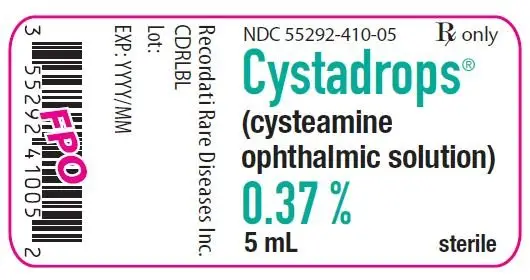
Drug Detail:Citrate of magnesia (Magnesium citrate [ mag-nee-see-um-sih-trate ])
Drug Class: Laxatives
| MAGNESIUM CITRATE
magnesium citrate liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - CVS (062312574) |
| Registrant - Aaron Industries, Inc. (030119387) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aaron Industries, Inc. | 030119387 | analysis, manufacture | |