammation. In addition, because these products may exacerbate inflammation, caution should be used in patients with active intraocular inflammation (e.g., uveitis).
5.4 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with bimatoprost ophthalmic solution. LUMIGAN® 0.01% should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
6. Adverse Reactions/Side Effects
The following adverse reactions are described elsewhere in the labeling:
- Pigmentation including blepharal pigmentation and iris hyperpigmentation [see Warnings and Precautions (5.1)]
- Eyelash Changes [see Warnings and Precautions (5.2)]
- Intraocular Inflammation [see Warnings and Precautions (5.3)]
- Macular Edema [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Contraindications (4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a 12-month clinical study with bimatoprost ophthalmic solutions 0.01%, the most common adverse reaction was conjunctival hyperemia (31%). Approximately 1.6% of patients discontinued therapy due to conjunctival hyperemia. Other adverse drug reactions (reported in 1 to 4% of patients) with LUMIGAN® 0.01% in this study included conjunctival edema, conjunctival hemorrhage, eye irritation, eye pain, eye pruritus, erythema of eyelid, eyelids pruritus, growth of eyelashes, hypertrichosis, instillation site irritation, punctate keratitis, skin hyperpigmentation, vision blurred, and visual acuity reduced.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of LUMIGAN® 0.01%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to LUMIGAN®, or a combination of these factors include: asthma-like symptoms, dizziness, dry eye, dyspnea, eye discharge, eye edema, foreign body sensation, headache, hypersensitivity including signs and symptoms of eye allergy and allergic dermatitis, hypertension, lacrimation increased, periorbital and lid changes associated with periorbital fat atrophy leading to skin tightness, deepening of the eyelid sulcus, eyelid ptosis, enophthalmos, and eyelid retraction; and photophobia.
8. Use In Specific Populations
| LUMIGAN
bimatoprost solution/ drops |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |